Exploring the Artemisia Genus: An Insight into the Phytochemical and Multi-Biological Potential of A. campestris subsp. lednicensis (Spreng.) Greuter & Raab-Straube
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolic and Flavonoid Content
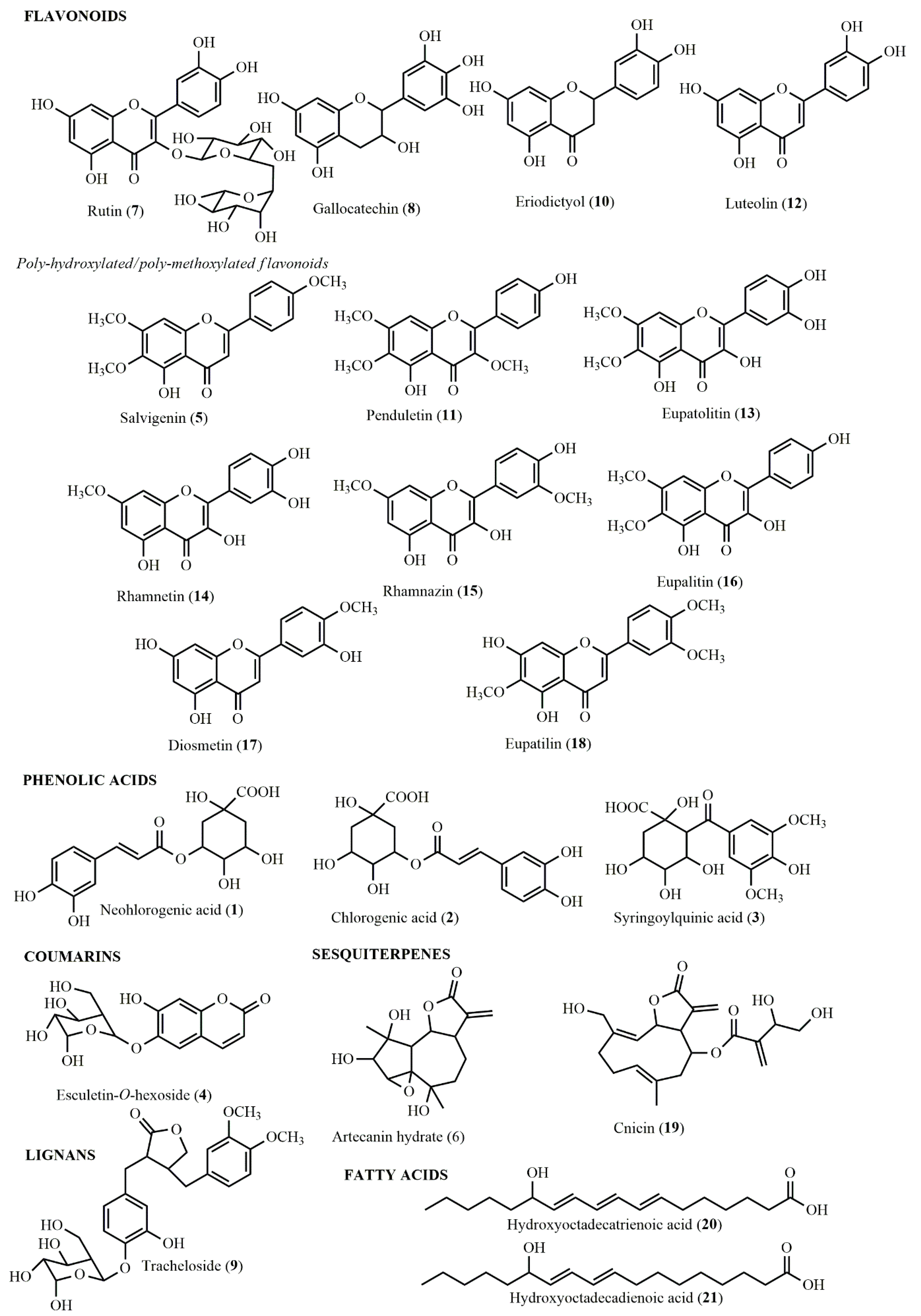
2.2. LC-HRMS/MS Metabolite Profiling
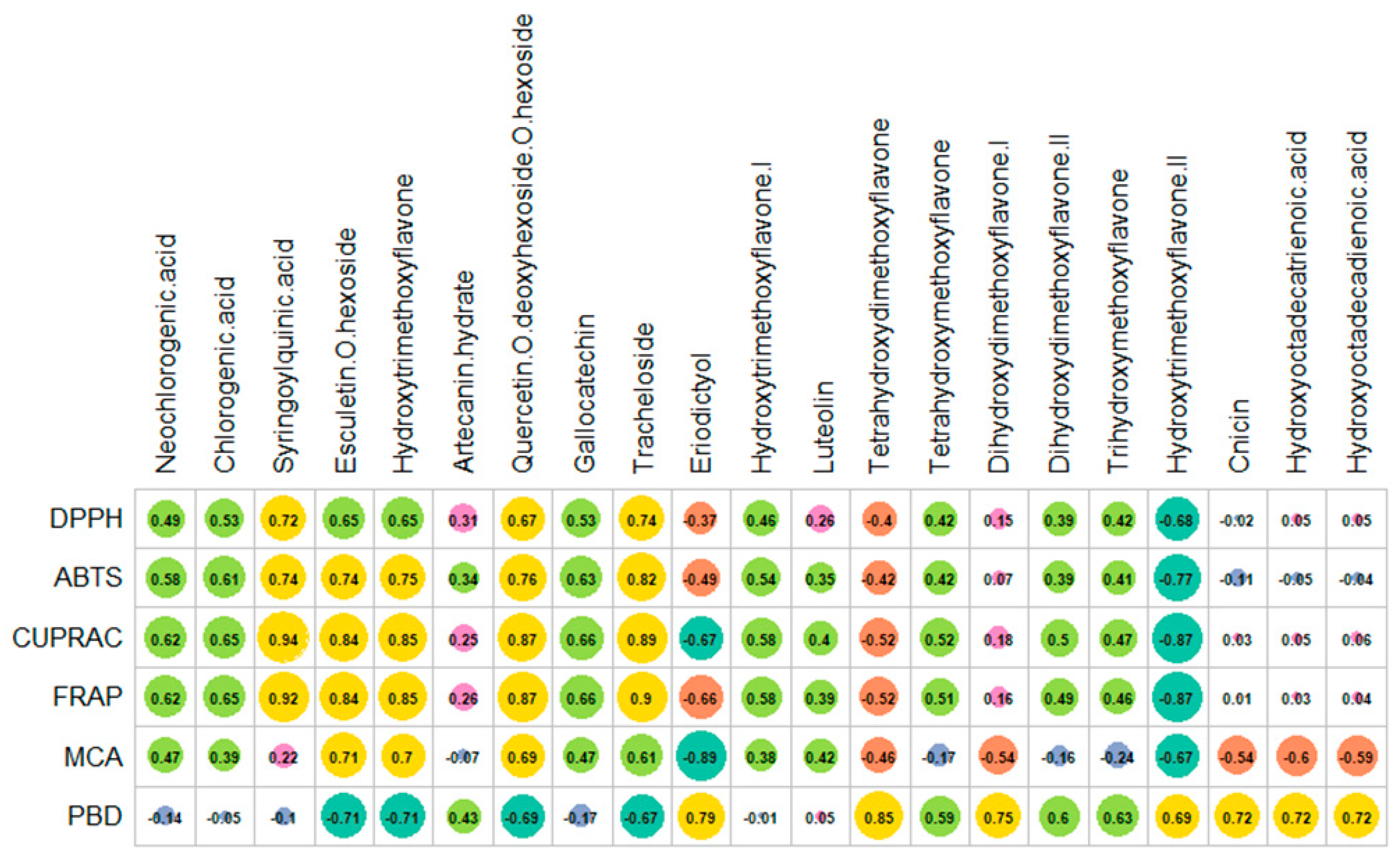
2.3. Antioxidant Properties
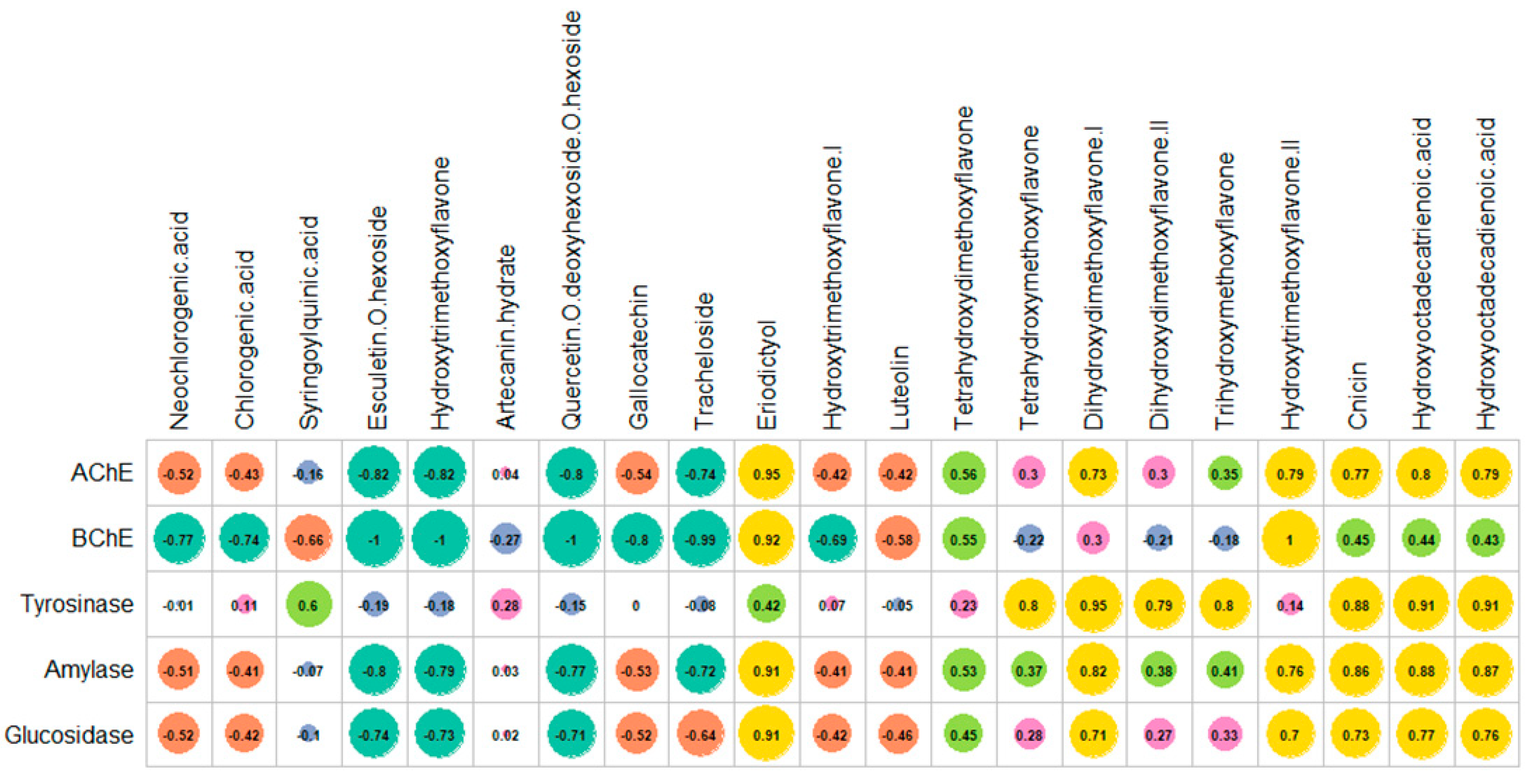
2.4. Enzyme Inhibitory Properties
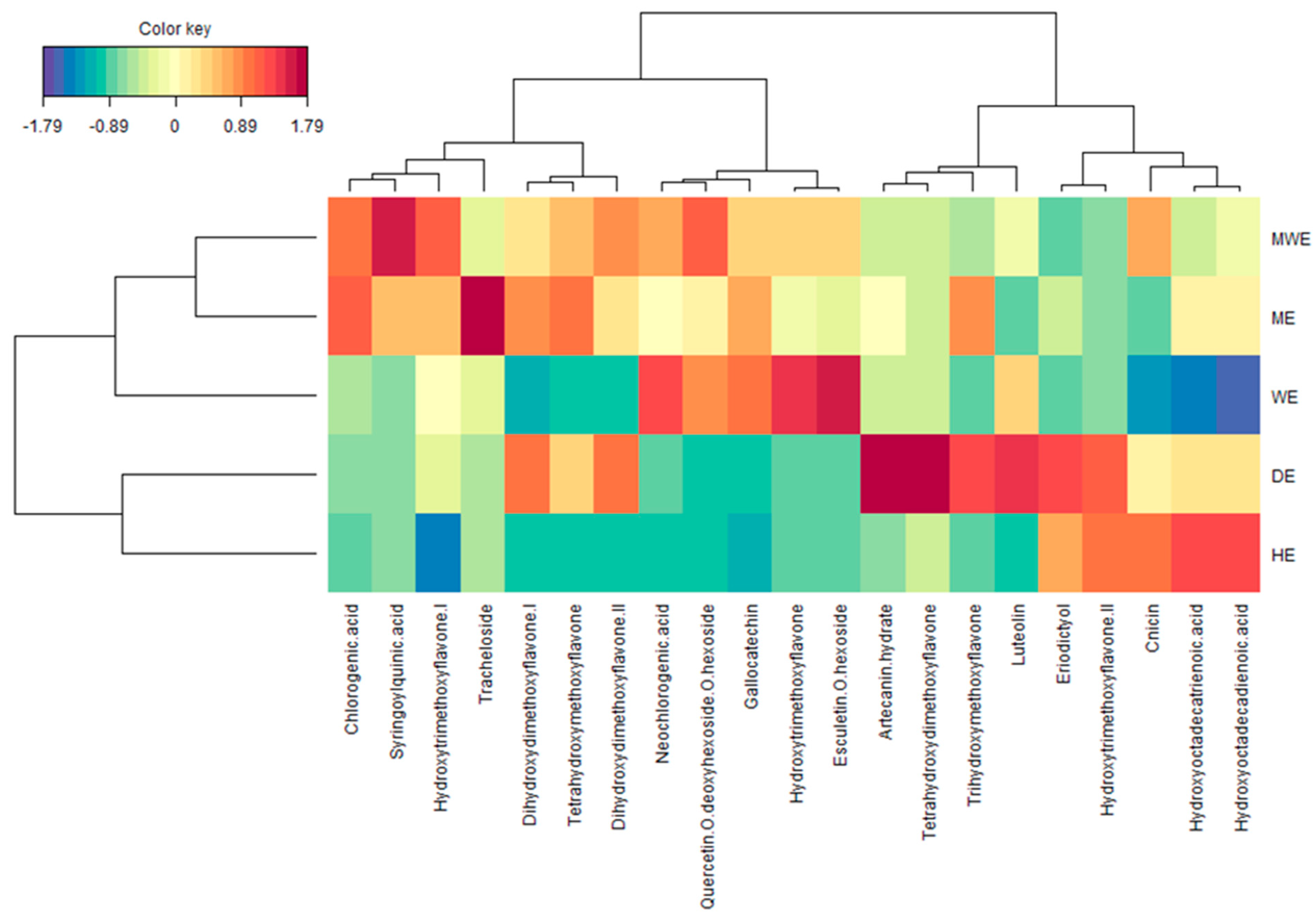
2.5. Multivariate Analysis
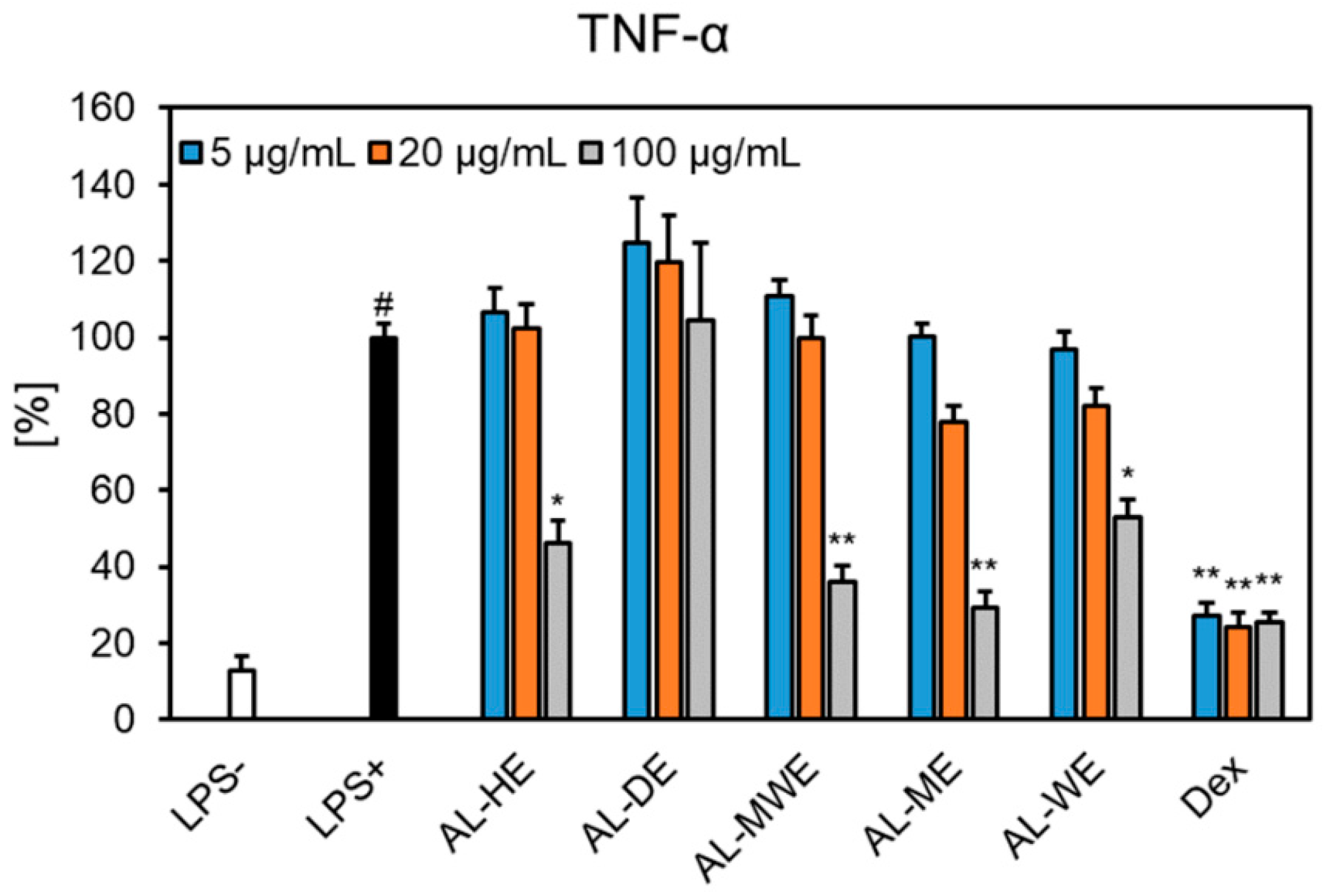
2.6. Inhibitory Activity on the Cytokine Secretion
2.7. Antimicrobial Properties
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material and Extraction
3.3. Phytochemical Analysis
3.4. Antioxidant Assays
3.5. Enzyme Inhibitory Assays
3.6. Cytokine Secretion in Human Neutrophils
3.7. Antimicrobial Assays
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [Green Version]
- Nigam, M.; Atanassova, M.; Mishra, A.P.; Pezzani, R.; Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Bioactive compounds and health benefits of Artemisia species. Nat. Prod. Commun. 2019, 14, 1934578X19850354. [Google Scholar]
- Trendafilova, A.; Moujir, L.M.; Sousa, P.M.; Seca, A.M. Research advances on health effects of edible Artemisia species and some sesquiterpene lactones constituents. Foods 2020, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Turi, C.E.; Shipley, P.R.; Murch, S.J. North American Artemisia species from the subgenus Tridentatae (Sagebrush): A phytochemical, botanical and pharmacological review. Phytochemistry 2014, 98, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Cao, S.; Qiu, F.; Zhang, B. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol. Ther. 2020, 216, 107650. [Google Scholar] [CrossRef] [PubMed]
- Bisht, D.; Kumar, D.; Kumar, D.; Dua, K.; Chellappan, D.K. Phytochemistry and pharmacological activity of the genus Artemisia. Arch. Pharm. Res. 2021, 44, 439–474. [Google Scholar] [CrossRef]
- Taleghani, A.; Emami, S.A.; Tayarani-Najaran, Z. Artemisia: A promising plant for the treatment of cancer. Bioorg. Med. Chem. 2020, 28, 115180. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, P. The genus Artemisia: A 2012–2017 literature review on chemical composition, antimicrobial, insecticidal and antioxidant activities of essential oils. Medicines 2017, 4, 68. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Abbasi, B.H.; Ahmad, N.; Khan, H.; Ali, G.S. Strategies to enhance biologically active-secondary metabolites in cell cultures of Artemisia–current trends. Crit. Rev. Biotechnol. 2017, 37, 833–851. [Google Scholar] [CrossRef]
- Ivanescu, B.; Lungu, C.; Vlase, L.; Gheldiu, A.M.; Grigorescu, C.; Corciova, A. Bioactive compounds from Artemisia campestris L. subsp. campestris. Dementia 2018, 2, 3. [Google Scholar] [CrossRef]
- Dib, I.; El Alaoui-Faris, F.E. Artemisia campestris L.: Review on taxonomical aspects, cytogeography, biological activities and bioactive compounds. Biomed. Pharmacother. 2019, 109, 1884–1906. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, H.; Laggoune, S.; Kabouche, A.; Semra, Z.; Kabouche, Z. Essential oil composition and antibacterial activity of Artemisia campestris L. from Khenchela (Algeria). Der Pharm. Lett. 2013, 5, 189–192. [Google Scholar]
