Foliar Application of Melatonin Improves the Salt Tolerance, Ion and Redox Homeostasis and Seed Oil Fatty Acid Profile in Camelina sativa
Abstract
:1. Introduction
2. Results
2.1. Leaf Photosynthetic Pigments
2.2. Leaf Gas Exchange Parameters
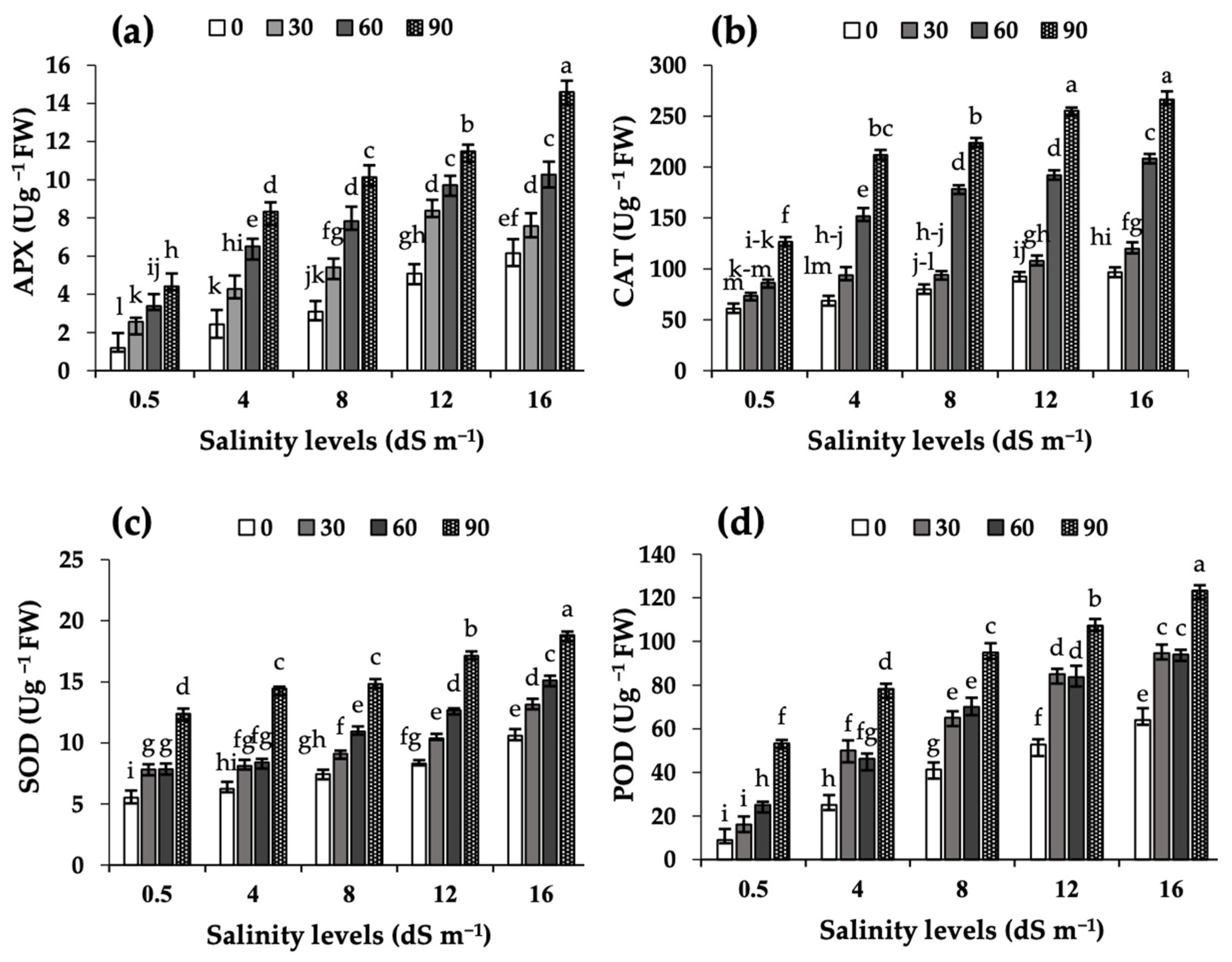
2.3. Antioxidant Enzyme Activities in Leaf Extracts
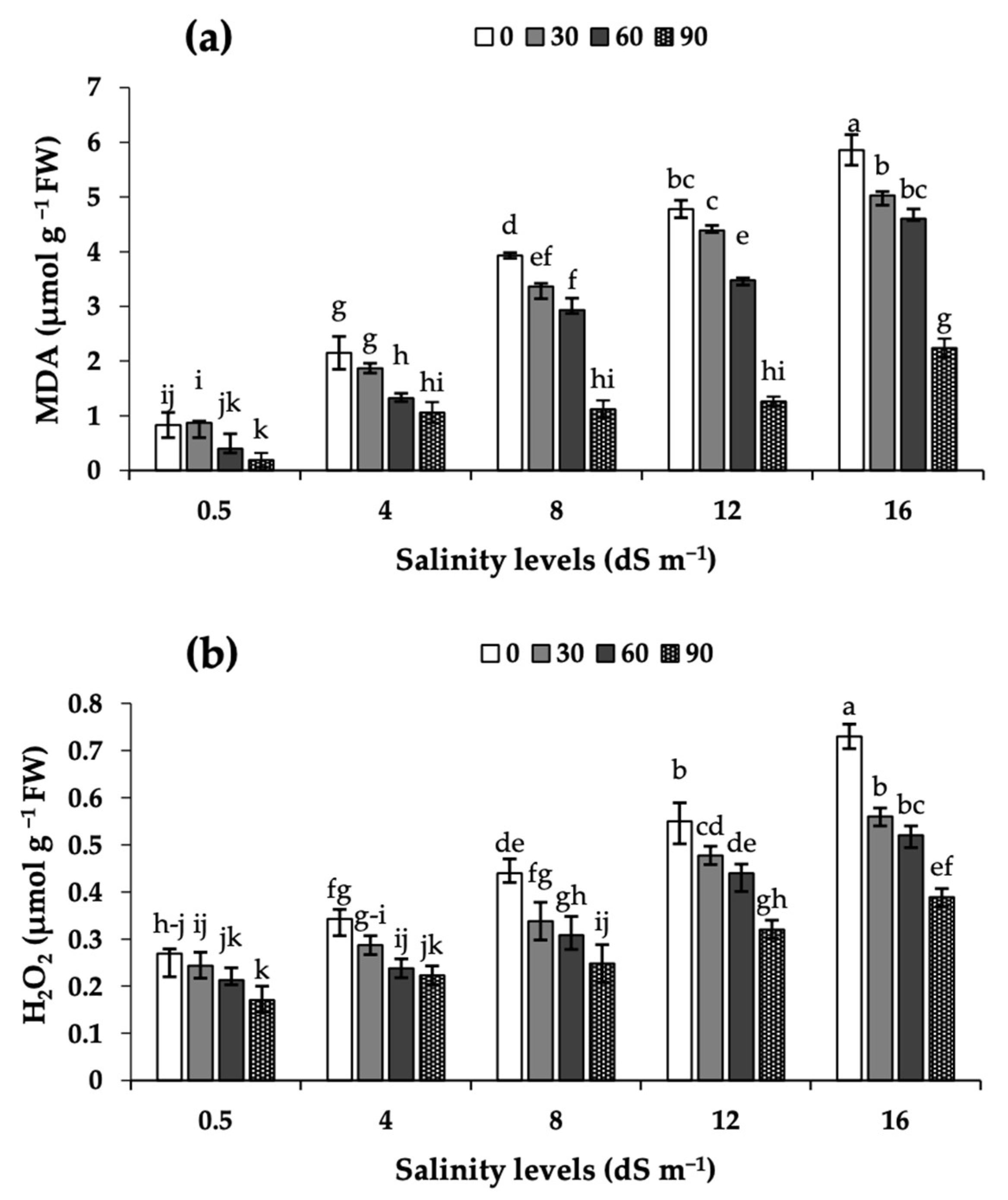
2.4. Malonyldialdehyde (MDA) and Hydrogen Peroxide (H2O2) in Leaves
2.5. Ion Concentrations, Translocation and Selective Transport (ST)
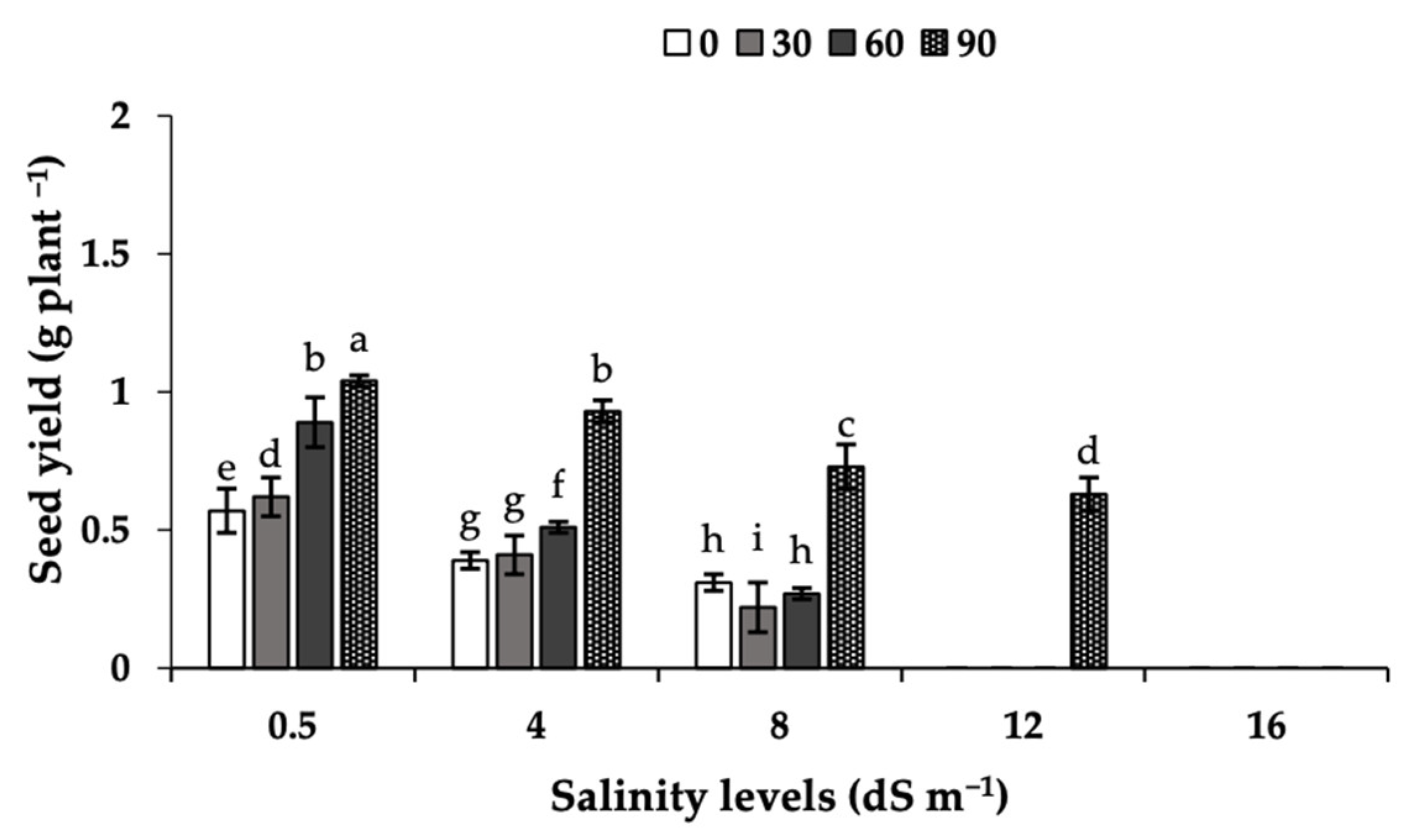
2.6. Seed Yield
2.7. Seed Fatty Acid Profile
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Design
4.3. Leaf Chlorophyll and Carotenoid Analysis
4.4. Photosynthetic Parameter Determination
4.5. Antioxidant Analysis
4.6. Malondialdehyde (MDA) and H2O2 Content Determination
4.7. Ion Concentrations, Translocation, and Selective Transport
4.8. Seed Yield and Fatty Acid Profile Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blackshaw, R.; Johnson, E.; Gan, Y.; May, W.; McAndrew, D.; Barthet, V.; McDonald, T.; Wispinski, D. Alternative oilseed crops for biodiesel feedstock on the Canadian prairies. Can. J. Plant Sci. 2011, 91, 889–896. [Google Scholar] [CrossRef]
- Li, X.; Mupondwa, E. Life cycle assessment of camelina oil derived biodiesel and jet fuel in the Canadian prairies. Sci. Total Environ. 2014, 481, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, K.J.; Sokolski, M.; Kordan, B. Camelina: Yield and quality response to nitrogen and sulfur fertilization in Poland. Ind. Crop. Prod. 2019, 141, 111776. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, M.F.; Salas, J.J.; Venegas-Caleron, M.; Garc´es, R.; Martínez Force, E. Molecular cloning and characterization of the genes encoding a microsomal oleate Δ12 desaturase (CsFAD2) and linoleate Δ15 desaturase (CsFAD3) from Camelina sativa. Ind. Crop. Prod. 2016, 89, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, H.M.; Akbari, P.; Paulose, B.; Schnell, D.; Qi, W.; Park, Y.; Dhankher, O.P. Transcriptome profiling of Camelina sativa to identify genes involved in triacylglycerol biosynthesis and accumulation in the developing seeds. Biotechnol. Biofuels 2016, 9, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heydarian, Z.; Yu, M.; Gruber, M.; Coutu, C.; Robinson, S.J.; Hegedus, D.D. Changes in gene expression in Camelina sativa roots and vegetative tissues in response to salinity stress. Sci. Rep. 2018, 8, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Shahzad, S.M.; Imtiaz, M.; Rizwan, M.S. Salinity effects on nitrogen metabolism in plants–focusing on the activities of nitrogen metabolizing enzymes: A review. J. Plant Nutr. 2018, 41, 1065–1081. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Wei, Z.; Liang, D.; Liu, C.; Yin, L.; Jia, D.; Fu, M.; Ma, F. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J. Pineal Res. 2012, 53, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015, 66, 647–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahedi, S.M.; Hosseini, M.S.; Abadía, J.; Marjani, M. Melatonin foliar sprays elicit salinity stress tolerance and enhance fruit yield and quality in strawberry (Fragaria × ananassa Duch.). Plant Physiol. Biochem. 2020, 149, 313–323. [Google Scholar] [CrossRef] [PubMed]
- El-Ghany, M.F.A.; Attia, M. Effect of exopolysaccharide-producing bacteria and melatonin on faba bean production in saline and non-saline soil. Agronomy 2020, 10, 316. [Google Scholar] [CrossRef] [Green Version]
- Alinia, M.; Kazemeini, S.A.; Sepehri, M.; Dadkhodaie, A. Simultaneous application of rhizobium strain and melatonin improves the photosynthetic capacity and induces antioxidant defense system in common bean (Phaseolus vulgaris L.) under salinity stress. J. Plant Growth Regul. 2022, 41, 1367–1381. [Google Scholar] [CrossRef]
- Khan, M.N.; Khan, Z.; Luo, T.; Liu, J.; Rizwan, M.; Zhang, J.; Xu, Z.; Wu, H.; Hu, L. Seed priming with gibberellic acid and melatonin in rapeseed: Consequences for improving yield and seed quality under drought and non-stress conditions. Ind. Crop. Prod. 2020, 156, 112850. [Google Scholar] [CrossRef]
- Dai, L.; Li, J.; Harmens, H.; Zheng, X.; Zhang, C. Melatonin enhances drought resistance by regulating leaf stomatal behaviour, root growth and catalase activity in two contrasting rapeseed (Brassica napus L.) genotypes. Plant Physiol. Biochem. 2020, 149, 86–95. [Google Scholar] [CrossRef]
