Comprehensive Genome-Wide Analysis and Expression Pattern Profiling of PLATZ Gene Family Members in Solanum Lycopersicum L. under Multiple Abiotic Stresses
Abstract
1. Introduction
2. Results
2.1. Identification and Sequence Analysis of PLATZ Family Members in Tomato
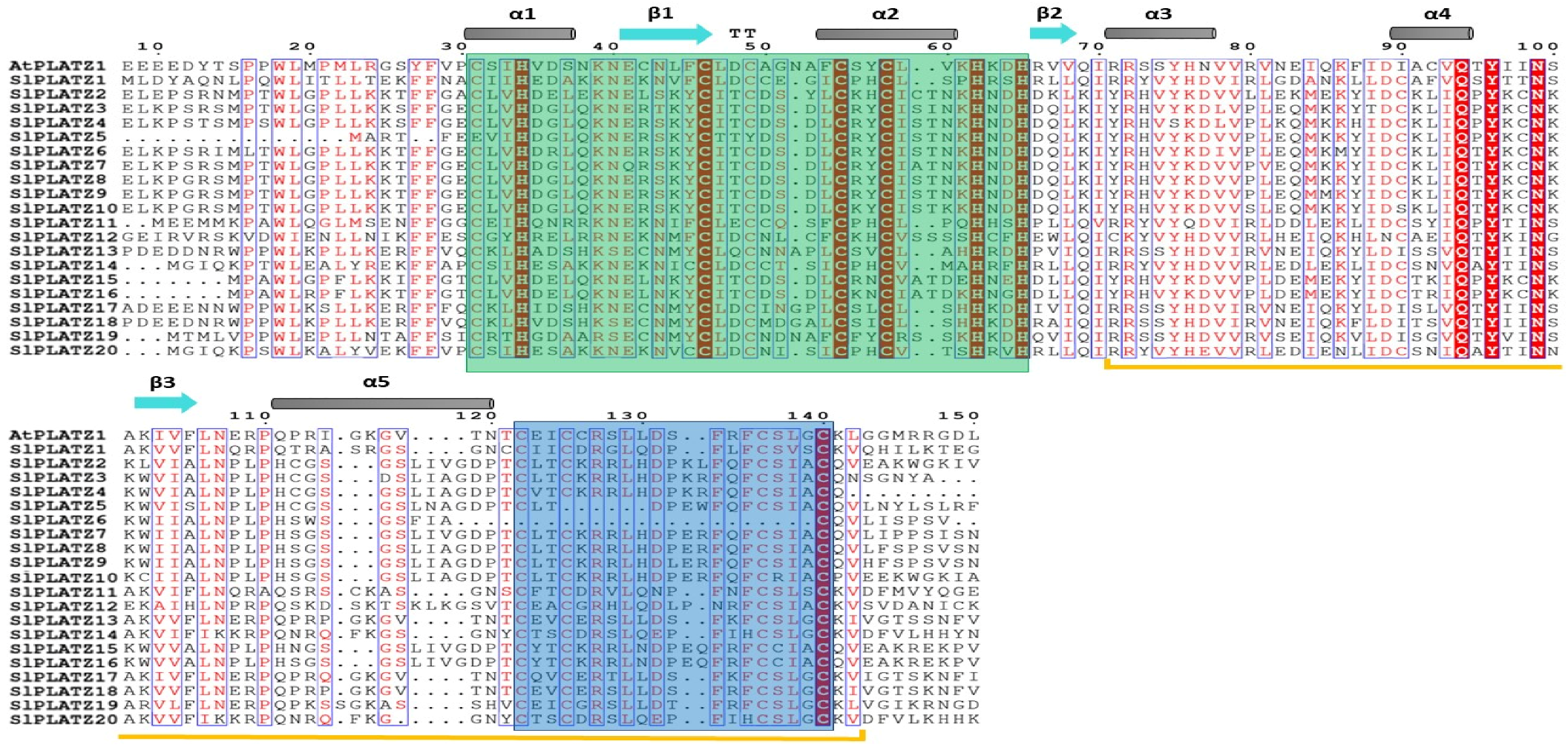
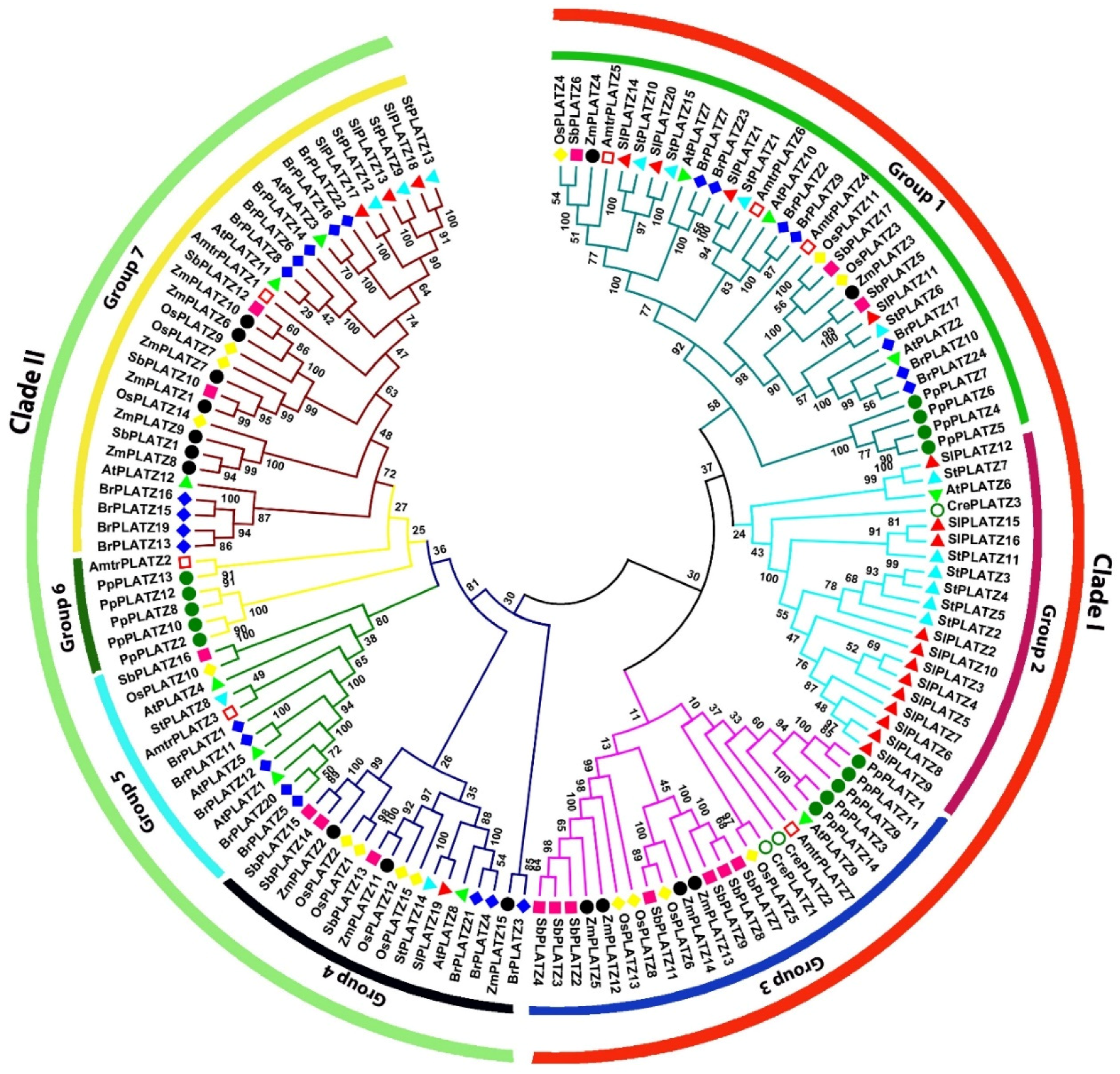
2.2. Multiple Protein Sequence Alignment and Phylogenetic Analysis
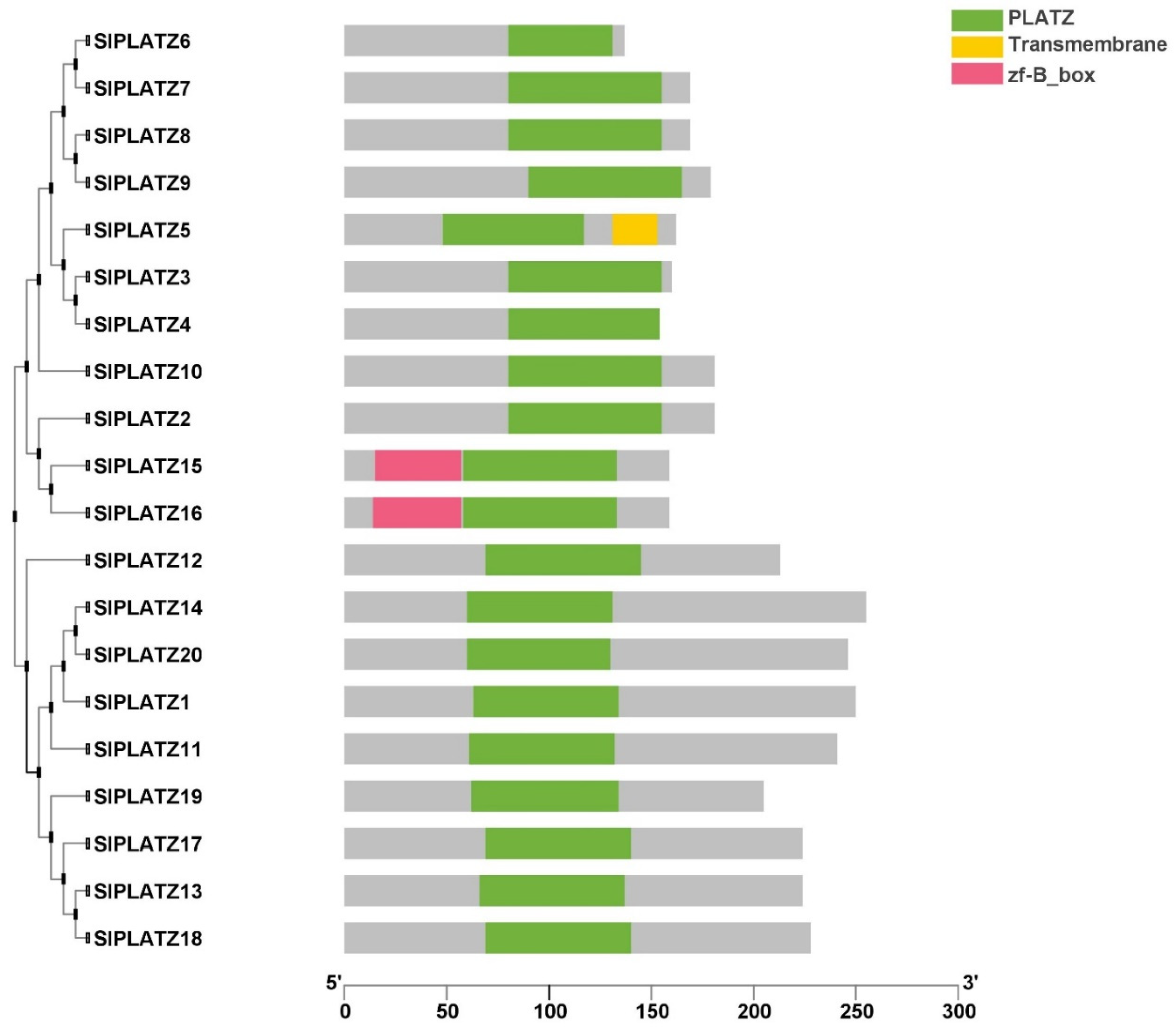
2.3. Conserved Domain Prediction, Gene Structure, and Motif Composition of SlPLATZ Genes
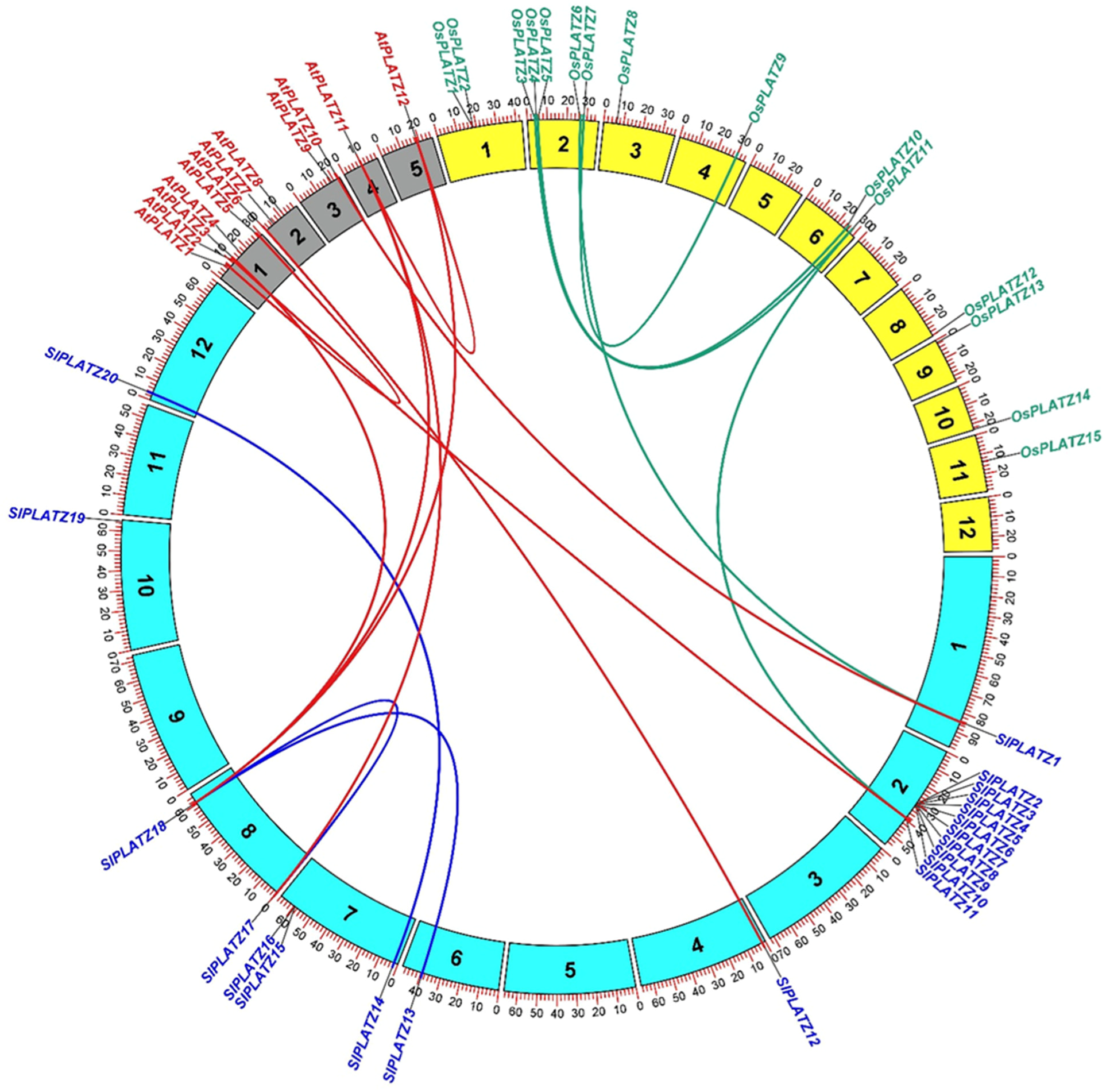
2.4. Chromosomal Distribution, Gene Duplication, and Microsynteny Analysis of SlPLATZ Genes
2.5. Analysis of Cis-Acting Elements and miRNA Target Sites in SlPLATZ Genes
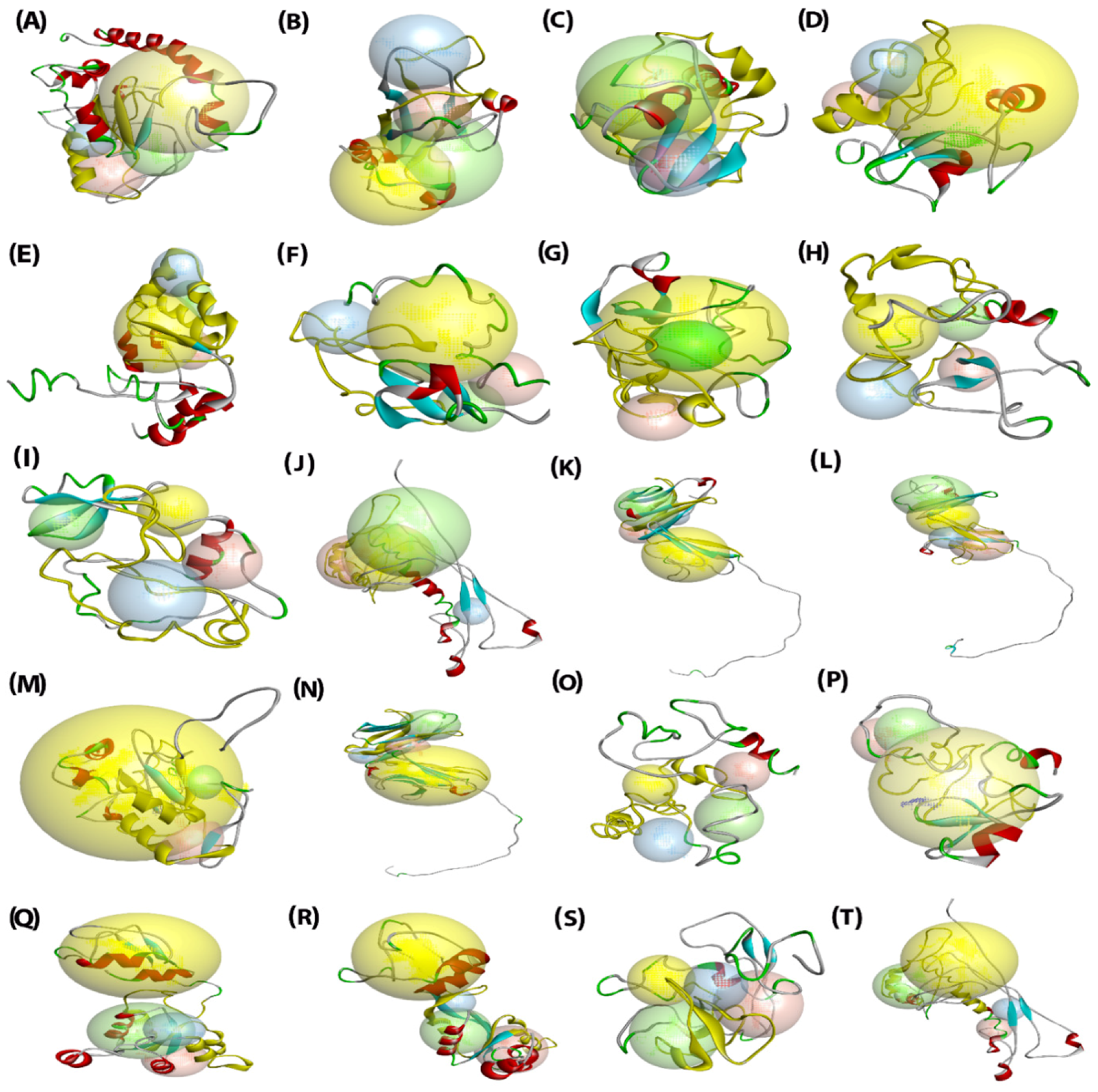
2.6. Analysis of Three-Dimensional Structures of Predicted SlPLATZ Proteins
2.7. Expression Profiling of Tomato PLATZ Genes in Various Organs
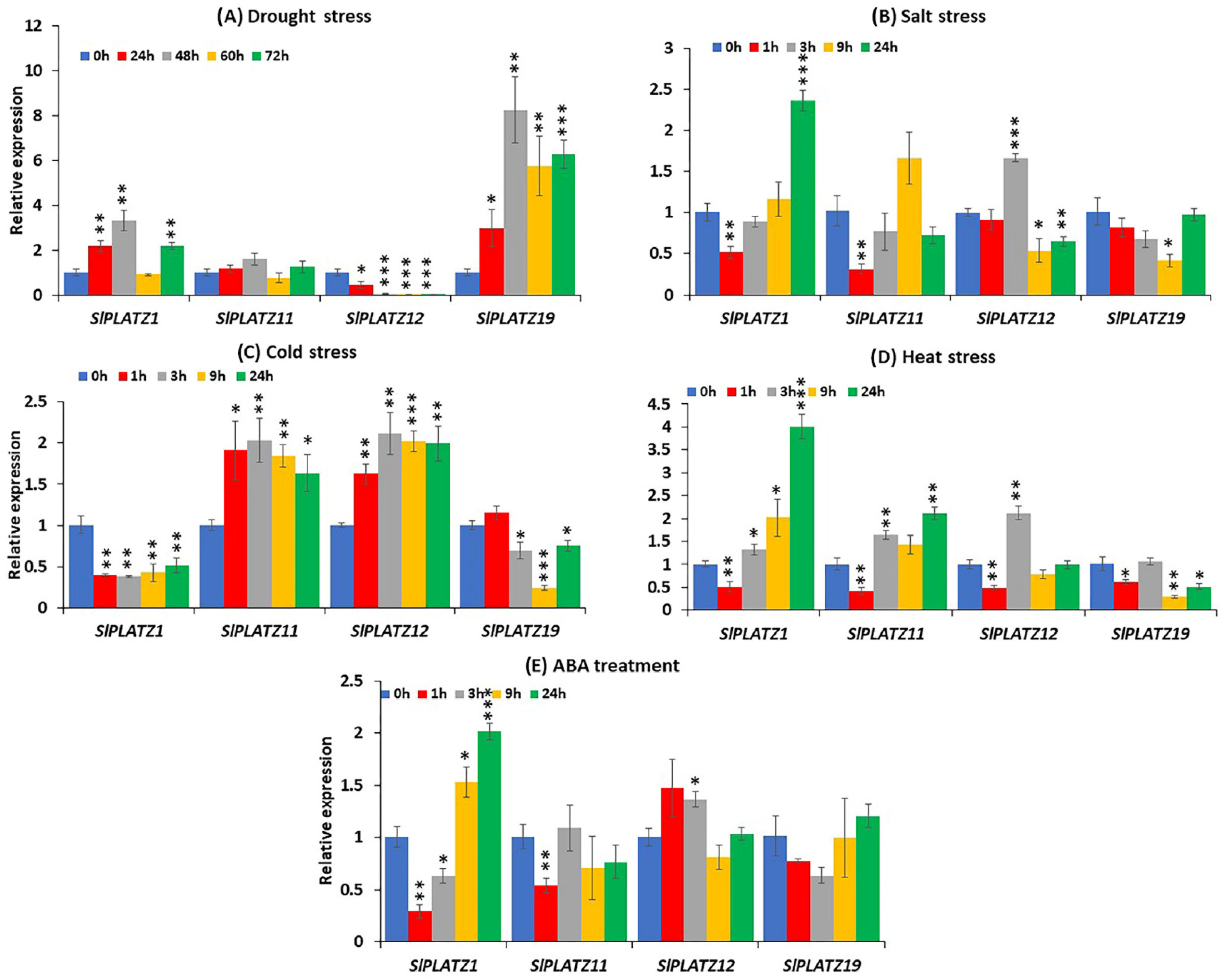
2.8. Expression Analysis of SlPLATZ Genes in Response to Abiotic Stresses and Phytohormone Treatment
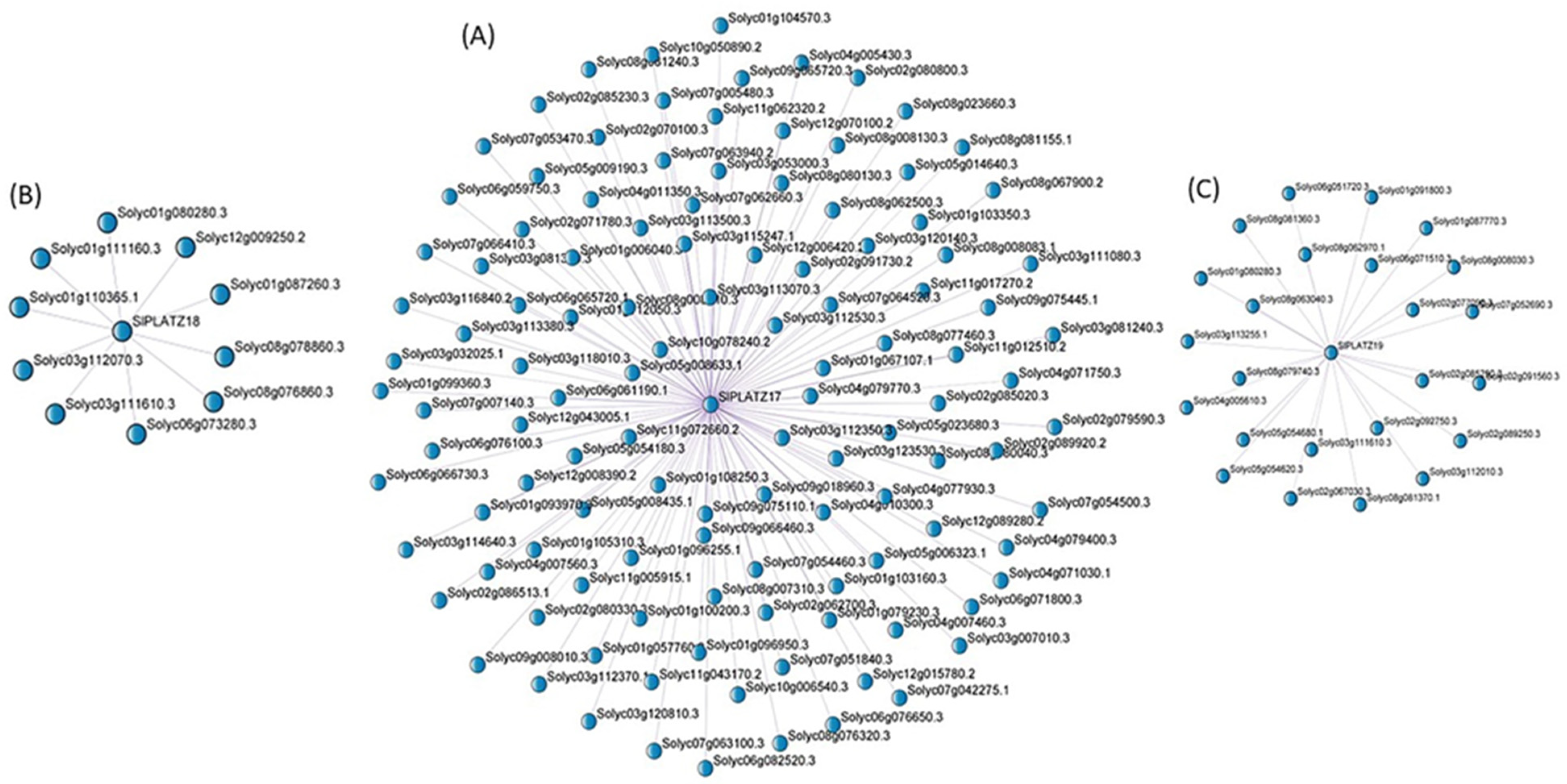
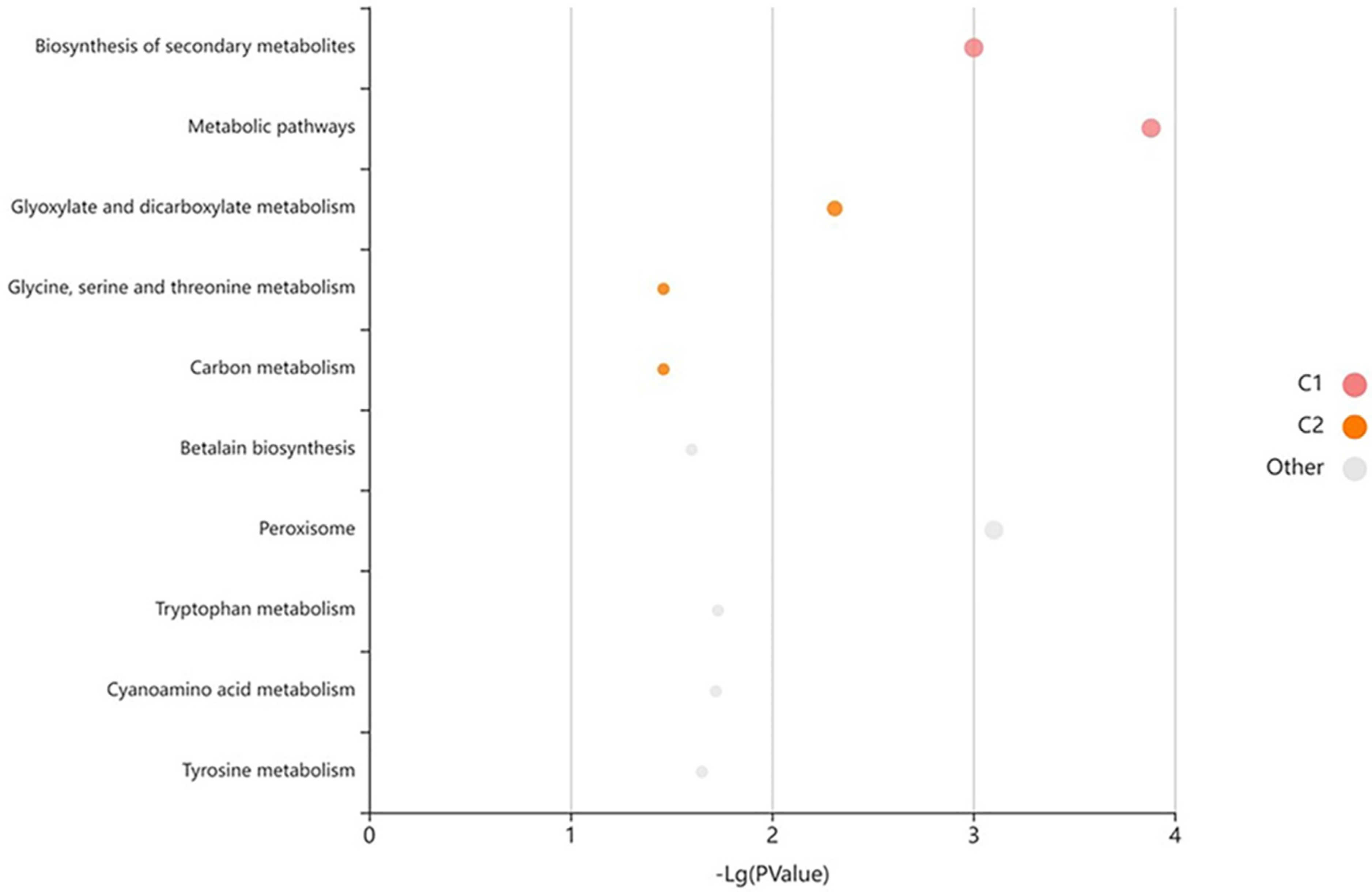
2.9. Weighted Gene Co-Expression Network (WGCNA) Analysis and Functional Enrichment Analysis
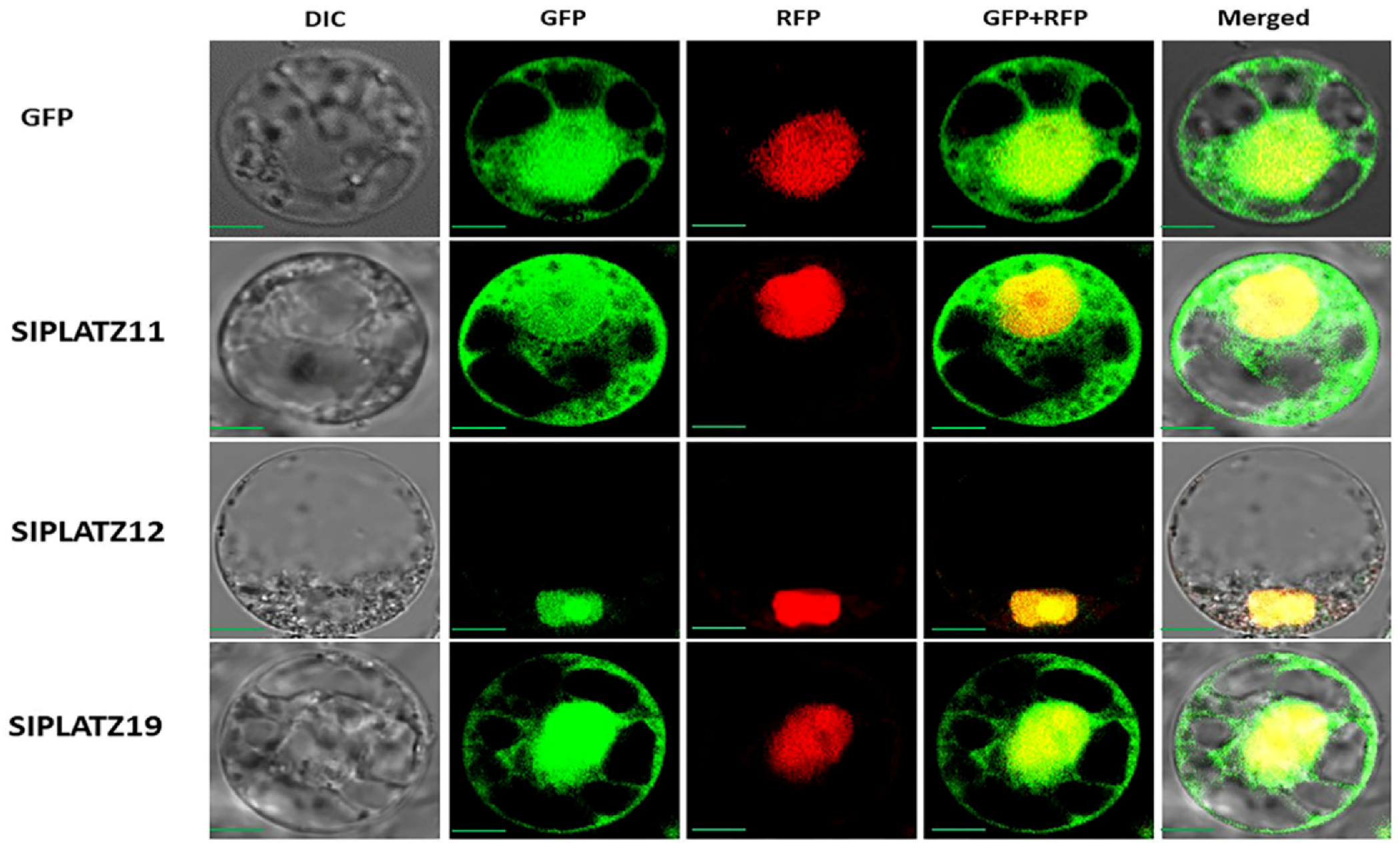
2.10. Subcellular Location Analysis of SlPLATZ Proteins
3. Discussion
4. Materials and Methods
4.1. Identification and Sequence Analysis of PLATZ Genes in the Tomato Genome
4.2. Phylogenetic Analysis of Tomato PLATZ Proteins
4.3. Chromosomal Locations, Gene Duplication, and Microsynteny Analysis
4.4. Prediction of Cis-Acting Elements and miRNA Target Sites
4.5. Homology Modeling of SlPLATZ Proteins
4.6. Plant Sample Collection and Stress Treatments
4.7. Expression Profiling of SlPLATZ Genes by qRT−PCR
4.8. Co-Expression Network Analysis of SlPLATZ Genes
4.9. Subcellular Localization
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Brkljacic, J.; Grotewold, E. Combinatorial control of plant gene expression. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.Z.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.J.; Samaha, R.R.; et al. Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Kizis, D.; Lumbreras, V.; Pagès, M. Role of AP2/EREBP transcription factors in gene regulation during abiotic stress. FEBS Lett. 2001, 498, 187–189. [Google Scholar] [CrossRef]
- Olsen, A.N.; Ernst, H.A.; Leggio, L.L.; Skriver, K. NAC transcription factors: Structurally distinct, functionally diverse. Trends Plant Sci. 2005, 10, 79–87. [Google Scholar] [CrossRef]
- Naeem, M.; Shahzad, K.; Saqib, S.; Shahzad, A.; Younas, M.; Afridi, M.I. The Solanum melongena COP1LIKE manipulates fruit ripening and flowering time in tomato (Solanum lycopersicum). Plant Growth Regul. 2022, 96, 369–382. [Google Scholar] [CrossRef]
- Nagano, Y.; Furuhashi, H.; Inaba, T.; Sasaki, Y. A novel class of plant-specific zinc-dependent DNA-binding protein that binds to a/T-rich DNA sequences. Nucleic Acids Res. 2001, 29, 4097–4105. [Google Scholar] [CrossRef]
- Griffiths, S.; Dunford, R.P.; Coupland, G.; Laurie, D.A. The evolution of CONSTANS-like gene families in barley, rice, and Arabidopsis. Plant Physiol. 2003, 131, 1855–1867. [Google Scholar] [CrossRef]
- Teakle, G.R.; Manfield, I.W.; Graham, J.F.; Gilmartin, P.M. Arabidopsis thaliana GATA factors: Organisation, expression and DNA-binding characteristics. Plant Mol. Biol. 2002, 50, 43–57. [Google Scholar] [CrossRef]