- Anibogwu, R.; Jesus, K.D.; Pradhan, S.; Pashikanti, S.; Mateen, S.; Sharma, K. Extraction, isolation and characterization of bioactive compounds from Artemisia and their biological significance: A review. Molecules 2021, 26, 6995. [Google Scholar] [CrossRef]
- The World Flora Online (WFO). Available online: http://www.worldfloraonline.org/taxon/wfo-0000097078 (accessed on 5 September 2022).
- Ciocârlan, V. Flora Ilustrată a României: Pteriodophyta et Spermatophyta; Ed. Ceres: Iasi, Romania, 2000; pp. 804–808. [Google Scholar]
- Clinciu, R.R.A.; Onofrei, V.; Robu, T.; Nastase, S. Study opportunities for spreading and use of certain species of Artemisia present in Moldova. An. Univ. Din Oradea Fasc. Protecția Mediu. 2015, 25, 19–24. [Google Scholar]
- Trifan, A.; Zengin, G.; Sinan, K.I.; Sieniawska, E.; Sawicki, R.; Maciejewska-Turska, M.; Skalikca-Woźniak, K.; Luca, S.V. Unveiling the Phytochemical Profile and Biological Potential of Five Artemisia Species. Antioxidants 2022, 11, 1017. [Google Scholar] [CrossRef] [PubMed]
- Dhifallah, A.; Selmi, H.; Ouerghui, A.; Sammeri, H.; Aouini, D.; Rouissi, H. Comparative Study of Phenolic Compounds and Antiradical Activities of Four Extracts of Tunisian Artemisia herba alba. Pharm. Chem. J. 2022, 56, 226–232. [Google Scholar] [CrossRef]
- Tayyaba Batool Kazmi, S.; Naz, I.; Saniya Zahra, S.; Nasar, H.; Fatima, H.; Shuja Farooq, A.; Haq, I.-u. Phytochemical analysis and comprehensive evaluation of pharmacological potential of Artemisia brevifolia Wall. ex DC. Saudi Pharm. J. 2022, 30, 793–814. [Google Scholar] [CrossRef]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of Ethanol/Water Solvents on Phenolic Profiles and Antioxidant Properties of Beijing Propolis Extracts. Evid. Based Complement. Alternat. Med. 2015, 2015, 595393. [Google Scholar] [CrossRef] [Green Version]
- Sepahpour, S.; Selamat, J.; Abdul Manap, M.Y.; Khatib, A.; Abdull Razis, A.F. Comparative Analysis of Chemical Composition, Antioxidant Activity and Quantitative Characterization of Some Phenolic Compounds in Selected Herbs and Spices in Different Solvent Extraction Systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef] [Green Version]
- Riahi, L.; Chograni, H.; Masmoudi, A.S.; Cherif, A. Variability of phenolic compounds accumulation and bioactivity among Tunisian Artemisia arborescens L. genetic resources. Res. J. Biotechnol. 2021, 16, 164–171. [Google Scholar]
- Nataraj, N.; Hussain, M.; Ibrahim, M.; Hausmann, A.E.; Rao, S.N.V.; Kaur, S.; Khazir, J.; Mir, B.A.; Olsson, S.B. Effect of Altitude on Volatile Organic and Phenolic Compounds of Artemisia brevifolia Wall ex Dc. From the Western Himalayas. Front. Ecol. Evol. 2022, 10, 864728. [Google Scholar] [CrossRef]
- Boukhalkhal, S.; Gourine, N.; Pinto, D.; Silva, A.M.S.; Yousfi, M. UHPLC-DAD-ESI-MSn profiling variability of the phenolic constituents of Artemisia campestris L. populations growing in Algeria. Biocatal. Agric. Biotechnol. 2020, 23, 101483. [Google Scholar] [CrossRef]
