Vigorous Growing of Donor Plantlets by Liquid Overlay in Subcultures Is the Key to Cryopreservation of Endangered Species Pogostemon yatabeanus
Abstract
1. Introduction
2. Results
2.1. Subculture Medium and Supplements in the Last Subculture Cycle (S-0)
2.1.1. Effect of Subculture Medium and Supplements on In Vitro Growth of Donor Plants
2.1.2. Droplet-Vitrification Procedure
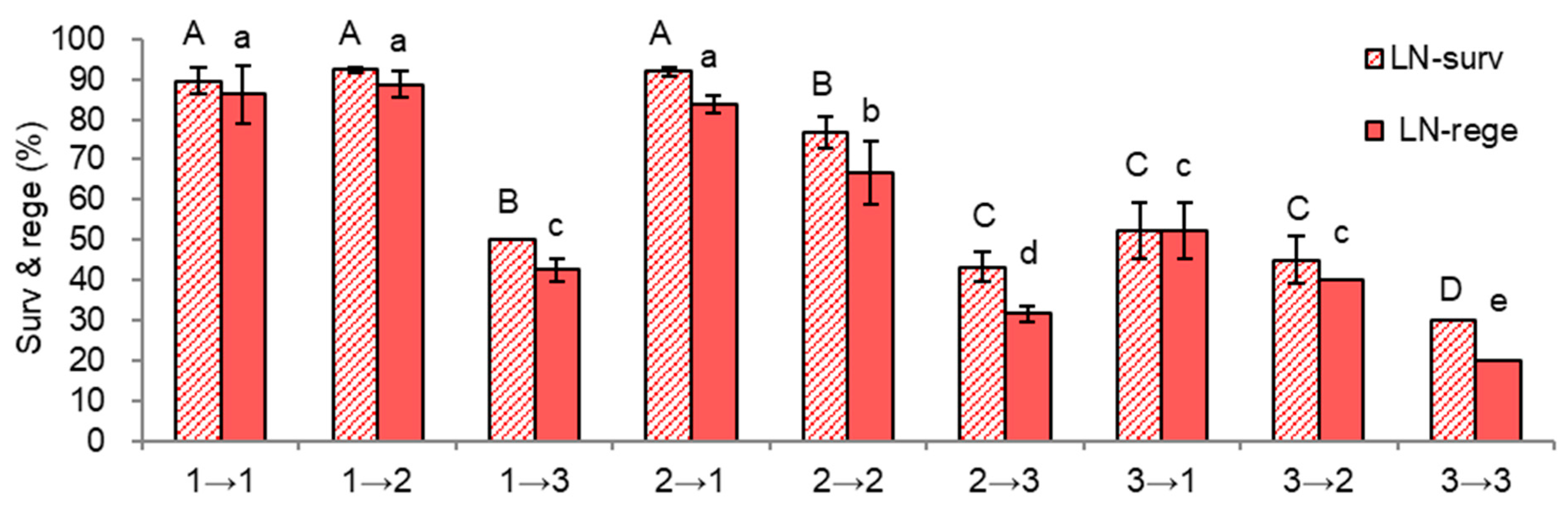
2.2. Revitalization of Donor Plants through the Subsequent Subcultures
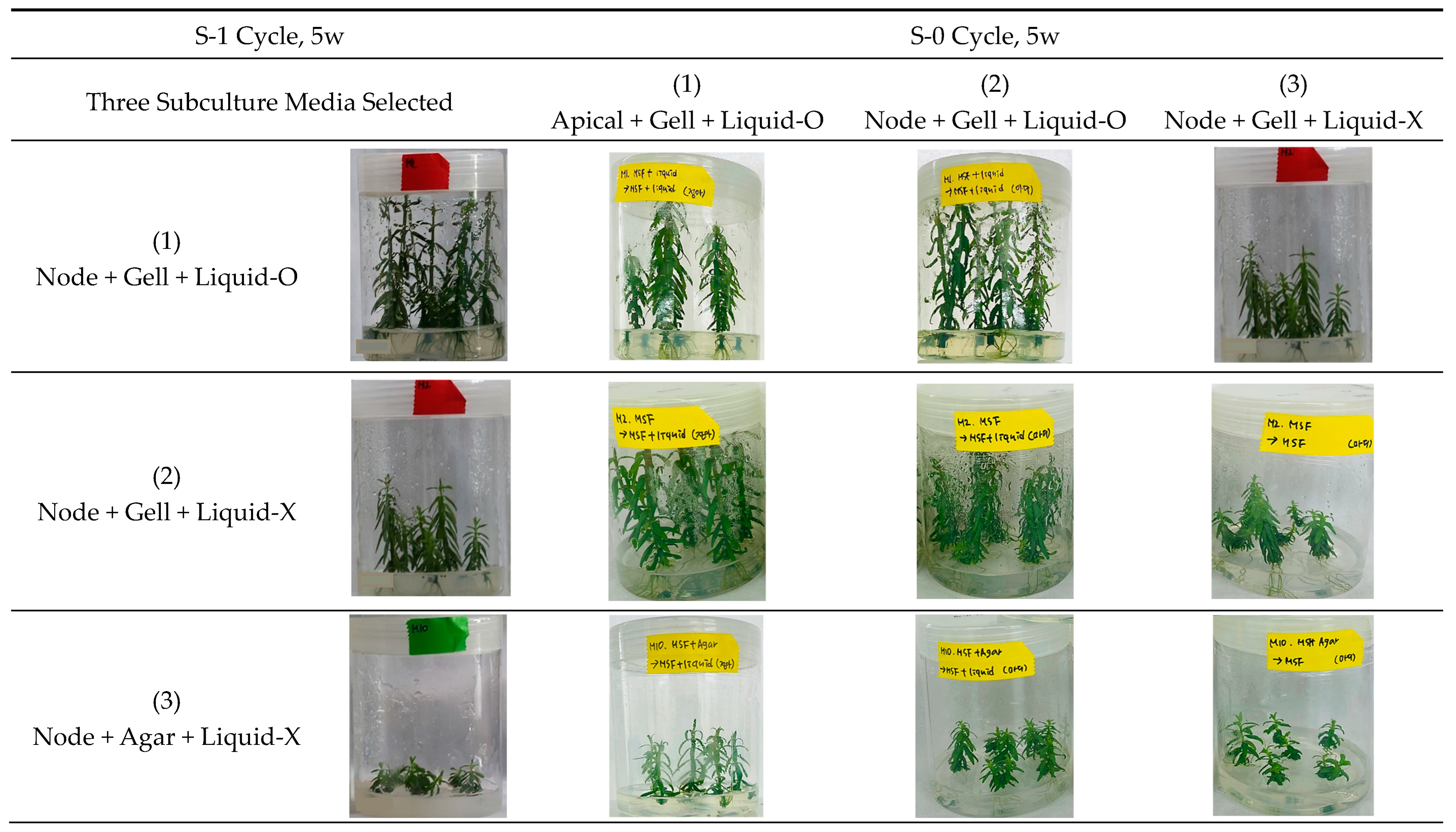
2.2.1. Selection of Three Subculture Variants
2.2.2. In Vitro Growth of Donor Plantlets during the Subsequent Subculture Cycles
2.2.3. Droplet-Vitrification Procedure Following the Subsequent Subculture Cycles
3. Discussion
3.1. In Vitro Subculture and Propagation System
3.2. Subculture Conditions and Post-Cryopreservation Regeneration
4. Materials and Methods
4.1. Plant Material, In Vitro Establish and Preparation of Mother Plants
4.2. Effect of Subculture Medium and Conditions in the Last Subculture Cycle (S-0)
4.2.1. In Vitro Growth of Donor Plants
4.2.2. Droplet-Vitrification Procedure
4.3. Revitalization of Donor Plants through the Subsequent Subcultures
4.3.1. In Vitro Growth of Donor Plants during the Subsequent Subculture Cycles, S-1→S-0
4.3.2. Droplet-Vitrification Procedure Following the Subsequent Cycles
4.4. Recovery Assessment and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WFO: World Flora Online. Published on the Internet. 2022. Available online: http://www.worldfloraonline.org (accessed on 4 November 2022).