- Noguero, M.; Atif, R.M.; Ochatt, S.; Thompson, R.D. The role of the DNA-binding One Zinc Finger (DOF) transcription factor family in plants. Plant Sci. 2013, 209, 32–45. [Google Scholar] [CrossRef]
- Schwabe, J.W.; Klug, A. Zinc mining for protein domains. Nat. Struct. Biol. 1994, 1, 345–349. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.F.; Zhao, T.T.; Ren, J.; Xu, X.Y. Research progress of plant C2H2 zinc finger protein. Mol. Plant Breed. 2018, 29, 1133–1141. [Google Scholar]
- Ciftci-Yilmaz, S.; Mittler, R. The zinc finger network of plants. Cell. Mol. Life Sci. 2008, 65, 1150–1160. [Google Scholar] [CrossRef]
- He, X.; Liu, M.; Fang, Z.; Ma, D.; Zhou, Y.; Yin, J. Genome-wide Analysis of a Plant AT-rich Sequence and Zinc-binding Protein (PLATZ) in Triticum Aestivum. Phyton 2021, 90, 971. [Google Scholar] [CrossRef]
- Wang, A.; Hou, Q.; Si, L.; Huang, X.; Luo, J.; Lu, D.; Zhu, J.; Shangguan, Y.; Miao, J.; Xie, Y.; et al. The PLATZ transcription factor GL6 affects grain length and number in rice. Plant Physiol. 2019, 180, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.R.; Xue, H.W. The rice PLATZ protein SHORT GRAIN6 determines grain size by regulating spikelet hull cell division. J. Integr. Plant Biol. 2020, 62, 847–864. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Ye, J.; Zheng, X.; Xiang, X.; Li, C.; Fu, M.; Wang, Q.; Zhang, Z.; Wu, Y. The maize imprinted gene Floury3 encodes a PLATZ protein required for tRNA and 5S rRNA transcription through interaction with RNA polymerase III. Plant Cell 2017, 29, 2661–2675. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Xiao, Q.; Luo, L.; Zhang, C.; Mao, C.; Du, J.; Long, T.; Cao, Y.; Yi, Q.; et al. Transcription factor ZmPLATZ2 positively regulate the starch synthesis in maize. Plant Growth Regul. 2021, 93, 291–302. [Google Scholar] [CrossRef]
- Chao, Q.; Gao, Z.F.; Zhang, D.; Zhao, B.G.; Dong, F.Q.; Fu, C.X.; Liu, L.J.; Wang, B.C. The developmental dynamics of the Populus stem transcriptome. Plant Biotechnol. J. 2019, 17, 206–219. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.; Jun, S.E.; Park, S.; Timilsina, R.; Kwon, D.S.; Kim, Y.; Park, S.J.; Hwang, J.Y.; Nam, H.G.; et al. ORESARA15, a PLATZ transcription factor, mediates leaf growth and senescence in Arabidopsis. New Phytol. 2018, 220, 609–623. [Google Scholar] [CrossRef]
- Dong, T.; Yin, X.; Wang, H.; Lu, P.; Liu, X.; Gong, C.; Wu, Y. ABA-INDUCED expression 1 is involved in ABA-inhibited primary root elongation via modulating ROS homeostasis in Arabidopsis. Plant Sci. 2021, 304, 110821. [Google Scholar] [CrossRef] [PubMed]
- Timilsina, R.; Kim, Y.; Park, S.; Park, H.; Park, S.J.; Kim, J.H.; Park, J.H.; Kim, D.; Park, Y.I.; Hwang, D.; et al. ORESARA 15, a PLATZ transcription factor, controls root meristem size through auxin and cytokinin signalling-related pathways. J. Exp. Bot. 2022, 73, 2511–2524. [Google Scholar] [CrossRef] [PubMed]
- González-Morales, S.I.; Chávez-Montes, R.A.; Hayano-Kanashiro, C.; Alejo-Jacuinde, G.; Rico-Cambron, T.Y.; de Folter, S.; Herrera-Estrella, L. Regulatory network analysis reveals novel regulators of seed desiccation tolerance in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2016, 113, E5232–E5241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lan, Y.; Wu, M.; Wang, L.; Liu, H.; Xiang, Y. PhePLATZ1, a PLATZ transcription factor in moso bamboo (Phyllostachys edulis), improves drought resistance of transgenic Arabidopsis thaliana. Plant Physiol. Biochem. 2022, 186, 121–134. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Ji, Z.; Zhang, L.; Li, C.; Huang, J.; Yang, G.; Yan, K.; Zhang, S.; Zheng, C.; et al. Regulation of drought tolerance in Arabidopsis involves PLATZ4-mediated transcriptional suppression of PIP2. bioRxiv. 2021. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, L.; Wei, J.; Wang, Y.; Chen, J.; Zhou, Y.; Chen, M.; Wang, F.; Ma, Y.; Xu, Z.S. The soybean PLATZ transcription factor GmPLATZ17 suppresses drought tolerance by interfering with stress-associated gene regulation of GmDREB5. Crop J. 2022, 10, 1014–1025. [Google Scholar] [CrossRef]
