AOX1a Expression in Arabidopsis thaliana Affects the State of Chloroplast Photoprotective Systems under Moderately High Light Conditions
Abstract
:1. Introduction
2. Results
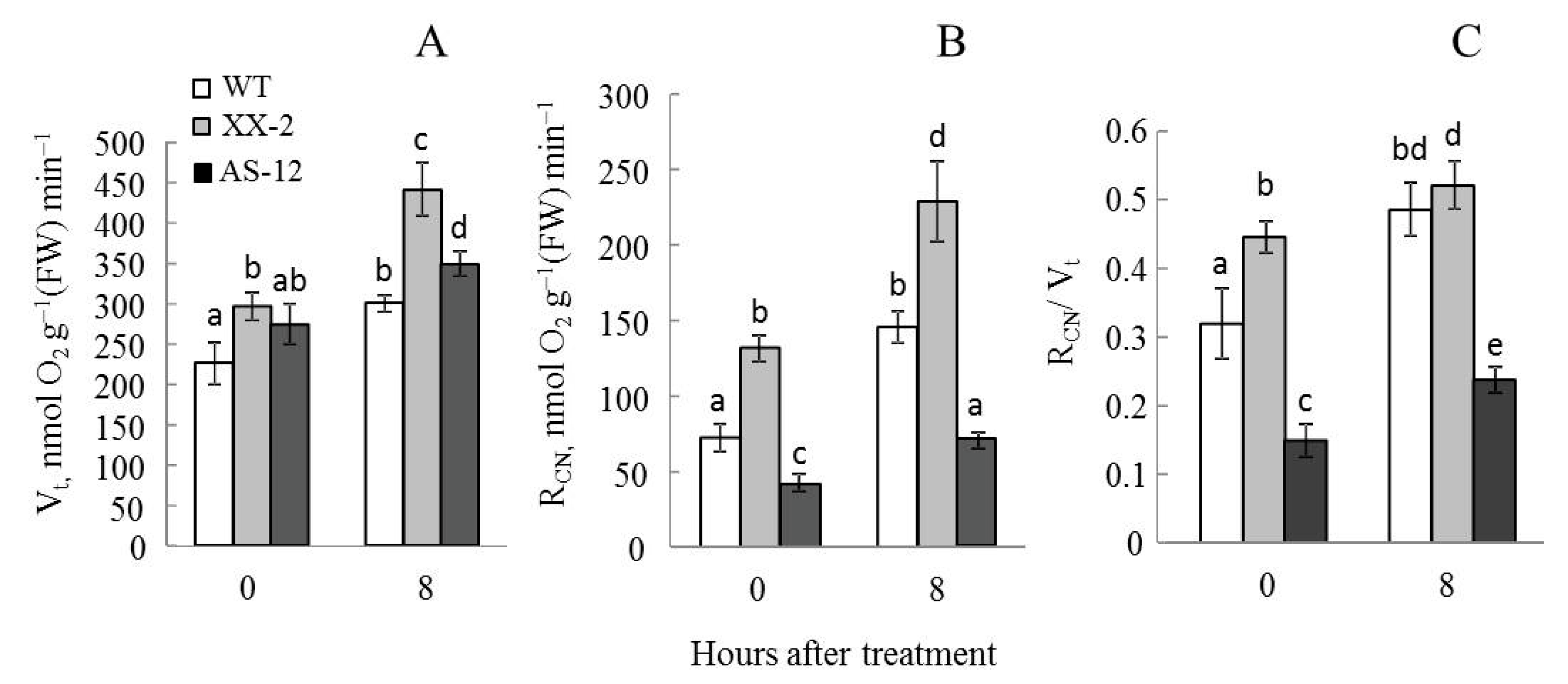
2.1. Plants with Altered AOX1a Expression Exhibit Different Respiration Responses to MHL
2.2. Specific Leaf Weight and Chl Content
2.3. Effects of MHL on Photosynthetic Apparatus Functioning
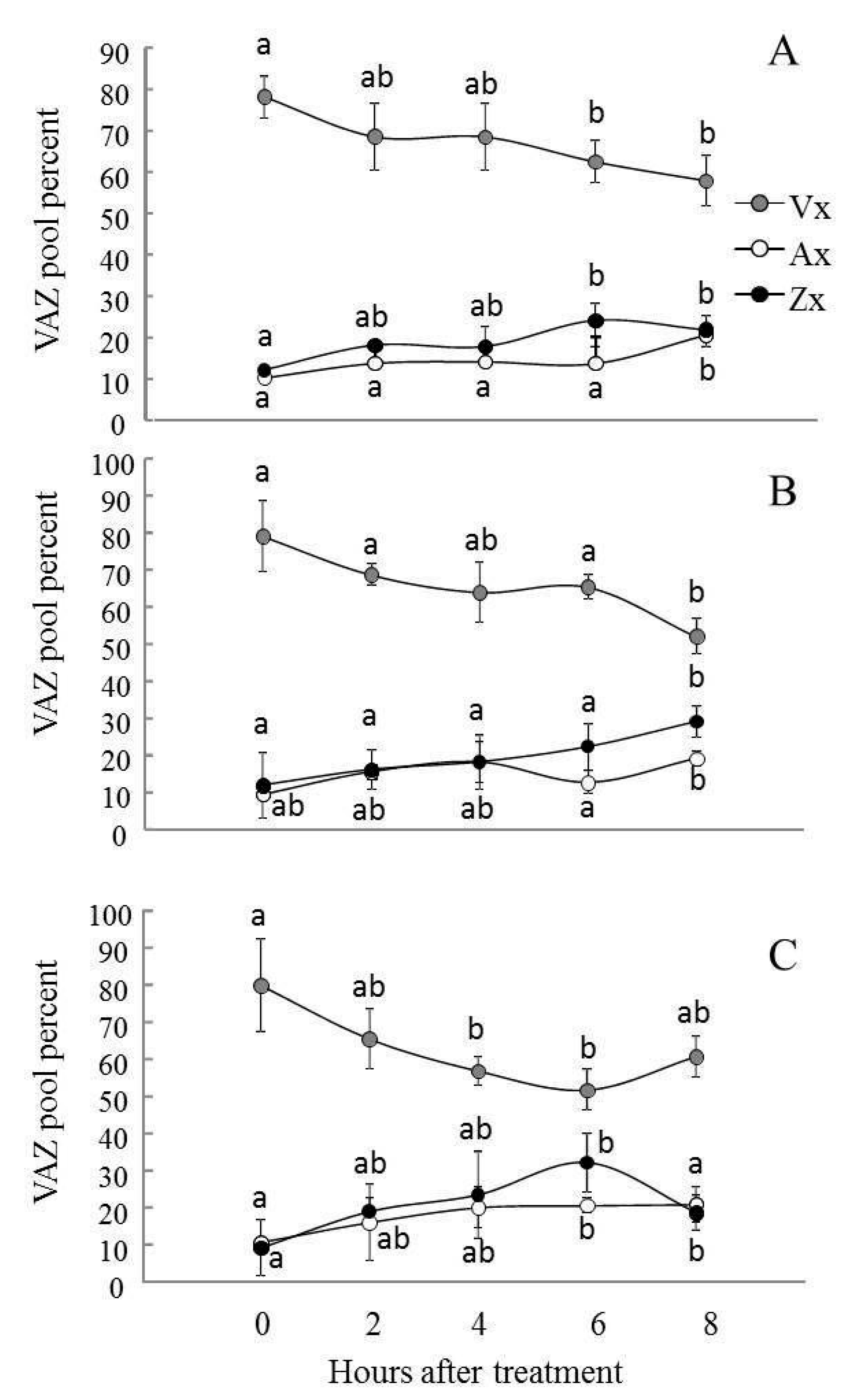
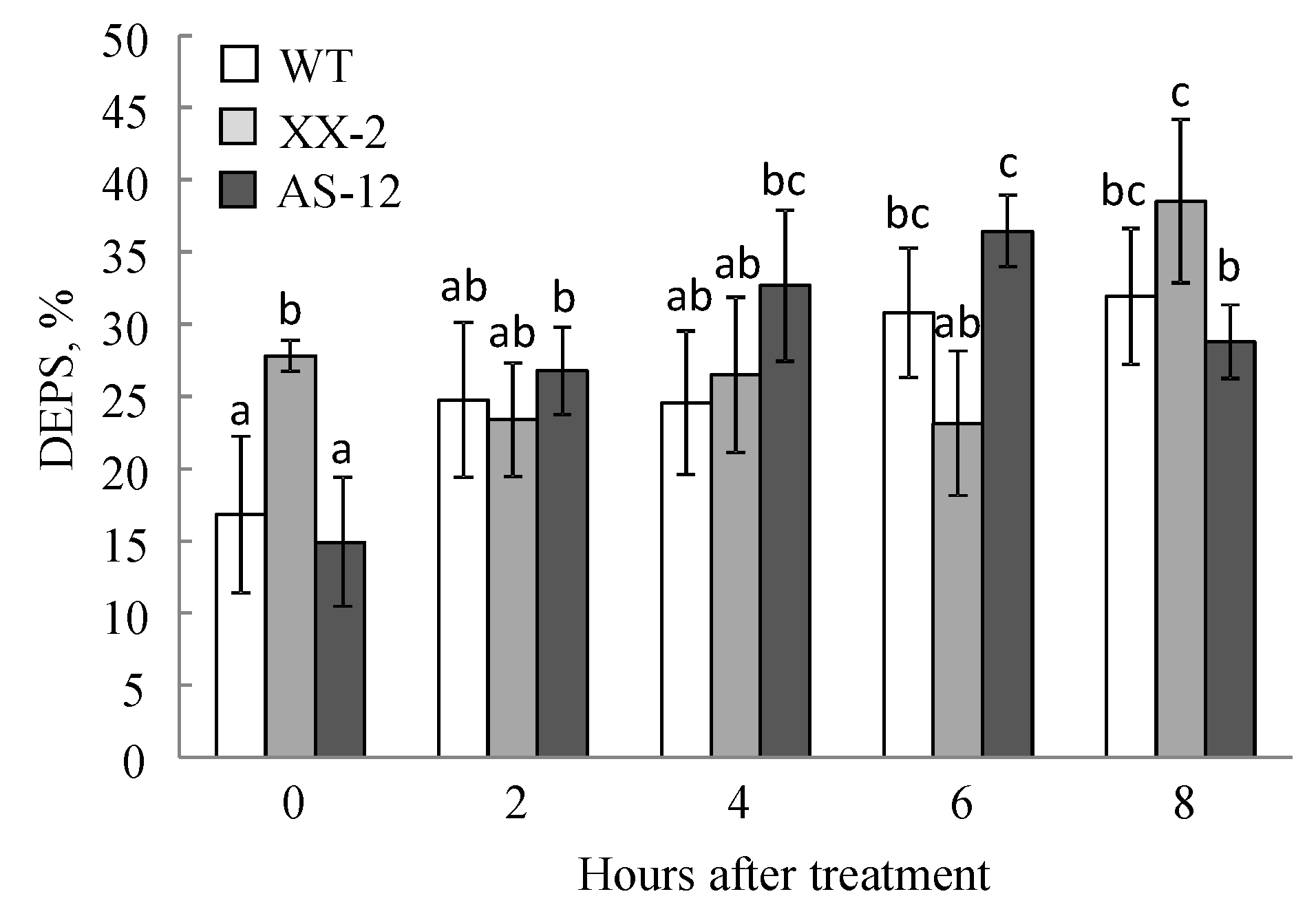
2.4. Violaxanthin De-Epoxidation and the Effectiveness of the Violaxanthin Cycle Decreased in AS-12 Plants after 8 h of MHL
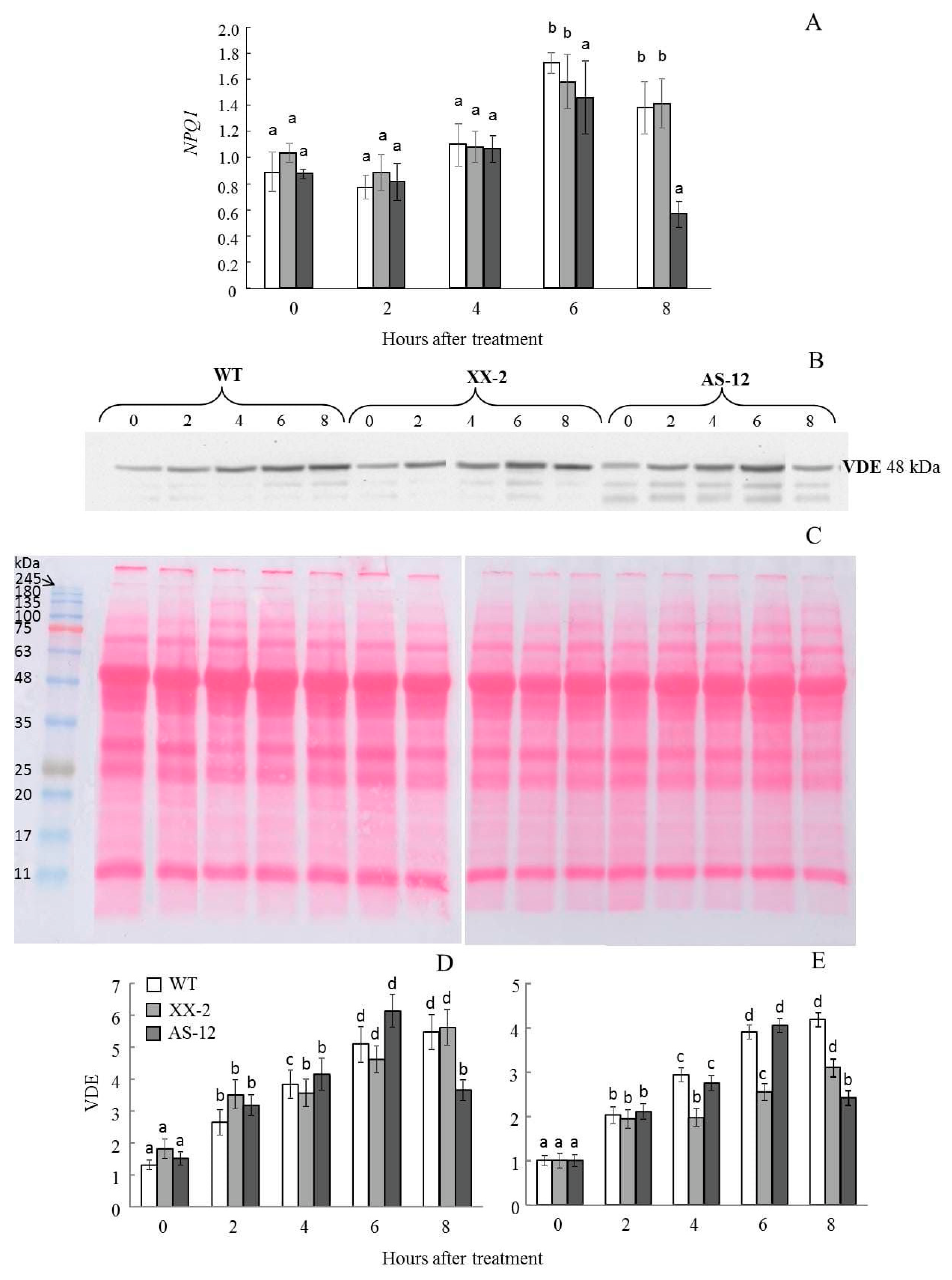
2.5. Violaxanthin De-Epoxidase Protein Content Follows the NPQ1 Expression
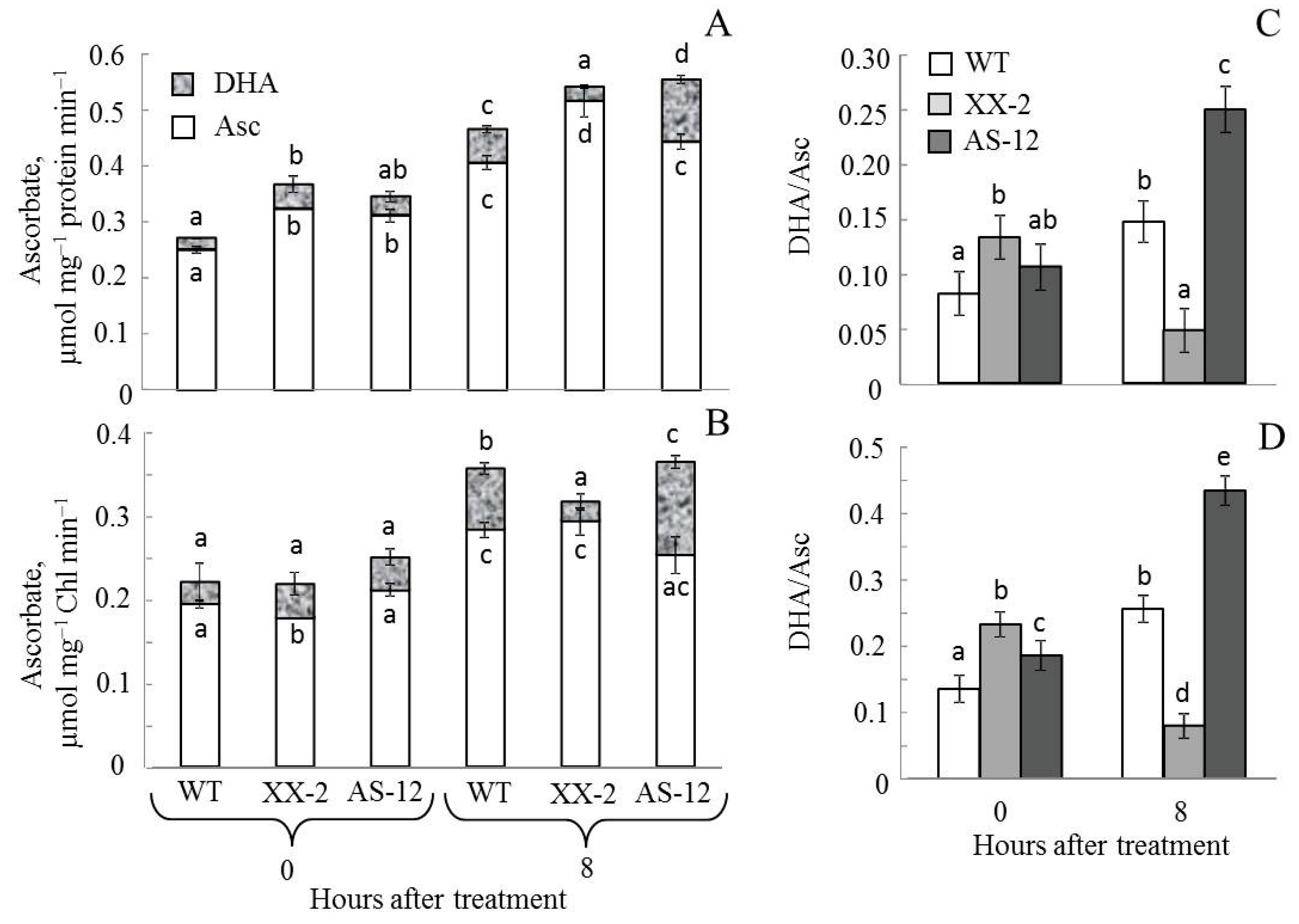
2.6. Changes in the Ascorbate Pool in Leaves and Chloroplasts of A. thaliana WT and Aox1a Lines after MHL
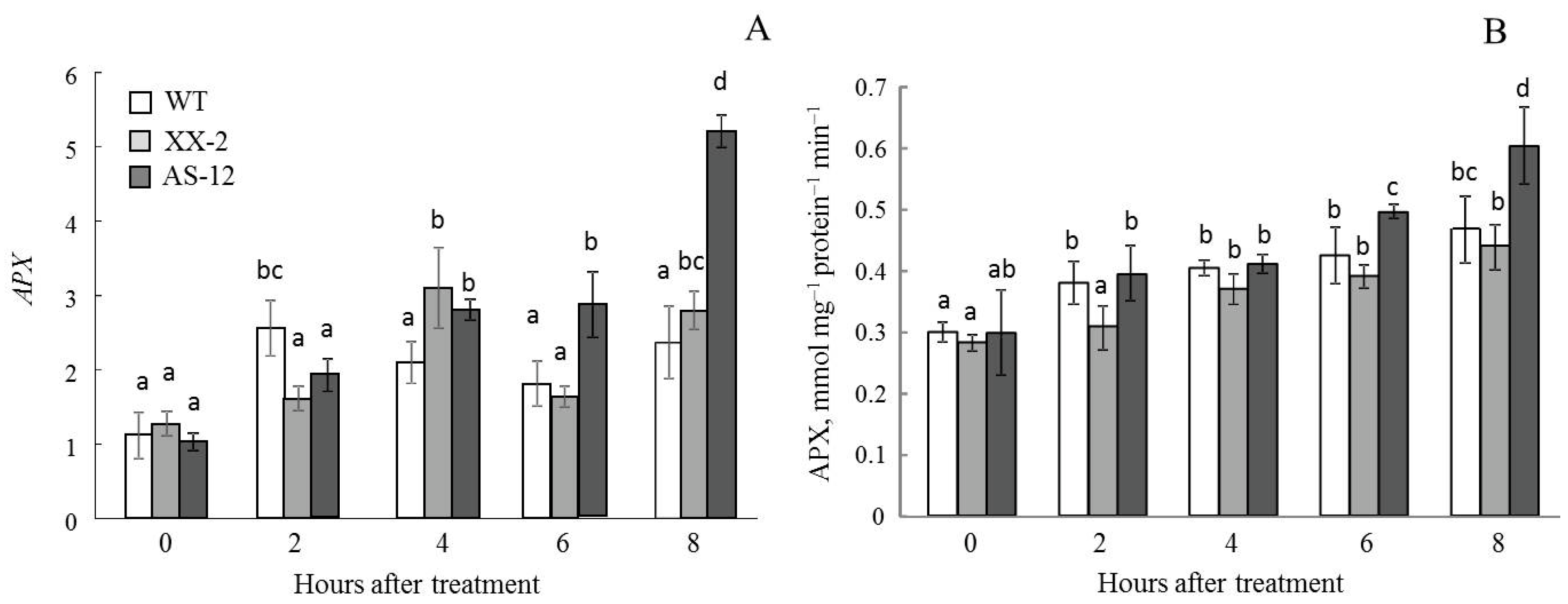
2.7. Gene Expression and Activity of Ascorbate Peroxidase in Leaves and Chloroplasts
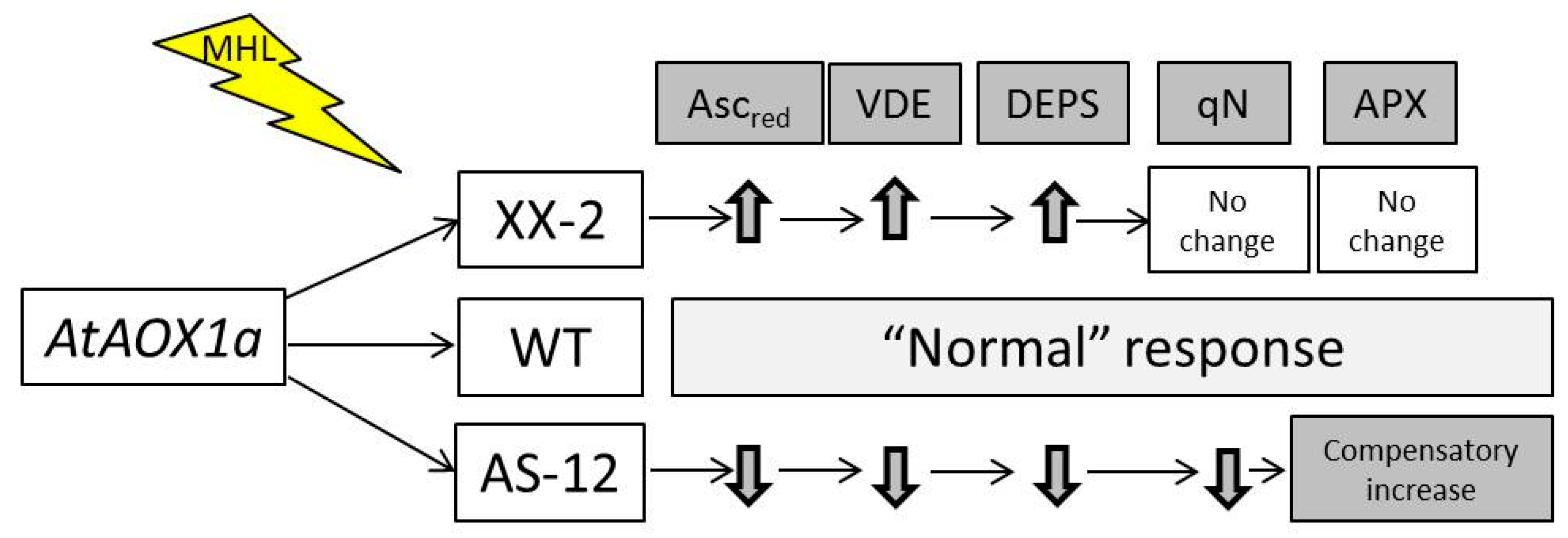
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Material and Growth Conditions
5.2. Specific Leaf Weight
5.3. O2 Exchange and CN-Resistant Respiration in Leaf Tissues
5.4. Gene Expression Analysis
5.5. SDS-PAGE and VDE Western Blot Analysis
5.6. Chl Extraction and Quantification
5.7. Carotenoid Analysis and Xanthophyll Cycle Activity Measurement
5.8. Chl Fluorescence Measurements
5.9. Measurement of Ascorbate and Dehydroascorbate Levels
5.10. Analysis of Ascorbate Peroxidase Activity
5.11. Chloroplast Isolation
5.12. Determination of Chl and Protein in Isolated Chloroplasts
5.13. Determination of Ascorbate and Dehydroascorbate Content in Isolated Chloroplasts