- Suh, Y.B.; Kim, Y.J. Korean Red List of Threatened Species, 2nd ed.; National Institute of Biological Resources: Incheon, Republic of Korea, 2014. [Google Scholar]
- Pence, V.C.; Ballesteros, D.; Walters, C.; Reed, B.M.; Philpott, M.; Dixon, K.W.; Pritchard, H.W.; Culley, T.M.; Vanhove, A.C. Cryobiotechnologies: Tools for expanding long-term ex situ conservation to all plant species. Biol. Conserv. 2020, 250, 108736. [Google Scholar] [CrossRef]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic plant species conservation: Biotechnological approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, H.; Popova, E.; Lee, Y.Y.; Park, S.U.; Kim, H.H. Ammonium-free medium is critical for regeneration of shoot tips of the endangered species Pogostemon yatabeanus cryopreserved using droplet-vitrification. CryoLetters 2021, 45, 290–299. [Google Scholar]
- Lee, H.E.; Popova, E.; Park, H.N.; Park, S.U.; Kim, H.H. Optimization of a cryopreservation method for the endangered Korean species Pogostemon yatabeanus using a systematic approach: The key role of ammonium and growth regulators. Plants 2021, 10, 2018. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.A.; Souza, E.H.; Max, D.A.S.; Rossi, M.L.; Villalobos-Olivera, A.; Ledo, C.A.S.; Martinez-Montero, M.E.; Souza, F.V.D. Morphoanatomical aspects of the starting material for the improvement of pineapple cryopreservation by the droplet-vitrification technique. An. Acad. Bras. Cienc. 2021, 93, e20190555. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Kim, H.H.; Ko, H.C.; Hwang, H.S.; Cho, E.G.; Sohn, J.K.; Engelmann, F. Cryopreservation of cultivated and wild potato varieties by droplet vitrification: Effect of subculture of mother-plants and of preculture of shoot tips. CryoLetters 2006, 27, 211–222. [Google Scholar]
- Engelmann, F. Cryopreservation of clonal crops: A review of key parameters. Acta Hortic. 2014, 1039, 31–39. [Google Scholar] [CrossRef]