- Jakovljevic, M.R.; Grujicic, D.; Vukajlovic, J.T.; Markovic, A.; Milutinovic, M.; Stankovic, M.; Vukovic, N.; Vukic, M.; Milosevic-Djordjevic, O. In vitro study of genotoxic and cytotoxic activities of methanol extracts of Artemisia vulgaris L. and Artemisia alba Turra. S. Afr. J. Bot. 2020, 132, 117–126. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety—Chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Singh, P.; Bajpai, V.; Khandelwal, N.; Varshney, S.; Gaikwad, A.N.; Srivastava, M.; Singh, B.; Kumar, B. Determination of bioactive compounds of Artemisia spp. plant extracts by LC–MS/MS technique and their in-vitro anti-adipogenic activity screening. J. Pharm. Biomed. Anal. 2021, 193, 113707. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Kashchenko, N.I.; Nikolaev, V.M.; Kim, S.-W.; Vennos, C. Bioactive phenolics of the genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS profile of the Siberian species and their inhibitory potential against α-amylase and α-glucosidase. Front. Pharmacol. 2018, 9, 756. [Google Scholar] [CrossRef]
- Mohamed, A.E.-H.H.; El-Sayed, M.; Hegazy, M.E.; Helaly, S.E.; Esmail, A.M.; Mohamed, N.S. Chemical constituents and biological activities of Artemisia herba-alba. Rec. Nat. Prod. 2010, 4, 1–25. [Google Scholar]
- Melguizo-Melguizo, D.; Diaz-de-Cerio, E.; Quirantes-Piné, R.; Švarc-Gajić, J.; Segura-Carretero, A. The potential of Artemisia vulgaris leaves as a source of antioxidant phenolic compounds. J. Funct. Foods 2014, 10, 192–200. [Google Scholar] [CrossRef]
- Han, J.; Ye, M.; Qiao, X.; Xu, M.; Wang, B.-r.; Guo, D.-A. Characterization of phenolic compounds in the Chinese herbal drug Artemisia annua by liquid chromatography coupled to electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2008, 47, 516–525. [Google Scholar] [CrossRef]
- Bourgou, S.; Rebey, I.B.; Mkadmini, K.; Isoda, H.; Ksouri, R.; Ksouri, W.M. LC-ESI-TOF-MS and GC-MS profiling of Artemisia herba-alba and evaluation of its bioactive properties. Food Res. Int. 2017, 99, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef] [Green Version]
- Han, M.H.; Park, C.; Lee, D.S.; Hong, S.H.; Choi, I.W.; Kim, G.Y.; Choi, S.H.; Shim, J.H.; Chae, J.I.; Yoo, Y.H.; et al. Cytoprotective effects of esculetin against oxidative stress are associated with the upregulation of Nrf2-mediated NQO1 expression via the activation of the ERK pathway. Int. J. Mol. Med. 2017, 39, 380–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasincu, A.; Luca, S.V.; Charalambous, C.; Neophytou, C.M.; Skalicka-Woźniak, K.; Miron, A. LC-HRMS/MS phytochemical profiling of Vernonia kotschyana Sch. Bip. ex Walp.: Potential involvement of highly-oxygenated stigmastane-type saponins in cancer cell viability, apoptosis and intracellular ROS production. S. Afr. J. Bot. 2022, 144, 83–91. [Google Scholar] [CrossRef]
- Shin, M.K.; Jeon, Y.D.; Hong, S.H.; Kang, S.H.; Kee, J.Y.; Jin, J.S. In Vivo and In Vitro effects of tracheloside on colorectal cancer cell proliferation and metastasis. Antioxidants 2021, 10, 513. [Google Scholar] [CrossRef]
- Pruccoli, L.; Morroni, F.; Sita, G.; Hrelia, P.; Tarozzi, A. Esculetin as a bifunctional antioxidant prevents and counteracts the oxidative stress and neuronal death induced by amyloid protein in SH-SY5Y cells. Antioxidants 2020, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Xu, L.; Yang, L.; Wang, X. Epigallocatechin gallate is the most effective catechin against antioxidant stress via hydrogen peroxide and radical scavenging activity. Med. Sci. Monit. 2018, 24, 8198–8206. [Google Scholar] [CrossRef]