5.14. Measurement of Ascorbate Peroxidase Activity in Isolated Chloroplasts
5.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vanlerberghe, G.C.; Dahal, K.; Alber, N.A.; Chadee, A. Photosynthesis, respiration and growth: A carbon and energy balancing act for alternative oxidase. Mitochondrion 2020, 52, 197–211. [Google Scholar] [CrossRef]
- Van Aken, O. Mitochondrial redox systems as central hubs in plant metabolism and signalling. Plant Physiol. 2021, 186, 36–52. [Google Scholar] [CrossRef]
- Noguchi, K.; Yoshida, K. Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion 2008, 8, 87–99. [Google Scholar] [CrossRef]
- Dinakar, C.; Raghavendra, A.S.; Padmasree, K. Importance of AOX pathway in optimizing photosynthesis under high light stress: Role of pyruvate and malate in activating AOX. Physiol. Plant. 2010, 139, 13–26. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Xu, F.; Zhang, Z.-W.; Chen, Y.-E.; Du, J.-B.; Jia, S.-D.; Yuan, S.; Lin, H.-H. Effects of light on cyanide-resistant respiration and alternative oxidase function in Arabidopsis seedlings. Plant Cell Environ. 2010, 33, 2121–2131. [Google Scholar] [CrossRef]
- Garmash, E.V.; Grabelnych, O.I.; Velegzhaninov, I.O.; Borovik, O.A.; Dalke, I.V.; Voinikov, V.K.; Golovko, T.K. Light regulation of mitochondrial alternative oxidase pathway during greening of etiolated wheat seedlings. J. Plant Physiol. 2017, 174, 75–84. [Google Scholar] [CrossRef]
- Giraud, E.; Ho, L.H.M.; Clifton, R.; Carroll, A.; Estavillo, G.; Tan, Y.-F.; Howell, K.A.; Ivanova, A.; Pogson, B.J.; Millar, A.H.; et al. The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol. 2008, 147, 595–610. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, C.K.A.; Yamori, W.; Takahashi, S.; Terashima, I.; Noguchi, K. Mitochondrial Alternative pathway-associated photoprotection of photosystem II is related to the photorespiratory pathway. Plant Cell Physiol. 2016, 57, 1426–1431. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Ozaki, H.; Noguchi, K. The Mitochondrial respiratory chain maintains the photosynthetic electron flow in Arabidopsis thaliana leaves under high-light stress. Plant Cell Physiol. 2020, 61, 283–295. [Google Scholar] [CrossRef]
- Zhang, D.; Yuan, S.; Xu, F.; Zhu, F.; Yuan, M.; Ye, H.; Guo, H.; Lv, X.; Yin, Y.; Lin, H. Light intensity affects chlorophyll synthesis during greening process by metabolite signal from mitochondrial alternative oxidase in Arabidopsis. Plant Cell Environ. 2016, 39, 12–25. [Google Scholar] [CrossRef]
- Yoshida, K.; Terashima, I.; Noguchi, K. How and why does the mitochondrial respiratory chain respond to light? Plant Signal. Behav. 2011, 6, 864–866. [Google Scholar] [CrossRef] [Green Version]
- Dahal, K.; Martyn, G.D.; Alber, N.A.; Vanlerberghe, G.C. Coordinated regulation of photosynthetic and respiratory components is necessary to maintain chloroplast energy balance in varied growth conditions. J. Exp. Bot. 2017, 68, 657–671. [Google Scholar] [CrossRef] [Green Version]
- Niyogi, K.K.; Grossman, A.R.; Björkman, O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell. 1998, 10, 1121–1134. [Google Scholar] [CrossRef] [Green Version]
- Jahns, P.; Latowski, D.; Strzalka, K. Mechanism and regulation of the violaxanthin cycle: The role of antenna proteins and membrane lipids. Biochim. Biophys. Acta 2009, 1787, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Bartoli, C.G.; Yu, J.; Gómez, F.; Fernández, L.; McIntosh, L.; Foyer, C.H. Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J. Exp. Bot. 2006, 57, 1621–1631. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Sweetlove, L.J.; Fernie, A.R. Operation and function of the tricarboxylic acid cycle in the illuminated leaf. Physiol. Plant. 2007, 129, 45–56. [Google Scholar] [CrossRef]
- Zhang, L.-T.; Zhang, Z.-S.; Gao, H.-Y.; Meng, X.-L.; Yang, C.; Liu, J.-G.; Meng, Q.-W. The mitochondrial alternative oxidase pathway protects the photosynthetic apparatus against photodamage in Rumex K-1 leaves. BMC Plant Biol. 2012, 12, 40. [Google Scholar] [CrossRef] [Green Version]
- Garmash, E.V.; Dymova, O.V.; Malyshev, R.V.; Plyusnina, S.N.; Golovko, T.K. Developmental changes in energy dissipation in etiolated wheat seedlings during the greening process. Photosynthetica 2013, 51, 497–508. [Google Scholar] [CrossRef]
- Dahal, K.; Vanlerberghe, G.C. Improved chloroplast energy balance during water deficit enhances plant growth: More crop per drop. J. Exp. Bot. 2018, 69, 1183–1197. [Google Scholar] [CrossRef] [Green Version]
- Ho, L.H.M.; Giraud, E.; Uggalla, V.; Lister, R.; Clifton, R.; Glen, A.; Thirkettle-Watts, D.; Van Aken, O.; Whelan, J. Identification of regulatory pathways controlling gene expression of stress-responsive mitochondrial proteins in Arabidopsis. Plant Physiol. 2008, 147, 1858–1873. [Google Scholar] [CrossRef]
- Garmash, E.V.; Velegzhaninov, I.O.; Ermolina, K.V.; Rybak, A.V.; Malyshev, R.V. Altered levels of AOX1a expression result in changes in metabolic pathways in Arabidopsis thaliana plants acclimated to low dose rates of ultraviolet B radiation. Plant Sci. 2020, 291, 110332. [Google Scholar] [CrossRef]