- Reed, B. Culture conditions are as important as the protocol in successful cryopreservation. Cryobiology 2018, 80, 170. [Google Scholar] [CrossRef]
- Niino, T.; Yamamoto, S.; Matsumoto, T.; Engelmann, F.; Valle Arizaga, M.; Tanaka, D. Development of V and D cryo-plate methods as effective protocols for cryobanking. Acta Hortic. 2019, 1234, 249–262. [Google Scholar] [CrossRef]
- Kaczmarczyk, A.; Turner, S.R.; Bunn, E.; Mancera, R.L.; Dixon, K.W. Cryopreservation of threatened native Australian species—What have we learned and where to from here? Vitr. Cell. Dev. Biol-Plant 2011, 47, 17–25. [Google Scholar] [CrossRef]
- Pence, V.C.; Winget, G.D.; Lindsey, K.M.; Plair, B.L.; Charls, S.M. In vitro propagation, cryopreservation, and genetic analysis of the endangered Hedeoma todsenii (Lamiaceae). Madroño 2009, 56, 221–228. [Google Scholar] [CrossRef]
- Jain, A.; Poling, M.D.; Smith, A.P.; Nagarajan, V.K.; Lahner, B.; Meagher, R.B.; Raghothama, K.G. Variations in the composition of gelling agents affect morphophysiological and molecular responses to deficiencies of phosphate and other nutrients. Plant Physiol. 2009, 150, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.M.; Amer, A.M.; Osman, N.H.; Sedikc, M.Z.; Hussein, M.H. Effects of different gelling agents on the different stages of rice regeneration in two rice cultivars. Saudi J. Biol. Sci. 2021, 28, 5738–5744. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.S.; Veeder, G.T.; Mirrasoul, P.J.; Kaneko, T.; Cottrell, I.W. Agar-like polysaccharide produced by a Pseudomonas species: Production and basic properties. Appl. Environ. Microbiol. 1982, 43, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Arregui, L.M.; Veramendi, J.; Mingo-Castel, A.M. Effect of gelling agents on in vitro tuberization of six potato cultivars. Amer. J. Potato Res. 2003, 80, 141–144. [Google Scholar] [CrossRef]