- Liu, S.; Yang, R.; Liu, M.; Zhang, S.; Yan, K.; Yang, G.; Huang, J.; Zheng, C.; Wu, C. PLATZ2 negatively regulates salt tolerance in Arabidopsis seedlings by directly suppressing the expression of the CBL4/SOS3 and CBL10/SCaBP8 genes. J. Exp. Bot. 2020, 71, 5589–5602. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, R.; Huo, Y.; Liu, S.; Yang, G.; Huang, J.; Zheng, C.; Wu, C. Expression of cotton PLATZ1 in transgenic Arabidopsis reduces sensitivity to osmotic and salt stress for germination and seedling establishment associated with modification of the abscisic acid, gibberellin, and ethylene signalling pathways. BMC Plant Biol. 2018, 18, 218. [Google Scholar] [CrossRef]
- So, H.A.; Choi, S.J.; Chung, E.; Lee, J.H. Molecular characterization of stress-inducible PLATZ gene from soybean (Glycine max L.). Plant Omics 2015, 8, 479–484. [Google Scholar]
- Li, T.; Xu, X.; Li, Y.; Wang, H.; Li, Z.; Li, Z. Comparative transcriptome analysis reveals differential transcription in heat-susceptible and heat-tolerant pepper (Capsicum annum L.) cultivars under heat stress. J. Plant Biol. 2015, 58, 411–424. [Google Scholar] [CrossRef]
- Zenda, T.; Liu, S.; Wang, X.; Liu, G.; Jin, H.; Dong, A.; Yang, Y.; Duan, H. Key maize drought-responsive genes and pathways revealed by comparative transcriptome and physiological analyses of contrasting inbred lines. Int. J. Mol. Sci. 2019, 20, 1268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lan, Y.; Shi, Y.; Gao, Y.; Wu, M.; Xu, Y.; Xiang, Y. Systematic analysis and functional characterization of the PLATZ transcription factors in Moso bamboo (Phyllostachys edulis). J. Plant Growth Regul. 2022, 130, 431–444. [Google Scholar] [CrossRef]
- Costa, J.M.; Heuvelink, E. The Global Tomato Industry. In Tomatoes; CABI Publishing: Wallingford, UK, 2018; pp. 276–313. ISBN 9781780641942. [Google Scholar]
- Kimura, S.; Sinha, N. Tomato (Solanum Lycopersicum): A model fruit-bearing crop. Cold Spring Harb. Protoc. 2008, 3, pdb-emo105. [Google Scholar] [CrossRef] [PubMed]
- Safder, I.; Shao, G.; Sheng, Z.; Hu, P.; Tang, S. Genome-wide identification studies–A primer to explore new genes in plant species. Plant Biol. 2022, 24, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ji, C.; Li, Q.; Zhou, Y.; Wu, Y. Genome-wide analysis of the plant-specific PLATZ proteins in maize and identification of their general role in interaction with RNA polymerase III complex. BMC Plant Biol. 2018, 18, 221. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Rong, H.; Tian, Y.; Qu, Y.; Xu, M.; Xu, L.A. Genome-Wide Identification of PLATZ Transcription Factors in Ginkgo biloba L. and Their Expression Characteristics During Seed Development. Front. Plant Sci. 2022, 13, 946194. [Google Scholar] [CrossRef] [PubMed]
- Azim, J.B.; Khan, M.F.H.; Hassan, L.; Robin, A.H.K. Genome-wide characterization and expression profiling of plant-specific PLATZ transcription factor family genes in Brassica rapa L. Plant Breed. Biotechnol. 2020, 8, 28–45. [Google Scholar] [CrossRef]