- Garmash, E.V.; Belykh, E.S.; Velegzhaninov, I.O. The gene expression profiles of mitochondrial respiratory components in Arabidopsis plants with differing amounts of ALTERNATIVE OXIDASE1a under high intensity light. Plant Signal. Behav. 2021, 16, 1864962. [Google Scholar] [CrossRef]
- Czechowski, T.; Stitt, M.; Altmann, T.; Udvardi, M.K.; Scheible, W.-R. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005, 139, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Umbach, A.L.; Fiorani, F.; Siedow, J.N. Characterization of transformed Arabidopsis with altered alternative oxidase levels and analysis of effects on reactive oxygen species in tissue. Plant Physiol. 2005, 139, 1806–1820. [Google Scholar] [CrossRef] [Green Version]
- Strodtkötter, I.; Padmasree, K.; Dinakar, C.; Speth, B.; Niazi, P.S.; Wojtera, J.; Voss, I.; Do, P.T.; Nunes-Nesi, A.; Fernie, A.R.; et al. Induction of the AOX1D isoform of alternative oxidase in A. thaliana T-DNA insertion lines lacking isoform AOX1A is insufficient to optimize photosynthesis when treated with Antimycin A. Mol. Plant 2009, 2, 284–297. [Google Scholar] [CrossRef]
- Selinski, J.; Hartmann, A.; Deckers-Hebestreit, G.; Day, D.A.; Whelan, J.; Scheibe, R. Alternative oxidase isoforms are differentially activated by tricarboxylic acid cycle intermediates. Plant Physiol. 2018, 176, 1423–1432. [Google Scholar] [CrossRef] [Green Version]
- Florez-Sarasa, I.; Flexas, J.; Rasmusson, A.G.; Umbach, A.L.; Siedow, J.N.; Ribas-Carbo, M. In vivo cytochrome and alternative pathway respiration in leaves of Arabidopsis thaliana plants with altered alternative oxidase under different light conditions. Plant Cell Environ. 2011, 34, 1373–1383. [Google Scholar] [CrossRef]