- Das, N.; Tripathi, N.; Basu, S.; Bose, C.; Maitra, S.; Khurana, S. Progress in the development of gelling agents for improved culturability of microorganisms. Front. Microbiol. 2015, 6, 698. [Google Scholar] [CrossRef]
- Buah, J.N.; Kawamitsu, Y.; Sato, S.; Murayama, S. Effects of different types and concentrations of gelling agents on the physical and chemical properties of media and the growth of banana (Musa spp.) in vitro. Plant Prod. Sci. 1999, 2, 138–145. [Google Scholar] [CrossRef]
- Huang, L.C.; Chi, D.L. Pivotal roles of picloram and gelrite in banana callus culture. Environ. Exp. Bot. 1988, 28, 249–258. [Google Scholar] [CrossRef]
- Veramendi, J.; Villafranca, M.J.; Sota, V.; Mingo-Castel, A.M. Gelrite as an alternative to agar for micropropagation and microtuberization of Solanum tuberosum L. cv. Baraka. Vitr. Cell. Dev. Biol-Plant 1997, 33, 195–199. [Google Scholar] [CrossRef]
- Fira, A.; Clapa, D. The influence of the gelling agent upon multiplication rate in Sequoia sempervirens. Bull. UASVM 2008, 65, 463. [Google Scholar]
- Benjelloun, J.; Taoufyq, A.; El Abidine Triqui, Z.; Alami, Q.L.; Layachi, R.; Smouni, A.; Bouzroud, S.; Guedira, A. Improvement of in vitro germination of Cycas revoluta zygotic embryos using gelrite as gelling agent. Adv. Hort. Sci. 2020, 34, 349–354. [Google Scholar] [CrossRef]
- Palanyandy, S.R.; Gantait, S.; Sinniah, U.R. Effects of some gelling agents and their concentrations on conversion of oil palm polyembryoids into plantlets. J. Genet. Eng. Biotechnol. 2020, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Scholten, H.J.; Pierik, R.L.M. Agar as a gelling agent: Chemical and physiological analysis. Plant Cell Rep. 1998, 17, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Repalli, S.K.; Geda, C.K.; Pradhan, N.S.N.; Rao, G.J.N. Influence of additional nutrients and gelling agents on in vitro response of selected indica rice varieties. Intl. J. Biol. 2019, 11, 26–35. [Google Scholar] [CrossRef]