- Fu, Y.; Cheng, M.; Li, M.; Guo, X.; Wu, Y.; Wang, J. Identification and characterization of PLATZ transcription factors in wheat. Int. J. Mol. Sci. 2020, 21, 8934. [Google Scholar] [CrossRef]
- Li, J.; Feng, S.; Zhang, Y.; Xu, L.; Luo, Y.; Yuan, Y.; Yang, Q.; Feng, B. Genome-wide identification and expression analysis of the plant-specific PLATZ gene family in Tartary buckwheat (Fagopyrum tataricum). BMC Plant Biol. 2022, 22, 160. [Google Scholar] [CrossRef]
- Wai, A.H.; Naing, A.H.; Lee, D.J.; Kim, C.K.; Chung, M.Y. Molecular genetic approaches for enhancing stress tolerance and fruit quality of tomato. Plant Biotechnol. Rep. 2020, 14, 515–537. [Google Scholar] [CrossRef]
- Hernández, Y.; Sanan-Mishra, N. miRNA mediated regulation of NAC transcription factors in plant development and environment stress response. Plant Gene 2017, 11, 190–198. [Google Scholar] [CrossRef]
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Maréchal-Drouard, L.; et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 2007, 318, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.H.; Gouy, M.; Yang, Y.W.; Sharp, P.M.; Li, W.H. Date of the monocot-dicot divergence estimated from chloroplast DNA sequence data. Proc. Natl. Acad. Sci. USA 1989, 86, 6201–6205. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, H.; Zhou, L.; Deng, L.; Lin, Y.; Peng, X.; Yan, H.; Cheng, B. Molecular evolution of the HD-ZIP I gene family in legume genomes. Gene 2014, 533, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Priest, H.D.; Filichkin, S.A.; Mockler, T.C. Cis-regulatory elements in plant cell signaling. Curr. Opin. Plant Biol. 2009, 12, 643–649. [Google Scholar] [CrossRef]
- Biłas, R.; Szafran, K.; Hnatuszko-Konka, K.; Kononowicz, A.K. Cis-regulatory elements used to control gene expression in plants. Plant Cell Tissue Organ Cult. 2016, 127, 269–287. [Google Scholar] [CrossRef]
- Wai, A.H.; Waseem, M.; Khan, A.M.M.; Nath, U.K.; Lee, D.J.; Kim, S.T.; Kim, C.K.; Chung, M.Y. Genome-wide identification and expression profiling of the PDI gene family reveals their probable involvement in abiotic stress tolerance in tomato (Solanum lycopersicum L.). Genes 2021, 12, 23. [Google Scholar] [CrossRef]
- Zhang, B. MicroRNA: A new target for improving plant tolerance to abiotic stress. J. Exp. Bot. 2015, 66, 1749–1761. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Wang, Y.; Tao, X.; Fan, Y.F.; Dai, Y.; Yang, H.; Ma, X.R. Genomic profiling of exogenous abscisic acid-responsive microRNAs in tomato (Solanum lycopersicum). BMC Genom. 2016, 17, 423. [Google Scholar] [CrossRef]
- Chen, L.; Meng, J.; Zhai, J.; Xu, P.; Luan, Y. MicroRNA396a-5p and-3p induce tomato disease susceptibility by suppressing target genes and upregulating salicylic acid. Plant Sci. 2017, 265, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, H.; Zhao, G.; Huang, Q.; Lu, Y.; Ouyang, B. Profiling of drought-responsive microRNA and mRNA in tomato using high-throughput sequencing. BMC Genom. 2017, 18, 481. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.H.; Lee, H.J.; Lee, J.H.; Wi, S.H.; Kim, S.K.; Hyun, T.K. Identification and Functional Prediction of Drought-Responsive Long Non-Coding RNA in Tomato. Agronomy 2019, 9, 629. [Google Scholar] [CrossRef]