- Kühn, K.; Yin, G.; Duncan, O.; Law, S.R.; Kubiszewski-Jakubiak, S.; Kaur, P.; Meyer, E.; Wang, Y.; Small, C.C.D.F.; Giraud, E.; et al. Decreasing electron flux through the cytochrome and/or alternative respiratory pathways triggers common and distinct cellular responses dependent on growth conditions. Plant Physiol. 2015, 167, 228–250. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Shibata, M.; Terashima, I.; Noguchi, K. Simultaneous determination of in vivo plastoquinone and ubiquinone redox states by HPLC-based analysis. Plant Cell Physiol. 2010, 51, 836–841. [Google Scholar] [CrossRef] [Green Version]
- Morales, L.M.M.; Silva, G.M.C.; Santana, D.B.; Pireda, S.F.; Cogo, A.J.D.; Heringer, A.S.; Oliveira, T.D.R.; Reis, R.S.; dos Prado, L.A.S.; Oliveira, A.V.; et al. Mitochondrial dysfunction associated with ascorbate synthesis in plants. Plant Physiol. Biochem. 2022, 185, 55–68. [Google Scholar] [CrossRef]
- Wang, D.; Wang, C.; Li, C.; Song, H.; Qin, J.; Chang, H.; Fu, W.; Wang, Y.; Wang, F.; Li, B.; et al. Functional relationship of Arabidopsis AOXs and PTOX revealed via transgenic analysis. Front. Plant. Sci. 2021, 12, 692847. [Google Scholar] [CrossRef]
- Müller-Moulé, P.; Havaux, M.; Niyogi, K.K. Zeaxanthin Deficiency Enhances the high light sensitivity of an ascorbate-deficient mutant of Arabidopsis. Plant Physiol. 2003, 133, 748–760. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Chew, O.; Whelan, J.; Millar, A.H. Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J. Biol. Chem. 2003, 278, 46869–46877. [Google Scholar] [CrossRef] [Green Version]
- Carrie, C.; Giraud, E.; Whelan, J. Protein Transport in Organelles: Dual targeting of proteins to mitochondria and chloroplasts. FEBS J. 2009, 276, 1187–1195. [Google Scholar] [CrossRef]