- Sah, S.K.; Kaur, A.; Jagdeep, S.S. High frequency embryogenic callus induction and whole plant regeneration in Japonica rice Cv. kitaake. J. Rice Res. 2014, 2, 125. [Google Scholar] [CrossRef]
- Toaima, N.; Bosila, H.; El-Ateeq, A.E. In vitro growth regulators, gelling agents and sucrose levels affect micropropagation of Gypsophila paniculate L. Mid. E. J. Agri. 2016, 5, 313. [Google Scholar]
- Siddiqui, Z.H.; Mujib, A.; Maqsood, M. Liquid overlaying improves somatic embryogenesis in Catharanthus roseus. Plant Cell Tiss. Organ Cult. 2011, 104, 247–256. [Google Scholar] [CrossRef]
- Pullman, G.S.; Skryabina, A. Liquid medium and liquid overlays improve embryogenic tissue initiation in conifers. Plant Cell Rep. 2007, 26, 873–887. [Google Scholar] [CrossRef]
- Hussien, F.A.; Osman, M.A.; Idris, T.I.M. The influence of liquid media support, gelling agents and liquid overlays on performance of in vitro cultures of ginger (Zingiber officinale). Intl. J. Sci. Res. Pub. 2014, 4, 2250–3153. [Google Scholar]
- Fujiwara, K.; Kira, S.; Kozai, T. Contribution of photosynthesis to dry weight increase of in vitro potato cultures under different CO2 concentrations. Acta Hortic. 1995, 393, 119–126. [Google Scholar] [CrossRef]
- Thomas, T.D. The role of activated charcoal in plant tissue culture. Biotech. Adv. 2008, 26, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Engelmann, F.; Zhao, Y.; Zhou, M.; Chen, S. Cryopreservation of apple shoot tips: Importance of cryopreservation technique and of conditioning of donor plants. CryoLetters 1999, 20, 121–130. [Google Scholar]