- Liu, M.; Yu, H.; Zhao, G.; Huang, Q.; Lu, Y.; Ouyang, B. Identification of drought-responsive microRNAs in tomato using high-throughput sequencing. Funct. Integr. Genom. 2018, 18, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Goswami, K.; Tiwari, M.; Mukherjee, S.K.; Sanan-Mishra, N. Identification and comparative analysis of microRNAs from tomato varieties showing contrasting response to ToLCV infections. Physiol. Mol. Biol. Plants 2018, 24, 185–202. [Google Scholar] [CrossRef] [PubMed]
- López-Galiano, M.J.; Sentandreu, V.; Martínez-Ramírez, A.C.; Rausell, C.; Real, M.D.; Camañes, G.; Ruiz-Rivero, O.; Crespo-Salvador, O.; García-Robles, I. Identification of stress associated microRNAs in Solanum lycopersicum by high-throughput sequencing. Genes 2019, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.P.; Jiang, F.L.; Wen, J.Q.; Cui, S.Y.; Zhou, Y.Z.; Wu, Z. MicroRNA319 family members play an important role in Solanum habrochaites and S. lycopersicum responses to chilling and heat stresses. Biol. Plant 2019, 63, 200–209. [Google Scholar] [CrossRef]
- Zhou, X.; Cui, J.; Meng, J.; Luan, Y. Interactions and links among the noncoding RNAs in plants under stresses. Theor. Appl. Genet. 2020, 133, 3235–3248. [Google Scholar] [CrossRef]
- Koutinas, N.; Pepelyankov, G.; Lichev, V. Flower induction and flower bud development in apple and sweet cherry. Biotechnol. Biotechnol. Equip. 2010, 24, 1549–1558. [Google Scholar] [CrossRef]
- Qin, F.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant Cell Physiol. 2011, 52, 1569–1582. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Abscisic-acid-dependent basic leucine zipper (bZIP) transcription factors in plant abiotic stress. Protoplasma 2017, 254, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Niu, C.Y.; Yang, C.R.; Jinn, T.L. The heat stress factor HSFA6b connects ABA signaling and ABA-mediated heat responses. Plant Physiol. 2016, 172, 1182–1199. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Bassil, E.; Hamilton, J.S.; Inupakutika, M.A.; Zandalinas, S.I.; Tripathy, D.; Luo, Y.; Dion, E.; Fukui, G.; Kumazaki, A.; et al. ABA is required for plant acclimation to a combination of salt and heat stress. PLoS ONE 2016, 11, e147625. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.L.; Ba, L.J.; Shan, W.; Kuang, J.F.; Lu, W.J.; Chen, J.Y. Involvement of WRKY transcription factors in abscisic-acid-induced cold tolerance of banana fruit. J. Agric. Food Chem. 2017, 65, 3627–3635. [Google Scholar] [CrossRef]
- Al-Abdallat, A.M.; Ali-Sheikh-Omar, M.A.; Alnemer, L.M. Overexpression of two ATNAC3-related genes improves drought and salt tolerance in tomato (Solanum lycopersicum L.). Plant Cell Tissue Organ Cult. 2015, 120, 989–1001. [Google Scholar] [CrossRef]
- Yang, Y.X.; Wang, M.M.; Yin, Y.L.; Onac, E.; Zhou, G.F.; Peng, S.; Xia, X.J.; Shi, K.; Yu, J.Q.; Zhou, Y.H. RNA-seq analysis reveals the role of red light in resistance against Pseudomonas syringae pv. tomato DC3000 in tomato plants. BMC Genom. 2015, 16, 120. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Han, Z.; Sun, X.; Li, H.; Zhang, J.; Lu, Y. Molecular analyses of tomato GS, GOGAT and GDH gene families and their response to abiotic stresses. Acta Physiol. Plant. 2016, 38, 1–14. [Google Scholar] [CrossRef]
- Howden, A.J.; Stam, R.; Martinez Heredia, V.; Motion, G.B.; Ten Have, S.; Hodge, K.; Marques Monteiro Amaro, T.M.; Huitema, E. Quantitative analysis of the tomato nuclear proteome during Phytophthora capsici infection unveils regulators of immunity. New Phytol. 2017, 215, 309–322. [Google Scholar] [CrossRef]
- Yu, C.; Song, L.; Song, J.; Ouyang, B.; Guo, L.; Shang, L.; Wang, T.; Li, H.; Zhang, J.; Ye, Z. ShCIGT, a Trihelix family gene, mediates cold and drought tolerance by interacting with SnRK1 in tomato. Plant Sci. 2018, 270, 140–149. [Google Scholar] [CrossRef]