- Chang, Y.; Fan, W.; Shi, H.; Feng, X.; Zhang, D.; Wang, L.; Zheng, Y.; Guo, L. Characterization of phenolics and discovery of α-glucosidase inhibitors in Artemisia argyi leaves based on ultra-performance liquid chromatography-tandem mass spectrometry and relevance analysis. J. Pharm. Biomed. Anal. 2022, 220, 114982. [Google Scholar] [CrossRef]
- Carvalho, I.S.; Cavaco, T.; Brodelius, M. Phenolic composition and antioxidant capacity of six Artemisia species. Ind. Crops Prod. 2011, 33, 382–388. [Google Scholar] [CrossRef]
- Xiao, J.-Q.; Liu, W.-Y.; Sun, H.-p.; Li, W.; Koike, K.; Kikuchi, T.; Yamada, T.; Li, D.; Feng, F.; Zhang, J. Bioactivity-based analysis and chemical characterization of hypoglycemic and antioxidant components from Artemisia argyi. Bioorg. Chem. 2019, 92, 103268. [Google Scholar] [CrossRef]
- Lomozová, Z.; Catapano, M.C.; Hrubša, M.; Karlíčková, J.; Macáková, K.; Kučera, R.; Mladěnka, P. Chelation of Iron and Copper by Quercetin B-Ring Methyl Metabolites, Isorhamnetin and Tamarixetin, and Their Effect on Metal-Based Fenton Chemistry. J. Agric. Food Chem. 2021, 69, 5926–5937. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron Chelation by the Powerful Antioxidant Flavonoid Quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef] [PubMed]
- Coulanges, V.; André, P.; Vidon, D.J.M. Esculetin antagonizes iron-chelating agents and increases the virulence of Listeria monocytogenes. Res. Microbiol. 1996, 147, 677–685. [Google Scholar] [CrossRef]
- Le Person, A.; Moncomble, A.; Cornard, J.-P. The Complexation of AlIII, PbII, and CuII Metal Ions by Esculetin: A Spectroscopic and Theoretical Approach. J. Phys. Chem. A 2014, 118, 2646–2655. [Google Scholar] [CrossRef]
- Muhammad, A.; Tel-Cayan, G.; Öztürk, M.; Nadeem, S.; Duru, M.E.; Anis, I.; Ng, S.W.; Shah, M.R. Biologically active flavonoids from Dodonaea viscosa and their structure–activity relationships. Ind. Crops Prod. 2015, 78, 66–72. [Google Scholar] [CrossRef]
- Nichols, E.; Szoeke, C.E.; Vollset, S.E.; Abbasi, N.; Abd-Allah, F.; Abdela, J.; Aichour, M.T.E.; Akinyemi, R.O.; Alahdab, F.; Asgedom, S.W. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef] [Green Version]
- Lalagkas, P.-N.; Polyzois, S.; Papanas, N.; Nena, E.; Vourli, N.; Kontogiorgis, C.; Constantinides, T. Prevalence of pharmacologically treated type 2 diabetes mellitus in 2012–2016 in Greece: Real-World Data. Prim. Care Diabetes 2022, 16, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Wang, Y.; Li, X.; Wang, S.; Wang, Z. Recent advance on carbamate-based cholinesterase inhibitors as potential multifunctional agents against Alzheimer’s disease. Eur. J. Med. Chem. 2022, 240, 114606. [Google Scholar] [CrossRef]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef]
- Uddin, M.J.; Russo, D.; Rahman, M.M.; Uddin, S.B.; Halim, M.A.; Zidorn, C.; Milella, L. Anticholinesterase Activity of Eight Medicinal Plant Species: In Vitro and In Silico Studies in the Search for Therapeutic Agents against Alzheimer’s Disease. Evid. Based Complement. Alternat. Med. 2021, 2021, 9995614. [Google Scholar] [CrossRef] [PubMed]