- Liu, Z.; Jun-song, C.; Jing-jing, L.; Guang-yuan, L.; Chun-sheng, L.; Gui-ping, F.; Xue-kun, Z.; Hai-qing, M.; Qing-yun, L.; Xi-ling, Z.; et al. Exogenous application of a low concentration of melatonin enhances salt tolerance in rapeseed (Brassica napus L.) seedlings. J. Integr. Agric. 2018, 17, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, I.A.; Shalby, N.; El-Badri, A.M.A.; Saleem, M.H.; Khan, M.N.; Nawaz, M.A.; Qin, M.; Agami, R.A.; Kuai, J.; Wang, B.; et al. Stomata and xylem vessels traits improved by melatonin application contribute to enhancing salt tolerance and fatty acid composition of Brassica napus L. plants. Agronomy 2020, 10, 1186. [Google Scholar] [CrossRef]
- Javeed, H.M.R.; Ali, M.; Skalicky, M.; Nawaz, F.; Qamar, R.; Rehman, A.U.; Faheem, M.; Mubeen, M.; Iqbal, M.M.; Rahman, M.H.; et al. Lipoic acid combined with melatonin mitigates oxidative stress and promotes root formation and growth in salt-stressed canola seedlings (Brassica napus L.). Molecules 2021, 26, 3147. [Google Scholar] [CrossRef] [PubMed]
- Steppuhn, H.; Falk, K.C.; Zhou, R. Emergence, height, grain yield and oil content of camelina and canola grown in saline media. Can. J. Soil Sci. 2010, 90, 151–164. [Google Scholar] [CrossRef]
- Rady, M.M.; Varma, B.; Howladar, S.M. Common bean (Phaseolus vulgaris L.) seedlings overcome NaCl stress as a result of presoaking in Moringa oleifera leaf extract. Sci. Hortic. 2013, 162, 63–70. [Google Scholar] [CrossRef]
- Amiripour, A.; Jahromi, M.G.; Soori, M.K.; Torkashvand, A.M. Changes in essential oil composition and fatty acid profile of coriander (Coriandrum sativum L.) leaves under salinity and foliar-applied silicon. Ind. Crop. Prod. 2021, 168, 113599. [Google Scholar] [CrossRef]
- Borzoo, S.; Mohsenzadeh, S.; Kahrizi, D. Water-deficit stress and genotype variation induced alteration in seed characteristics of Camelina sativa. Rhizosphere 2021, 20, 100427. [Google Scholar] [CrossRef]
- Liu, N.; Lin, Z.; Guan, L.; Gaughan, G.; Lin, G. Antioxidant enzymes regulate reactive oxygen species during pod elongation in Pisum sativum and Brassica chinensis. PLoS ONE 2014, 9, e87588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Desoky, E.S.M.; Merwad, A.R.M.; El-Maati, M.F.A.; Mansour, E.; Arnaout, S.M.; Awad, M.F.; Ramadan, M.F.; Ibrahim, S.A. Physiological and biochemical mechanisms of exogenously applied selenium for alleviating destructive impacts induced by salinity stress in bread wheat. Agronomy 2021, 11, 926. [Google Scholar] [CrossRef]