- Yang, L.; Miao, M.; Lyu, H.; Cao, X.; Li, J.; Li, Y.; Li, Z.; Chang, W. Genome-wide identification, evolution, and expression analysis of RING finger gene family in Solanum lycopersicum. Int. J. Mol Sci. 2019, 20, 4864. [Google Scholar] [CrossRef]
- Pei, T.; Bao, Y.; Wu, T.; Wang, Z.; Wang, Y.; Liu, Q.; Yang, H.; Jiang, J.; Zhang, H.; Li, J.; et al. Silencing of the SlZF-31 gene decreases the salt stress tolerance and drought tolerance of tomato. Plant Cell Tissue Organ Cult. 2021, 146, 191–201. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, X.; Wang, C.; Yun, F.; Huang, D.; Yao, Y.; Gao, R.; Ye, F.; Liu, X.; Liao, W. Genome-wide identification and expression analysis of serine hydroxymethyl transferase (SHMT) gene family in tomato (Solanum lycopersicum). PeerJ 2022, 10, e12943. [Google Scholar] [CrossRef] [PubMed]
- Nveawiah-Yoho, P. Mechanisms for Salt Tolerance and Susceptibility in Tomato. Ph.D. Thesis, Tennessee State University, Nashville, TN, USA, 2012. [Google Scholar]
- Okekeogbu, I.; Ye, Z.; Sangireddy, S.R.; Li, H.; Bhatti, S.; Hui, D.; Zhou, S.; Howe, K.J.; Fish, T.; Yang, Y.; et al. Effect of aluminum treatment on proteomes of radicles of seeds derived from Al-treated tomato plants. Proteomes 2014, 2, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhao, T.; Xu, X.; Li, J. Genome-wide identification and characterization of GRAS transcription factors in tomato (Solanum lycopersicum). PeerJ 2017, 5, e3955. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; Liu, L.; Wang, Z.; Li, Y.; Guo, L.; Li, Y.; Zhang, X.; Ren, S.; Zhao, B.; et al. SlTLFP8 reduces water loss to improve water-use efficiency by modulating cell size and stomatal density via endoreduplication. Plant Cell Environ. 2020, 43, 2666–2679. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Zhu, N.; Zhong, S.; Bouzayen, M.; Li, Z. SlGRAS4 mediates a novel regulatory pathway promoting chilling tolerance in tomato. Plant Biotechnol. J. 2020, 18, 1620–1633. [Google Scholar] [CrossRef]
- Basim, H.; Basim, E.; Tombuloglu, H.; Unver, T. Comparative transcriptome analysis of resistant and cultivated tomato lines in response to Clavibacter michiganensis subsp. michiganensis. Genomics 2021, 113, 2455–2467. [Google Scholar] [CrossRef]
- Mu, X.; Zhang, L.; Wang, H.; Cao, S.; Yao, J.; Li, T.; Xia, X. Growth and transcriptome analysis of tomato under seawater stress. S. Afr. J. Bot. 2021, 137, 463–474. [Google Scholar] [CrossRef]
- Pereyra-Bistraín, L.I.; Ovando-Vázquez, C.; Rougon-Cardoso, A.; Alpuche-Solís, Á.G. Comparative RNA-Seq Analysis Reveals Potentially Resistance-Related Genes in Response to Bacterial Canker of Tomato. Genes 2021, 12, 1745. [Google Scholar] [CrossRef]
- Qiu, J.; Ni, L.; Xia, X.; Chen, S.; Zhang, Y.; Lang, M.; Li, M.; Liu, B.; Pan, Y.; Li, J.; et al. Genome-Wide Analysis of the Protein Phosphatase 2C Genes in Tomato. Genes 2022, 13, 604. [Google Scholar] [CrossRef]
- Song, C.; Wu, M.; Zhou, Y.; Gong, Z.; Yu, W.; Zhang, Y.; Yang, Z. NAC-mediated membrane lipid remodeling negatively regulates fruit cold tolerance. Hortic. Res. 2022, 9, uhac039. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucl. Acids Res. 2014, 42, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Nei, M.; Gojobori, T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 1986, 3, 418–426. [Google Scholar]