- Uriarte-Pueyo, I.; Calvo, M. Flavonoids as Acetylcholinesterase Inhibitors. Curr. Med. Chem. 2011, 18, 5289–5302. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Biswas, R.; Sharma, A.; Banerjee, S.; Biswas, S.; Katiyar, C.K. Validation of medicinal herbs for anti-tyrosinase potential. J. Herb. Med. 2018, 14, 1–16. [Google Scholar] [CrossRef]
- Gupta, M.K.; Senthilkumar, S.; Chiranjivi, A.K.; Banik, K.; Girisa, S.; Kunnumakkara, A.B.; Dubey, V.K.; Rangan, L. Antioxidant, anti-tyrosinase and anti-inflammatory activities of 3, 5-dihydroxy-4′, 7-dimethoxyflavone isolated from the leaves of Alpinia nigra. Phytomedicine Plus 2021, 1, 100097. [Google Scholar] [CrossRef]
- Arroo, R.R.; Sari, S.; Barut, B.; Özel, A.; Ruparelia, K.C.; Şöhretoğlu, D. Flavones as tyrosinase inhibitors: Kinetic studies in vitro and in silico. Phytochem. Anal. 2020, 31, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Patel, K. Therapeutic importance of eriodictyol in the medicine for the treatment of diabetes and associated complication through its insulin secretagogue properties. Metab. Clin. Exp. 2022, 128, 155056. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Rocchetti, G.; Domínguez, R.; Rocha, J.M.; Lorenzo, J.M. Application of metabolomics to decipher the role of bioactive compounds in plant and animal foods. Curr. Opin. Food Sci. 2022, 46, 100851. [Google Scholar] [CrossRef]
- Kaiser, H.F. A note on Guttman’s lower bound for the number of common factors. Br. J. Math. Stat. Psychol. 1961, 14, 1–2. [Google Scholar] [CrossRef]
- Czerwińska, M.E.; Dudek, M.K.; Pawłowska, K.A.; Pruś, A.; Ziaja, M.; Granica, S. The influence of procyanidins isolated from small-leaved lime flowers (Tilia cordata Mill.) on human neutrophils. Fitoterapia 2018, 127, 115–122. [Google Scholar] [CrossRef]
- Luca, S.V.; Kulinowski, Ł.; Ciobanu, C.; Zengin, G.; Czerwińska, M.E.; Granica, S.; Xiao, J.; Skalicka-Woźniak, K.; Trifan, A. Phytochemical and multi-biological characterization of two Cynara scolymus L. varieties: A glance into their potential large scale cultivation and valorization as bio-functional ingredients. Ind. Crops Prod. 2022, 178, 114623. [Google Scholar] [CrossRef]
- Trifan, A.; Skalicka-Woźniak, K.; Granica, S.; Czerwińska, M.E.; Kruk, A.; Marcourt, L.; Wolfender, J.-L.; Wolfram, E.; Esslinger, N.; Grubelnik, A. Symphytum officinale L.: Liquid-liquid chromatography isolation of caffeic acid oligomers and evaluation of their influence on pro-inflammatory cytokine release in LPS-stimulated neutrophils. J. Ethnopharmacol. 2020, 262, 113169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; An, J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007, 45, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majdan, M.; Kiss, A.K.; Hałasa, R.; Granica, S.; Osińska, E.; Czerwińska, M.E. Inhibition of neutrophil functions and antibacterial effects of tarragon (Artemisia dracunculus L.) infusion—phytochemical characterization. Front. Pharmacol. 2020, 11, 947. [Google Scholar] [CrossRef] [PubMed]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene lactones from Artemisia genus: Biological activities and methods of analysis. J. Anal. Methods Chem. 2015, 2015, 247685. [Google Scholar] [CrossRef] [Green Version]