- Huang, P.; He, L.; Abbas, A.; Hussain, S.; Hussain, S.; Du, D.; Hafeez, B.M.; Balooch, S.; Zahra, N.; Ren, X.; et al. Seed priming with sorghum water extract improves the performance of camelina (Camelina sativa (L.) crantz.) under salt stress. Plants 2021, 10, 749. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Li, H.; Li, X.; Cao, Y.; Zhang, H.; Li, S.; Zhang, L.; Qi, Y.; Ren, S.; et al. Melatonin improved anthocyanin accumulation by regulating gene expressions and resulted in high reactive oxygen species scavenging capacity in cabbage. Front. Plant Sci. 2016, 7, 197. [Google Scholar] [CrossRef]
- Chen, Y.E.; Mao, J.J.; Sun, L.Q.; Huang, B.; Ding, C.B.; Gu, Y.; Liao, J.-Q.; Hu, C.; Zhang, Z.-W.; Yuan, S.; et al. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plant 2018, 164, 349–363. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Zhu, T.; Zhao, C.; Li, L.; Chen, M. The role of melatonin in salt stress responses. Int. J. Mol. Sci. 2019, 20, 1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.; Cui, Q.; Feng, K.; Xu, D.; Li, C.; Zheng, Q. Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta Physiol. Plant. 2016, 38, 82. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Al-Khaishany, M.Y.; Khan, M.N.; Al-Amri, A.; Ali, H.M.; Alaraidh, I.A.; Alsahli, A.A. Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int. J. Mol. Sci. 2019, 20, 353. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Wang, S.; Deng, X.; Yin, L.; Xiong, B.; Wang, X. Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol. Plant. 2016, 38, 48. [Google Scholar] [CrossRef]
- Yan, F.; Wei, H.; Ding, Y.; Li, W.; Chen, L.; Ding, C.; Tang, S.; Jiang, Y.; Liu, G.; Li, G. Melatonin enhances Na+/K+ homeostasis in rice seedlings under salt stress through increasing the root H+-pump activity and Na+/K+ transporters sensitivity to ROS/RNS. Environ. Exp. Bot. 2021, 182, 104328. [Google Scholar] [CrossRef]