- Jimenez, A.; Hernandez, J.A.; del Rio, L.A.; Sevilla, F. Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of Pea leaves. Plant Physiol. 1997, 114, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Vanlerberghe, G.C. A lack of mitochondrial alternative oxidase compromises capacity to recover from severe drought stress. Physiol. Plant. 2013, 149, 461–473. [Google Scholar] [CrossRef]
- Boyes, D.C.; Zayed, A.M.; Ascenzi, R.; McCaskill, A.J.; Hoffman, N.E.; Davis, K.R.; Gorlach, J. Growth, stage-based phenotypic analysis of Arabidopsis: A model for high throughput functional genomics in plants. Plant Cell 2001, 13, 1499–1510. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, R.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinf. 2012, 13, 134. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Plant Cell Membranes; Academic Press: San Diego, CA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Schindler, C.; Lichtenthaler, H.K. Photosynthetic CO2-assimilation, chlorophyll fluorescence and zeaxanthin accumulation in field grown maple trees in the course of a sunny and a cloudy day. J. Plant Physiol. 1996, 148, 399–412. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Kley, J.; Heil, M.; Muck, A.; Svatos, A.; Boland, W. Isolating intact chloroplasts from small Arabidopsis samples for proteomic studies. Anal. Biochem. 2010, 398, 198–202. [Google Scholar] [CrossRef]
- Law, M.Y.; Charles, S.A.; Halliwell, B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat. Biochem. J. 1983, 210, 899–903. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.A.; Asada, K. Inactivation of ascorbate peroxidase in spinach chloroplasts on dark addition of hydrogen peroxide: Its protection by ascorbate. Plant Cell Physiol. 1984, 25, 1285–1295. [Google Scholar] [CrossRef]