- Svanborg, C.; Godaly, G.; Hedlund, M. Cytokine responses during mucosal infections: Role in disease pathogenesis and host defence. Curr. Opin. Microbiol. 1999, 2, 99–103. [Google Scholar] [CrossRef]
- de Oliveira Lima, M.; de Medeiros, A.A.; Silva, K.S.; Cardoso, G.; de Oliveira Lima, E.; de Oliveira Pereira, F. Investigation of the antifungal potential of linalool against clinical isolates of fluconazole resistant Trichophyton rubrum. J. Mycol. Med. 2017, 27, 195–202. [Google Scholar] [CrossRef]
- Vega, A.; Wendel, G.; Maria, A.; Pelzer, L. Antimicrobial activity of Artemisia douglasiana and dehydroleucodine against Helicobacter pylori. J. Ethnopharmacol. 2009, 124, 653–655. [Google Scholar] [CrossRef]
- Palacios-Espinosa, J.F.; Núñez-Aragón, P.N.; Gomez-Chang, E.; Linares, E.; Bye, R.; Romero, I. Anti-Helicobacter pylori activity of Artemisia ludoviciana subsp. mexicana and two of its bioactive components, Estafiatin and Eupatilin. Molecules 2021, 26, 3654. [Google Scholar]
- Zengin, G.; Aktumsek, A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant to Turkey. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, 9–15. [Google Scholar]
- Arendrup, M.C.; Kahlmeter, G.; Guinea, J.; Meletiadis, J.; Testing, S.o.A.S.T.o.t.E.E.C.f.A.S. How to: Perform antifungal susceptibility testing of microconidia-forming dermatophytes following the new reference EUCAST method E. Def 11.0, exemplified by Trichophyton. Clin. Microbiol. Infect. 2021, 27, 55–60. [Google Scholar] [CrossRef]





| Sample | Yield (%) | Total Phenolic Content (mg GAE/g) | Total Flavonoid Content (mg RE/g) |
|---|---|---|---|
| AL-HE | 4.33 | 17.11 ± 0.27 e | 9.57 ± 0.14 c |
| AL-DE | 6.20 | 20.67 ± 0.52 d | 16.43 ± 0.37 b |
| AL-ME | 12.60 | 84.42 ± 0.33 b | 23.13 ± 0.73 a |
| AL-MWE | 22.59 | 104.00 ± 0.69 a | 15.08 ± 0.26 b |
| AL-WE | 23.55 | 71.73 ± 0.78 c | 8.92 ± 0.31 c |
| No. | Proposed Identity | Class | TR (min) | [M-H]− (m/z) | MF | MS/MS (m/z) | AL-HE | AL-DE | AL-ME | AL-MWE | AL-WE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Neochlorogenic acid * | Phenolic acid | 9.8 | 353.0893 | C16H18O9 | 191.0484, 179.0252, 135.0370 | – | × | × | × | × |
| 2 | Chlorogenic acid * | Phenolic acid | 13.2 | 353.0893 | C16H18O9 | 191.0568, 173.0429, 135.0461 | – | × | × | × | × |
| 3 | Syringoylquinic acid | Phenolic acid | 14.6 | 371.0969 | C16H20O10 | 353.0882, 339.0740, 209.0675, 179.0358 | – | – | × | × | – |
| 4 | Esculetin-O-hexoside | Coumarin | 14.9 | 339.0715 | C15H16O9 | 177.0233, 149.0157, 133.0217, 105.0327 | – | – | × | × | × |
| 5 | Hydroxytrimethoxyflavone (e.g., salvigenin) | Flavonoid | 16.4 | 327.0847 | C18H16O6 | 241.0099, 177.0415, 151.0075 | – | – | × | × | × |
| 6 | Artecanin hydrate | Sesquiterpene | 19.6 | 295.1169 | C15H20O6 | 251.1300, 207.1409, 189.1280, 151.0831 | × | × | × | × | × |
| 7 | Quercetin-O-deoxyhexoside-O-hexoside (e.g., rutin) | Flavonoid | 23.1 | 609.1476 | C27H30O16 | 300.0287, 271.0255, 151.0035 | – | – | × | × | × |
| 8 | Gallocatechin | Flavonoid | 25.0 | 305.0671 | C15H14O7 | 225.1144, 147.0823 | – | × | × | × | × |
| 9 | Tracheloside | Lignan | 26.9 | 549.1985 | C27H34O12 | 387.1681, 207.1037, 179.0375, 161.0251 | – | – | × | × | × |