- Shafi, A.; Singh, A.K.; Zahoor, I. Melatonin: Role in abiotic stress resistance and tolerance. In Plant Growth Regulators, 2nd ed.; Aftab, T., Hakeem, K.R., Eds.; Springer: Cham, Malaysia, 2021; pp. 239–273. [Google Scholar] [CrossRef]
- Carmo-Silva, A.E.; Salvucci, M.E. The temperature response of CO2 assimilation, photochemical activities and Rubisco activation in Camelina sativa, a potential bioenergy crop with limited capacity for acclimation to heat stress. Planta 2012, 236, 1433–1445. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts, polyphenoloxidase in Beta vulgaris L. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [Green Version]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Havir, E.A.; McHale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Sergiev, I.; Alexieva, V.; Karanov, E. Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Compt. Rend. Acad. Bulg. Sci. 1997, 51, 121–124. [Google Scholar]
- Horneck, D.A.; Hanson, D. Handbook of reference methods for plant analysis. In Determination of Potassium and Sodium by Flame Emission Spectrophotometry, 1st ed.; Kalra, P.Y., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 153–157. [Google Scholar] [CrossRef]
- Malik, R.N.; Husain, S.Z.; Nazir, I. Heavy metal contamination and accumulation in soil and wild plant species from industrial area of Islamabad. Pak. J. Bot. 2010, 42, 291–301. [Google Scholar]
- Wang, S.; Zhao, G.; Gao, Y.; Tang, Z.; Zhang, C. Puccinellia Tenuiflora exhibits stronger selectivity for K+ over Na+ than wheat. J. Plant Nutr. 2005, 27, 1841–1857. [Google Scholar] [CrossRef]
- Golmakani, M.T.; Mendiola, J.A.; Rezaei, K.; Ibáñez, E. Expanded ethanol with CO2 and pressurized ethyl lactate to obtain fractions enriched in γ- Linolenic Acid from Arthrospira platensis (Spirulina). J. Supercrit. Fluids. 2012, 62, 109–115. [Google Scholar] [CrossRef]
- López, M.V.; de la Vega, M.; Gracia, R.; Claver, A.; Alfonso, M. Agronomic potential of two European pennycress accessions as a winter crop under European Mediterranean conditions. Ind. Crop. Prod. 2021, 159, 113107. [Google Scholar] [CrossRef]
| Salinity Level (dS m−1) | MEL (µM) | Total Chl (mg g−1 FW) | Carotenoids (mg g−1 FW) | Pn (µmol CO2 m−2 s−1) | Tr (mmol H2O m−2 s−1) | gs (mol H2O m−2 s−1) | Ci (µmol CO2 mol −1) |