| Gene Name | AGI No. | Gene Description ** | Forward Primer (5′~3′) Reverse Primer (5′~3′) | Product Length, bp |
|---|---|---|---|---|
| NPQ1 | At1g08550 | Non-photochemical quenching 1 | CGAGTGTGCTGTGTCGAGAA TGGCAGTCGAAGGCATCAAA | 168 |
| APX1 | At1g07890 | Ascorbate peroxidase 1, cytosolic | TGGCCCTGACATTCCTTTCC ATGTGGGCCTCAGCGTAATC | 393 |
| SAPX | At4g08390 | Stromal ascorbate peroxidase | GCGTCGGTGAATCGGAGTTT CCACCTCTTTGTGGCCATTC | 135 |
| AT2G28390 * | At2g28390 | SAND family protein | AACTCTATGCAGCATTTGATCCACT TGATTGCATATCTTTATCGCCATC | 61 |
| AT4G34270 * | At4g34270 | TIP41-like family protein | GTGAAAACTGTTGGAGAGAAGCAA TCAACTGGATACCCTTTCGCA | 61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garmash, E.V.; Dymova, O.V.; Silina, E.V.; Malyshev, R.V.; Belykh, E.S.; Shelyakin, M.A.; Velegzhaninov, I.O. AOX1a Expression in Arabidopsis thaliana Affects the State of Chloroplast Photoprotective Systems under Moderately High Light Conditions. Plants 2022, 11, 3030. https://doi.org/10.3390/plants11223030
Garmash EV, Dymova OV, Silina EV, Malyshev RV, Belykh ES, Shelyakin MA, Velegzhaninov IO. AOX1a Expression in Arabidopsis thaliana Affects the State of Chloroplast Photoprotective Systems under Moderately High Light Conditions. Plants. 2022; 11(22):3030. https://doi.org/10.3390/plants11223030
Chicago/Turabian StyleGarmash, Elena V., Olga V. Dymova, Ekaterina V. Silina, Ruslan V. Malyshev, Elena S. Belykh, Mikhail A. Shelyakin, and Ilya O. Velegzhaninov. 2022. "AOX1a Expression in Arabidopsis thaliana Affects the State of Chloroplast Photoprotective Systems under Moderately High Light Conditions" Plants 11, no. 22: 3030. https://doi.org/10.3390/plants11223030