- Pathirana, R.; Mathew, L.; McLachlan, A. A simplified method for high recovery of kiwifruit (Actinidia spp.) shoot tips after droplet vitrification cryopreservation suitable for long-term conservation. Plant Cell Tiss. Organ. Cult. 2021, 144, 97–102. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco cell cultures. Physiol Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
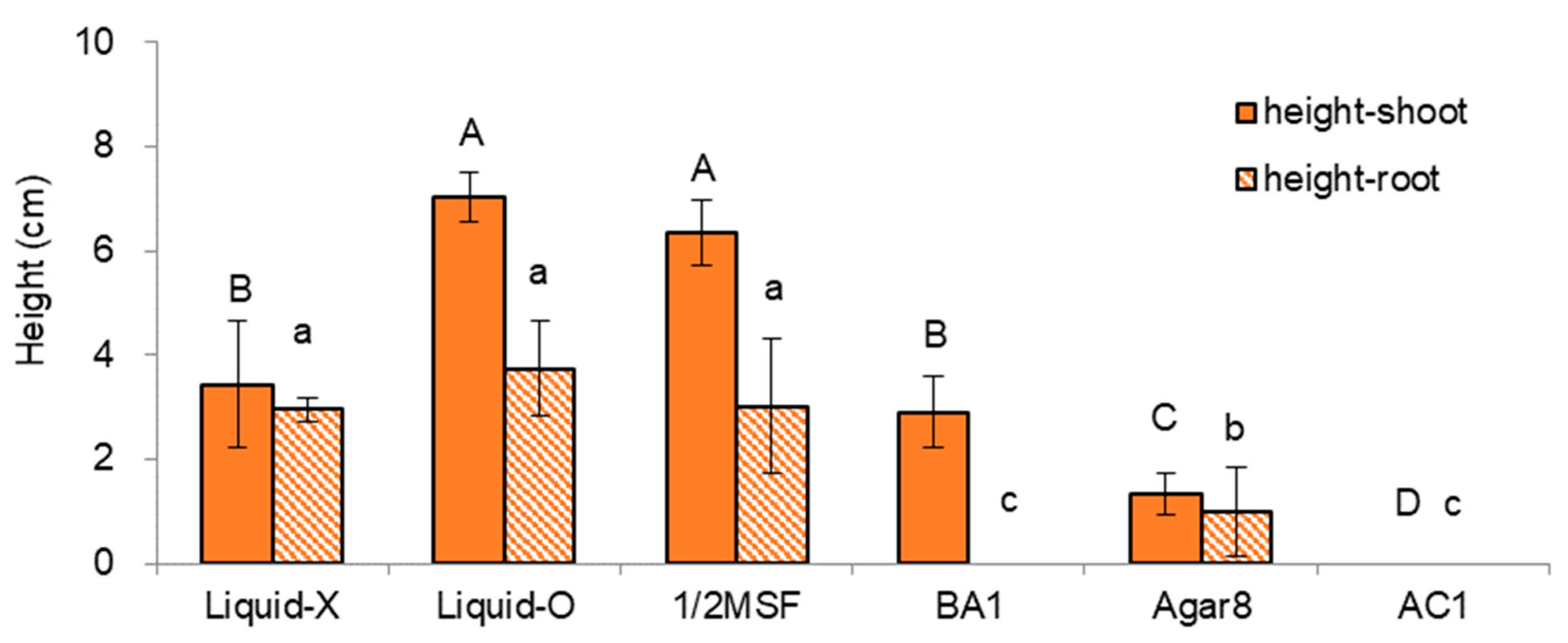
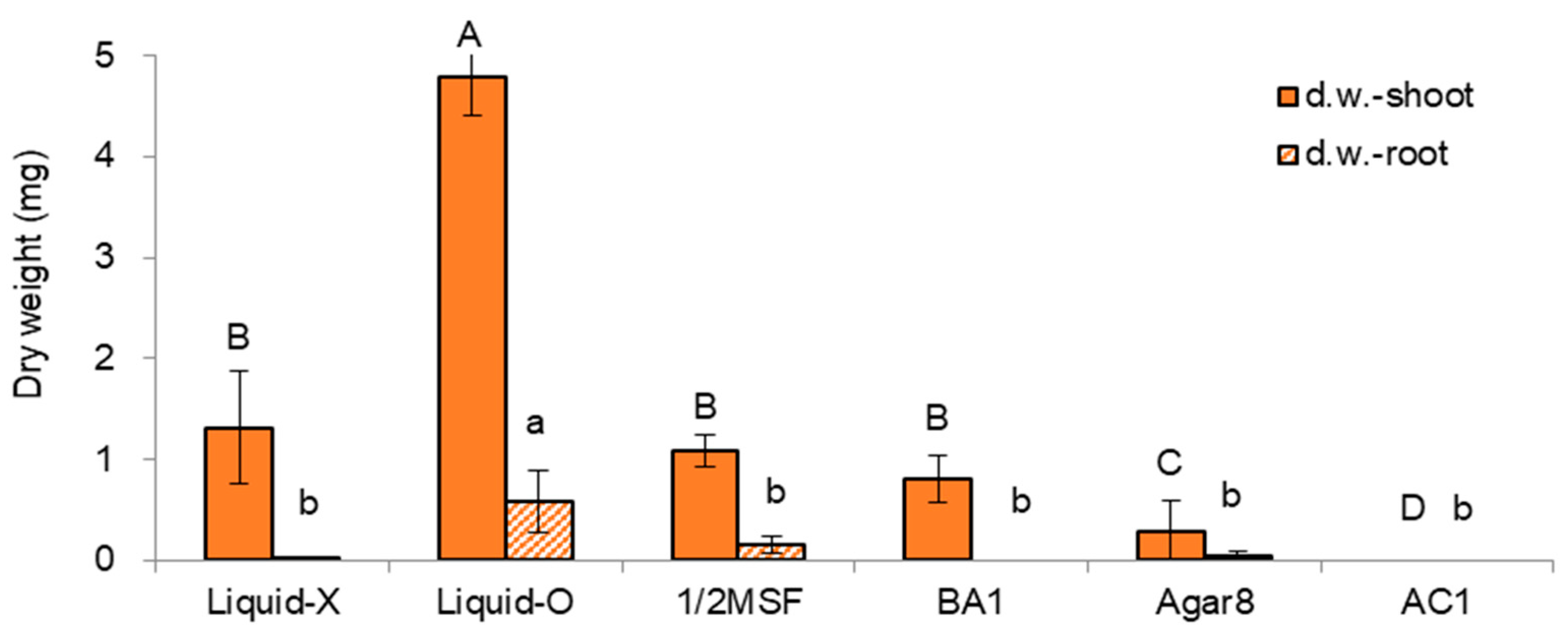
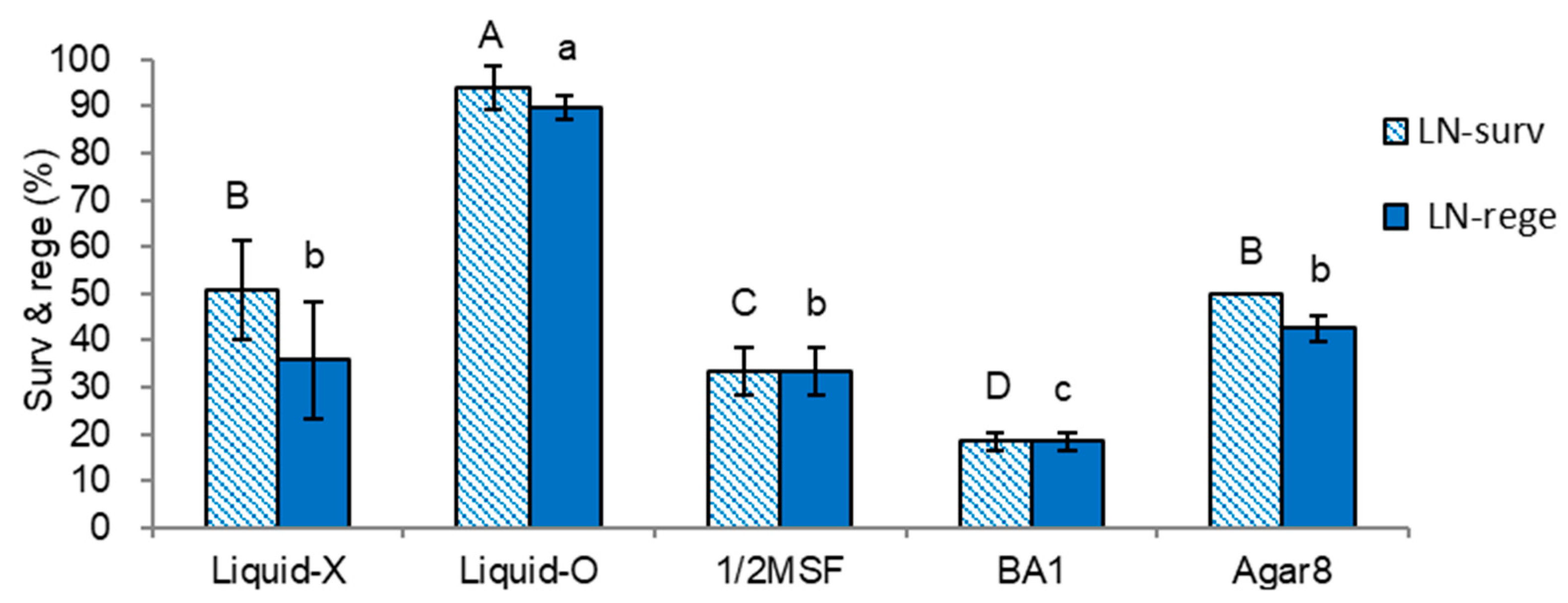


| Treatment Sets | Regrowth Steps (5d-3w-2w) | Height | Dry Weight | ||
|---|---|---|---|---|---|
| Shoots | Roots | Shoots | Roots | ||
| Subculture medium and supplements | RM1-RM2-MSF * | 0.49 | 0.62 | 0.85 | 0.90 |
| RM2-RM2-MSF | 0.75 | 0.74 | 0.95 | 0.98 | |
| Code | Subsequent Subculture Cycle | Mean of Three Subculture Combinations each (S-1→S-0) | ||
|---|---|---|---|---|
| S-1, 5 Weeks | S-0, 5 Weeks | LN Survival (%) | LN Regeneration (%) | |
| 1→1, 2, 3 | Node + Gell + Liquid-O | Apical + Gell + Liquid-O Node + Gell + Liquid-O Node + Agar + Liquid-X | 77.3 | 72.4 |
| 2→1, 2, 3 | Node + Gell + Liquid-X | Apical + Gell + Liquid-O Node + Gell + Liquid-O Node + Agar + Liquid-X | 70.6 | 60.7 |
| 3→1, 2, 3 | Node + Agar + Liquid-X | Apical + Gell + Liquid-O Node + Gell + Liquid-O Node + Agar + Liquid-X | 42.4 | 37.4 |
| 1, 2, 3→1 | Node + Gell + Liquid-O Node + Gell + Liquid-X Node + Agar + Liquid-X | Apical+Gell+Liquid-O | 77.9 | 74.1 |
| 1, 2, 3→2 | Node + Gell + Liquid-O Node + Gell + Liquid-X Node + Agar + Liquid-X | Node+Gell+Liquid-O | 71.3 | 65.1 |
| 1, 2, 3→3 | Node + Gell + Liquid-O Node + Gell + Liquid-X Node + Agar + Liquid-X | Node+Agar+Liquid-X | 41.1 | 31.4 |
| No. | Medium | Gelling Agent | Liquid Overlay | Hormone | AC | Code | Remarks |
|---|---|---|---|---|---|---|---|
| (g L−1) | (mg L−1) | (g L−1) | |||||
| 1 | MS | Gellan gum 3 | Liquid-O | - | - | Liquid-O | |
| 2 | MS | Gellan gum 3 | Liquid-X | - | - | Liquid-X | standard |
| 3 | MS | Agar 8 | Liquid-X | - | - | Agar8 | |