|---|---|---|---|---|---|---|---|
| 0.5 | 0 | 2.88 ab | 1.76 c | 3.65 i | 3.38 f–h | 0.21 d–g | 353.33 e |
| 30 | 2.89 ab | 2.34 b | 8.56 de | 4.04 c–e | 0.27 a–c | 326.00 f | |
| 60 | 2.89 ab | 2.40 b | 9.28 d | 5.06 ab | 0.30 ab | 296.50 gh | |
| 90 | 2.95 a | 2.85 a | 13.25 a | 5.22 a | 0.32 a | 264.50 i | |
| 4 | 0 | 2.51ef | 0.91 f–h | 3.00 ij | 2.69 i–k | 0.14 i–k | 376.33 d |
| 30 | 2.63 de | 0.96 e–h | 7.91 ef | 3.35 gh | 0.20 d–h | 349.00 e | |
| 60 | 2.65 cd | 1.36 d | 8.63 de | 4.37 cd | 0.23 c–e | 319.50 f | |
| 90 | 2.77 bc | 1.82 c | 12.78 a | 4.53 bc | 0.25 b–d | 287.50 fg | |
| 8 | 0 | 2.45 f–h | 0.64 i–k | 2.54 jk | 2.35 j–l | 0.11 j–l | 399.33 bc |
| 30 | 2.45 f–h | 0.74 h–j | 7.45 fg | 3.01 hi | 0.17 f–i | 372.00 d | |
| 60 | 2.47 fg | 1.15 d–g | 8.17 ef | 4.03 c–e | 0.20 d–h | 342.50 e | |
| 90 | 2.46 f–h | 1.20 de | 12.32 ab | 4.19 c–e | 0.22 c–f | 310.50 fg | |
| 12 | 0 | 2.14 jk | 0.50 jk | 1.85 kl | 2.25 kl | 0.09 kl | 411.33 b |
| 30 | 2.23 ij | 0.59 jk | 6.76 gh | 2.91 h–j | 0.15 h–j | 384.00 cd | |
| 60 | 2.35 g–i | 1.00 e-h | 7.48 fg | 3.93 d–f | 0.18 e–i | 354.50 e | |
| 90 | 2.32 hi | 1.17 d–f | 11.63 bc | 4.09 c–e | 0.20 d–h | 322.50 f | |
| 16 | 0 | 1.73 n | 0.42 k | 1.31 l | 2.05 l | 0.07 l | 434.33 a |
| 30 | 1.86 mn | 0.52 jk | 6.22 h | 2.71 i–k | 0.13 i–k | 407.00 b | |
| 60 | 1.97 lm | 0.95 e–h | 6.94 gh | 3.73 e–g | 0.16 g–j | 377.50 d | |
| 90 | 2.08 kl | 0.88 g–i | 11.09 c | 3.89 d–g | 0.18 e–i | 345.50 e | |
| Significance | |||||||
| Salinity | ** | ** | ** | ** | ** | ** | |
| MEL | ** | ** | ** | ** | ** | ** | |
| Salinity × MEL | * | ** | * | * | ** | * | |
| Salinity Level (dS m−1) | MEL Concentrations (µM) | K (mg g−1 DW) | Na (mg g−1 DW) | K/Na | Translocation | Selective Transport | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | Shoot | Root | K (mg g−1 DW) | Na (mg g−1 DW) | |||
| 0.5 | 0 | 8.78 c–e | 2.89 b | 2.00 f | 3.46 f | 4.39 d | 0.83 c | 3.29 e | 0.57 g | 4.94 fg |
| 30 | 9.37 bc | 2.91 b | 1.20 fg | 2.65 fg | 7.88 c | 1.10 b | 3.89 de | 0.45 h | 4.14 g | |
| 60 | 9.69 b | 2.49 d | 0.71 g | 2.17 g | 13.71 b | 1.14 b | 3.21 ef | 0.32 i | 4.54 fg | |
| 90 | 11.36 a | 3.44 a | 0.48 g | 1.94 g | 23.57 a | 1.77 a | 3.03 ef | 0.24 j | 5.62 fg | |