- Nekrutenko, A.; Makova, K.D.; Li, W.H. The KA/KS ratio test for assessing the protein-coding potential of genomic regions: An empirical and simulation study. Genome Res. 2002, 12, 198–202. [Google Scholar] [CrossRef]
- Koch, M.A.; Haubold, B.; Mitchell-Olds, T. Comparative evolutionary analysis of chalcone synthase and alcohol dehydrogenase loci in Arabidopsis, Arabis, and related genera (Brassicaceae). Mol. Biol. Evol. 2000, 17, 1483–1498. [Google Scholar]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y. Improving the physical realism and structural accuracy of protein models by a two-step atomic-level energy minimization. Biophys. J. 2011, 101, 2525–2534. [Google Scholar] [CrossRef]
- Ayaz, A.; Huang, H.; Zheng, M.; Zaman, W.; Li, D.; Saqib, S.; Zhao, H.; Lü, S. Molecular Cloning and Functional Analysis of GmLACS2-3 Reveals Its Involvement in Cutin and Suberin Biosynthesis along with Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 9175. [Google Scholar] [CrossRef] [PubMed]
- Wai, A.H.; Waseem, M.; Cho, L.H.; Kim, S.T.; Lee, D.J.; Kim, C.K.; Chung, M.Y. Comprehensive genome-wide analysis and expression pattern profiling of the SlHVA22 gene family unravels their likely involvement in the abiotic stress adaptation of tomato. Int. J. Mol. Sci. 2022, 23, 12222. [Google Scholar] [CrossRef] [PubMed]
- Wai, A.H.; Cho, L.H.; Peng, X.; Waseem, M.; Lee, D.J.; Lee, J.M.; Kim, C.K.; Chung, M.Y. Genome-wide identification and expression profiling of Alba gene family members in response to abiotic stress in tomato (Solanum lycopersicum L.). BMC Plant Biol. 2021, 21, 530. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, R.; Gómez-Ariza, J.; Lanfranco, L.; Bonfante, P. Laser microdissection reveals that transcripts for five plant and one fungal phosphate transporter genes are contemporaneously present in arbusculated cells. Mol. Plant Microbe Interact. 2007, 20, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Andrews, S. FastQC A quality control tool for high throughput sequence data. In Babraham Bioinformatics; Babraham Institute: Cambridge, UK, 2010. [Google Scholar]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general-purpose read summarization program. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef]
- Smyth, G.K. Limma: Linear Models for Microarray Data. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor; Springer: New York, NY, USA, 2005; pp. 397–420. [Google Scholar]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, D.Y.; Yang, J.I.; Moon, S.; An, G. Cloning vectors for rice. J. Plant Biol. 2009, 52, 73. [Google Scholar] [CrossRef]
- Page, M.T.; Parry, M.A.; Carmo-Silva, E. A high-throughput transient expression system for rice. Plant Cell Environ. 2019, 42, 2057–2064. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Locus Name | ORF (bp) | Chromosome No. | Protein | Subcellular Localization | No. of Introns | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Length (aa) | PLATZ Domain Start-End (aa) | MW (kDa) | pI | GRAVY | ||||||
| SlPLATZ1 | Solyc01g091000 | 753 | 1 | 250 | 63–134 | 27.76 | 8.76 | −0.272 | Nucleus | 3 |
| SlPLATZ2 | Solyc02g033100 | 546 | 2 | 181 | 80–155 | 20.88 | 9.2 | −0.528 | Nucleus | 3 |
| SlPLATZ3 | Solyc02g033110 | 483 | 2 | 160 | 80–155 | 18.41 | 8.69 | −0.628 | Nucleus | 3 |
| SlPLATZ4 | Solyc02g033120 | 462 | 2 | 154 | 80–154 | 17.54 | 8.92 | −0.568 | Nucleus | 2 |
| SlPLATZ5 | Solyc02g036120 | 489 | 2 | 162 | 48–117 | 19.19 | 8.26 | −0.217 | Cytoplasm | 1 |
| SlPLATZ6 | Solyc02g036130 | 414 | 2 | 137 | 80–131 | 15.94 | 8.61 | −0.382 | Nucleus | 2 |