| 10 | Eriodictyol | Flavonoid | 29.0 | 287.0567 | C15H12O6 | 151.0046, 135.0479 | × | × | × | × | × |
| 11 | Dihydroxytrimethoxyflavone I (e.g., penduletin) | Flavonoid | 30.1 | 343.0813 | C18H16O7 | 328.0382, 313.0382, 298.0133, 285.0421, 270.0199, 255.0318, 242.0284 | – | × | × | × | × |
| 12 | Luteolin * | Flavonoid | 31.0 | 285.0400 | C15H10O6 | 175.0386, 133.0313 | – | × | × | × | × |
| 13 | Tetrahydroxydimethoxyflavone (e.g., eupatolitin) | Flavonoid | 31.5 | 345.0602 | C17H14O8 | 330.0402, 315.0188, 287.0296, 259.0301, 259.0301, 215.0351, 175.0091, 149.0308, 121.0326 | – | × | – | – | – |
| 14 | Tetrahydroxymethoxyflavone (e.g., rhamnetin) | Flavonoid | 31.7 | 315.0509 | C16H12O7 | 300.0327, 271.0269, 255.0312, 243.0322, 227.0356, 215.0350, 171.0409, 147.0202 | – | × | × | × | – |
| 15 | Dihydroxydimethoxyflavone I (e.g., rhamnazin) | Flavonoid | 33.9 | 329.0678 | C17H14O7 | 314.0456, 299.0241, 271.0279, 271.0272, 243.0312, 227.0430, 215.0360, 199.0421, 185.0236, 161.0264, 151.0068, 133.0347 | × | × | × | × | – |
| 16 | Dihydroxydimethoxyflavone II (e.g., eupalitin) | Flavonoid | 35.4 | 329.0678 | C17H14O7 | 314.0456, 299.0241, 271.0279, 271.0272, 243.0312, 227.0430, 215.0360, 199.0421, 185.0236, 161.0264, 151.0068, 133.0347 | – | × | × | – | – |
| 17 | Trihydroxymethoxyflavone (e.g., diosmetin) | Flavonoid | 37.0 | 299.0566 | C16H12O6 | 284.0259, 255.0179, 239.0292, 227.0330, 151.0077, 133.0252 | – | × | × | × | – |
| 18 | Dihydroxytrimethoxyflavone II (e.g., eupatilin) | Flavonoid | 37.6 | 343.0813 | C18H16O7 | 328.0382, 313.0382, 298.0133, 285.0421, 270.0199, 255.0318, 242.0284 | × | × | – | – | – |
| 19 | Cnicin | Sesquiterpene | 39.4 | 377.1617 | C20H26O7 | 295.1213, 251.1322, 189.1257, 151.07060 | × | × | × | × | – |
| 20 | Hydroxyoctadecatrienoic acid | Fatty acid | 46.8 | 293.2118 | C18H30O3 | 275.1973, 224.1359, 195.1381 | × | × | × | × | – |
| 21 | Hydroxyoctadecadienoic acid | Fatty acid | 49.3 | 295.2269 | C18H32O3 | 277.2162, 195.1407, 171.1029 | × | × | × | × | – |
| Sample | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | Metal Chelating (mg EDTAE/g) | Phosphomolybdenum (mmol TE/g) |
|---|---|---|---|---|---|---|
| AL-HE | 0.71 ± 0.07 d | 20.57 ± 1.06 d | 38.56 ± 0.62 d | 21.68 ± 0.51 d | 13.64 ± 1.52 b | 1.46 ± 0.13 b |
| AL-DE | 7.63 ± 0.49 c | 44.96 ± 1.95 c | 44.00 ± 0.94 d | 25.04 ± 0.77 d | 12.88 ± 0.99 b | 2.11 ± 0.11 a |
| AL-ME | 213.68 ± 7.30 a | 356.35 ± 9.46 a | 311.21 ± 3.66 a | 202.34 ± 3.26 a | 13.35 ± 0.33 b | 1.42 ± 0.02 b |
| AL-MWE | 61.74 ± 0.12 b | 152.40 ± 0.21 b | 275.79 ± 0.47 b | 166.59 ± 3.85 b | 22.25 ± 0.24 a | 1.41 ± 0.06 b |
| AL-WE | 58.78 ± 0.09 b | 151.76 ± 0.15 b | 143.32 ± 1.08 c | 91.93 ± 0.30 c | 21.61 ± 0.34 a | 0.92 ± 0.01 c |
| Sample | AChE (mg GALAE/g) | BChE (mg GALAE/g) | Tyrosinase (mg KAE/g) | Amylase (mmol ACAE/g) | Glucosidase (mmol ACAE/g) |
|---|---|---|---|---|---|
| AL-HE | 3.26 ± 0.06 b | 3.18 ± 0.06 a | 35.89 ± 2.11 c | 0.35 ± 0.01 a | 2.16 ± 0.03 a |
| AL-DE | 3.64 ± 0.09 a | 2.82 ± 0.15 b | 41.53 ± 0.34 b | 0.38 ± 0.02 a | 2.21 ± 0.02 a |
| AL-ME | 2.66 ± 0.08 c | n.a. | 48.60 ± 0.67 a | 0.29 ± 0.02 b | 2.06 ± 0.13 b |
| AL-MWE | 1.21 ± 0.02 d | n.a. | 40.38 ± 0.48 b | 0.20 ± 0.01 c | 0.88± 0.02 c |
| AL-WE | 0.15 ± 0.05 e | n.a. | 18.62 ± 1.28 d | 0.05 ± 0.00 d | 0.43 ± 0.03 d |