| 4 | 0 | 7.97 d–f | 1.73 g | 6.53 c | 7.99 c | 1.22 e–h | 0.21 g | 3.38 e | 0.81 b–d | 4.80 fg |
| 30 | 7.92 e–f | 2.37 e | 6.03 cd | 7.49 cd | 1.31 e–h | 0.31 f | 3.58 de | 0.80 cd | 5.82 ef | |
| 60 | 8.83 b–d | 2.46 d | 3.83 e | 5.29 e | 2.31 ef | 0.46 e | 3.33 e | 0.72 e | 5.24 fg | |
| 90 | 9.05 bc | 2.67 c | 3.23 e | 4.68 e | 2.87 de | 0.57 d | 4.60 d | 0.68 f | 7.46 d | |
| 8 | 0 | 6.03 h | 0.74 k | 7.87 ab | 9.33 ab | 0.77 f–h | 0.08 j | 3.76 de | 0.84 ab | 4.95 fg |
| 30 | 5.55 h | 0.87 j | 6.68 c | 8.14 c | 0.83 f–h | 0.10 ij | 4.04 de | 0.82 bc | 5.09 fg | |
| 60 | 7.82 fg | 1.93 f | 4.15 e | 5.61 e | 1.88 e–h | 0.34 f | 6.38 c | 0.74 e | 7.15 de | |
| 90 | 8.92 bc | 2.37 e | 4.13 e | 5.59 e | 2.17 e–g | 0.42 e | 8.08 b | 0.73 e | 7.30 de | |
| 12 | 0 | 5.60 h | 0.60 lm | 7.74 b | 9.20 b | 0.72 f–h | 0.06 j | 6.66 c | 0.84 ab | 5.46 fg |
| 30 | 7.38 fg | 0.75 k | 5.95 cd | 7.40 cd | 1.24 e–h | 0.10 ij | 6.76 c | 0.80 cd | 7.77 d | |
| 60 | 6.95 g | 1.95 f | 5.50 d | 6.83 d | 1.32 e–h | 0.10 ij | 9.76 a | 0.78 d | 8.35 cd | |
| 90 | 7.89 f | 1.21 h | 5.34 d | 6.79 d | 1.51 e–h | 0.17 gh | 9.43 a | 0.78 d | 9.59 c | |
| 16 | 0 | 3.29 i | 0.52 m | 8.88 a | 10.34 a | 0.37 h | 0.05 j | 4.54 d | 0.85 a | 11.20 b |
| 30 | 4.10 i | 0.67 kl | 8.02 ab | 9.48 ab | 0.51 gh | 0.07 j | 2.12 f | 0.84 ab | 12.15 ab | |
| 60 | 4.15 i | 1.04 i | 7.81 b | 9.27 b | 0.53 gh | 0.21 gh | 6.30 c | 0.84 ab | 11.89 ab | |
| 90 | 5.50 h | 1.18 h | 5.73 cd | 7.19 cd | 0.96 f–h | 0.16 gh | 6.26 c | 0.79 ab | 13.26 a | |
| Significance | ||||||||||
| Salinity | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| MEL | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| Salinity × MEL | * | * | * | ** | ** | ** | ** | ** | ** | |
| Salinity Level(dS m−1) | MEL Concentrations (µM) | SFA | MUFA | PUFA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C16: 0 | C18: 0 | C18: 1 | C20: 1 | C22: 1 | C18: 2 | C18: 3 | C20: 2 | C20: 3 | ||
| 0.5 | 0 | 10.37 e | 1.44 f | 16.34 b–d | 2.33 e | 0.25 d | 23.55 e | 47.66 b | 0.36 g–i | 0.18 fg |
| 30 | 10.86 e | 0.64 g | 16.85 bc | 3.85 d | 0.38 c | 29.00 d | 47.66 b | 1.49 e | 0.82 c | |
| 60 | 10.87 e | 0.52 g | 17.39 b | 5.02 c | 1.42 b | 30.29 c | 49.30 a | 3.77 c | 1.30 b | |