| SlPLATZ7 | Solyc02g036140 | 510 | 2 | 169 | 80–155 | 19.29 | 8.7 | −0.359 | Nucleus | 2 |
| SlPLATZ8 | Solyc02g036170 | 510 | 2 | 169 | 80–155 | 19.34 | 8.95 | −0.397 | Nucleus | 2 |
| SlPLATZ9 | Solyc02g036200 | 540 | 2 | 179 | 90–165 | 20.49 | 8.86 | −0.335 | Nucleus | 3 |
| SlPLATZ10 | Solyc02g036230 | 546 | 2 | 181 | 80–155 | 20.86 | 9.48 | −0.709 | Cytoplasm | 3 |
| SlPLATZ11 | Solyc02g068510 | 726 | 2 | 241 | 61–132 | 27.39 | 6.64 | −0.445 | Nucleus | 3 |
| SlPLATZ12 | Solyc04g008090 | 642 | 4 | 213 | 69–145 | 24.73 | 9.18 | −0.57 | Nucleus | 2 |
| SlPLATZ13 | Solyc06g061240 | 675 | 6 | 224 | 66–137 | 25.34 | 9.54 | −0.651 | Nucleus | 2 |
| SlPLATZ14 | Solyc07g007320 | 768 | 7 | 255 | 60–131 | 29.16 | 8.55 | −0.433 | Nucleus | 2 |
| SlPLATZ15 | Solyc07g049120 | 480 | 7 | 159 | 58–133 | 18.58 | 8.89 | −0.61 | Cytoplasm | 2 |
| SlPLATZ16 | Solyc07g049130 | 480 | 7 | 159 | 58–133 | 18.58 | 9.22 | −0.633 | Nucleus | 2 |
| SlPLATZ17 | Solyc08g005100 | 675 | 8 | 224 | 69–140 | 25.62 | 9.37 | −0.498 | Nucleus | 2 |
| SlPLATZ18 | Solyc08g076860 | 687 | 8 | 228 | 69–140 | 25.57 | 9.39 | −0.393 | Nucleus | 2 |
| SlPLATZ19 | Solyc10g085800 | 618 | 10 | 205 | 62–134 | 22.9 | 9.08 | −0.393 | Nucleus | 2 |
| SlPLATZ20 | Solyc12g010470 | 741 | 12 | 246 | 60–130 | 28.02 | 8.45 | −0.427 | Nucleus | 2 |
| Duplicated Gene Pair | Ka | Ks | Ka/Ks | Duplication Type | Types of Selection | Time (MYA) |
|---|---|---|---|---|---|---|
| SlPLATZ2 vs. SlPLATZ3 | 0.1130 | 0.2189 | 0.5162 | tandem | Purifying selection | 7.30 |
| SlPLATZ3 vs. SlPLATZ4 | 0.0279 | 0.0315 | 0.8857 | tandem | Purifying selection | 1.05 |
| SlPLATZ6 vs. SlPLATZ7 | 0.0414 | 0.0468 | 0.8855 | tandem | Purifying selection | 1.56 |
| SlPLATZ15 vs. SlPLATZ16 | 0.0218 | 0.0704 | 0.3092 | tandem | Purifying selection | 2.35 |
| SlPLATZ13 vs. SlPLATZ18 | 0.0948 | 0.7982 | 0.1188 | Segmental | Purifying selection | 26.61 |
| SlPLATZ14 vs. SlPLATZ20 | 0.1004 | 0.8476 | 0.1184 | Segmental | Purifying selection | 28.25 |
| SlPLATZ17 vs. SlPLATZ18 | 0.1477 | 0.9430 | 0.1567 | Segmental | Purifying selection | 31.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wai, A.H.; Rahman, M.M.; Waseem, M.; Cho, L.-H.; Naing, A.H.; Jeon, J.-S.; Lee, D.-j.; Kim, C.-K.; Chung, M.-Y. Comprehensive Genome-Wide Analysis and Expression Pattern Profiling of PLATZ Gene Family Members in Solanum Lycopersicum L. under Multiple Abiotic Stresses. Plants 2022, 11, 3112. https://doi.org/10.3390/plants11223112
Wai AH, Rahman MM, Waseem M, Cho L-H, Naing AH, Jeon J-S, Lee D-j, Kim C-K, Chung M-Y. Comprehensive Genome-Wide Analysis and Expression Pattern Profiling of PLATZ Gene Family Members in Solanum Lycopersicum L. under Multiple Abiotic Stresses. Plants. 2022; 11(22):3112. https://doi.org/10.3390/plants11223112
Chicago/Turabian StyleWai, Antt Htet, Md Mustafizur Rahman, Muhammad Waseem, Lae-Hyeon Cho, Aung Htay Naing, Jong-Seong Jeon, Do-jin Lee, Chang-Kil Kim, and Mi-Young Chung. 2022. "Comprehensive Genome-Wide Analysis and Expression Pattern Profiling of PLATZ Gene Family Members in Solanum Lycopersicum L. under Multiple Abiotic Stresses" Plants 11, no. 22: 3112. https://doi.org/10.3390/plants11223112
APA StyleWai, A. H., Rahman, M. M., Waseem, M., Cho, L.-H., Naing, A. H., Jeon, J.-S., Lee, D.-j., Kim, C.-K., & Chung, M.-Y. (2022). Comprehensive Genome-Wide Analysis and Expression Pattern Profiling of PLATZ Gene Family Members in Solanum Lycopersicum L. under Multiple Abiotic Stresses. Plants, 11(22), 3112. https://doi.org/10.3390/plants11223112