| Microorganism | MIC (mg/L) | |||||
|---|---|---|---|---|---|---|
| AL-HE | AL-DE | AL-ME | AL-MWE | AL-WE | Positive Control | |
| Gram-positive bacteria | Vancomycin | |||||
| Staphylococcus aureus ATCC 25923 | >5000 | 5000 | 5000 | >5000 | 5000 | 0.98 |
| Staphylococcus epidermidis ATCC 12228 | >5000 | 5000 | 5000 | >5000 | 5000 | 0.98 |
| Micrococcus luteus ATCC 10240 | >5000 | 5000 | 5000 | >5000 | 2500 | 0.12 |
| Enterococcus faecalis ATCC 29212 | >5000 | >5000 | >5000 | >5000 | >5000 | 1.95 |
| Bacillus subtilis ATCC 6633 | >5000 | >5000 | >5000 | >5000 | 5000 | 0.24 |
| Bacillus cereus ATCC 10876 | >5000 | 5000 | >5000 | >5000 | 5000 | 0.98 |
| Gram-negative bacteria | Ciprofloxacin | |||||
| Salmonella Typhimurium ATCC 14028 | >5000 | >5000 | >5000 | >5000 | >5000 | 0.061 |
| Escherichia coli ATCC 25922 | >5000 | >5000 | >5000 | >5000 | >5000 | 0.015 |
| Proteus mirabilis ATCC 12453 | >5000 | 5000 | >5000 | >5000 | >5000 | 0.030 |
| Klebsiella pneumoniae ATCC 13883 | >5000 | >5000 | >5000 | >5000 | >5000 | 0.122 |
| Pseudomonas aeruginosa ATCC 90271 | >5000 | >5000 | >5000 | >5000 | >5000 | 0.488 |
| Helicobacter pylori ATCC 43504 | 625 | 2500 | 5000 | 625 | 625 | 1.0 (Ofloxacin) |
| Yeasts | Nystatin | |||||
| Candida albicans ATCC 2091 | 5000 | 5000 | 5000 | 2500 | 5000 | 0.48 |
| Candida parapsilosis ATCC 22019 | 5000 | 5000 | 5000 | 2500 | 2500 | 0.24 |
| Candida glabrata ATCC 90030 | 10,000 | 10,000 | 10,000 | 10,000 | 10,000 | 0.24 |
| Dermatophytes | Terbinafine | |||||
| Trichophyton rubrum ATCC 28188 | >1000 | >1000 | >1000 | >1000 | >1000 | 0.031 |
| Trichophyton mentagrophytes ATCC 9533 | >1000 | >1000 | >1000 | >1000 | >1000 | 0.031 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifan, A.; Czerwińska, M.E.; Mardari, C.; Zengin, G.; Sinan, K.I.; Korona-Glowniak, I.; Skalicka-Woźniak, K.; Luca, S.V. Exploring the Artemisia Genus: An Insight into the Phytochemical and Multi-Biological Potential of A. campestris subsp. lednicensis (Spreng.) Greuter & Raab-Straube. Plants 2022, 11, 2874. https://doi.org/10.3390/plants11212874
Trifan A, Czerwińska ME, Mardari C, Zengin G, Sinan KI, Korona-Glowniak I, Skalicka-Woźniak K, Luca SV. Exploring the Artemisia Genus: An Insight into the Phytochemical and Multi-Biological Potential of A. campestris subsp. lednicensis (Spreng.) Greuter & Raab-Straube. Plants. 2022; 11(21):2874. https://doi.org/10.3390/plants11212874
Chicago/Turabian StyleTrifan, Adriana, Monika E. Czerwińska, Constantin Mardari, Gokhan Zengin, Kouadio Ibrahime Sinan, Izabela Korona-Glowniak, Krystyna Skalicka-Woźniak, and Simon Vlad Luca. 2022. "Exploring the Artemisia Genus: An Insight into the Phytochemical and Multi-Biological Potential of A. campestris subsp. lednicensis (Spreng.) Greuter & Raab-Straube" Plants 11, no. 21: 2874. https://doi.org/10.3390/plants11212874
APA StyleTrifan, A., Czerwińska, M. E., Mardari, C., Zengin, G., Sinan, K. I., Korona-Glowniak, I., Skalicka-Woźniak, K., & Luca, S. V. (2022). Exploring the Artemisia Genus: An Insight into the Phytochemical and Multi-Biological Potential of A. campestris subsp. lednicensis (Spreng.) Greuter & Raab-Straube. Plants, 11(21), 2874. https://doi.org/10.3390/plants11212874