| 90 | 10.64 e | 1.36 f | 21.13 a | 7.00 a | 1.61 a | 37.74 a | 49.46 b | 4.00 b | 1.81 a | |
| 4 | 0 | 13.24 d | 1.77 e | 13.75 f | 0.55 g | 0.00 e | 22.30 f | 34.13 g | 0.23 hi | 0.16 g |
| 30 | 14.73 c | 1.39 f | 15.32 de | 1.23 f | 0.00 e | 28.33 d | 35.42 f | 0.41 gh | 0.37 de | |
| 60 | 12.48 d | 1.40 f | 16.08 cd | 3.85 d | 0.38 c | 30.24 c | 41.14 cd | 2.63 d | 0.40 d | |
| 90 | 10.26 e | 1.95 de | 17.06 bc | 5.86 b | 0.47 c | 31.66 b | 48.82 a | 5.32 a | 1.33 b | |
| 8 | 0 | 15.69 c | 2.49 bc | 3.90 g | 0.52 gh | 0.00 e | 21.59 f | 15.61 h | 0.23 hi | 0.05 h |
| 30 | 23.38 a | 2.08 d | 13.90 f | 0.63 g | 0.00 e | 22.14 f | 37.28 e | 0.20 i | 0.22 f | |
| 60 | 18.89 b | 2.37 c | 14.56 ef | 2.86 e | 0.00 e | 23.72 e | 40.69 d | 0.42 gh | 0.33 e | |
| 90 | 13.42 d | 2.61 b | 14.56 ef | 4.77 c | 0.20 d | 23.72 e | 42.17 c | 0.82 f | 0.42 d | |
| 12 | 0 | - | - | - | - | - | - | - | - | - |
| 30 | - | - | - | - | - | - | - | - | - | |
| 60 | - | - | - | - | - | - | - | - | - | |
| 90 | 1.72 f | 26.68 a | 4.43 g | 2.84 e | 0.00 e | 20.11 g | 41.26 cd | 0.52 g | 0.33 e | |
| Significance | ||||||||||
| Salinity | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| MEL | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| Salinity × MEL | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakyani, M.R.F.; Alinia, M.; Kazemeini, S.A.; Abadía, J.; Dadkhodaie, A. Foliar Application of Melatonin Improves the Salt Tolerance, Ion and Redox Homeostasis and Seed Oil Fatty Acid Profile in Camelina sativa. Plants 2022, 11, 3113. https://doi.org/10.3390/plants11223113
Bakyani MRF, Alinia M, Kazemeini SA, Abadía J, Dadkhodaie A. Foliar Application of Melatonin Improves the Salt Tolerance, Ion and Redox Homeostasis and Seed Oil Fatty Acid Profile in Camelina sativa. Plants. 2022; 11(22):3113. https://doi.org/10.3390/plants11223113
Chicago/Turabian StyleBakyani, Mohammad Reza Forozan, Mozhgan Alinia, Seyed Abdolreza Kazemeini, Javier Abadía, and Ali Dadkhodaie. 2022. "Foliar Application of Melatonin Improves the Salt Tolerance, Ion and Redox Homeostasis and Seed Oil Fatty Acid Profile in Camelina sativa" Plants 11, no. 22: 3113. https://doi.org/10.3390/plants11223113