| 4 | 1/2 MS | Gellan gum 3 | Liquid-X | - | - | 1/2 MSF | |
| 5 | MS | Gellan gum 3 | Liquid-X | BA 1 | - | BA1 | |
| 6 | MS | Gellan gum 3 | Liquid-X | - | AC 1 | AC1 |
| Repeated Standard Subcultures for PropAgation (S-2 *, 6w) | Subculture Treatment (Gelling Agent and Liquid Overlay, S-1 ** Cycle, 5w) | Subculture Treatment (Section, Gelling Agent, and Liquid Overlay) for Rejuvenation (S-0 ***, 5w) | Code (S-1→S-0 Cycle) |
|---|---|---|---|
| Node + Gell + Liquid-O | (1) Node + Gell + Liquid-O | (1) Apical + Gell + Liquid-O (2) Node + Gell + Liquid-O (3) Node + Gell + Liquid-X | 1→1 1→2, standard 1→3 |
| (2) Node + Gell + Liquid-X | (1) Apical + Gell + Liquid-O (2) Node + Gell + Liquid-O (3) Node + Gell + Liquid-X | 2→1 2→2 2→3 | |
| (3) Node + Agar + Liquid-X | (1) Apical + Gell + Liquid-O (2) Node + Gell + Liquid-O (3) Node + Gell + Liquid-X | 3→1 3→2 3→3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Kim, H. Vigorous Growing of Donor Plantlets by Liquid Overlay in Subcultures Is the Key to Cryopreservation of Endangered Species Pogostemon yatabeanus. Plants 2022, 11, 3127. https://doi.org/10.3390/plants11223127
Lee H, Kim H. Vigorous Growing of Donor Plantlets by Liquid Overlay in Subcultures Is the Key to Cryopreservation of Endangered Species Pogostemon yatabeanus. Plants. 2022; 11(22):3127. https://doi.org/10.3390/plants11223127
Chicago/Turabian StyleLee, Hyoeun, and Haenghoon Kim. 2022. "Vigorous Growing of Donor Plantlets by Liquid Overlay in Subcultures Is the Key to Cryopreservation of Endangered Species Pogostemon yatabeanus" Plants 11, no. 22: 3127. https://doi.org/10.3390/plants11223127
APA StyleLee, H., & Kim, H. (2022). Vigorous Growing of Donor Plantlets by Liquid Overlay in Subcultures Is the Key to Cryopreservation of Endangered Species Pogostemon yatabeanus. Plants, 11(22), 3127. https://doi.org/10.3390/plants11223127






