Synergistic Effects of Some Methoxyflavones Extracted from Rhizome of Kaempferia parviflora Combined with Gentamicin against Carbapenem-Resistant Strains of Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii
Abstract
1. Introduction
2. Results
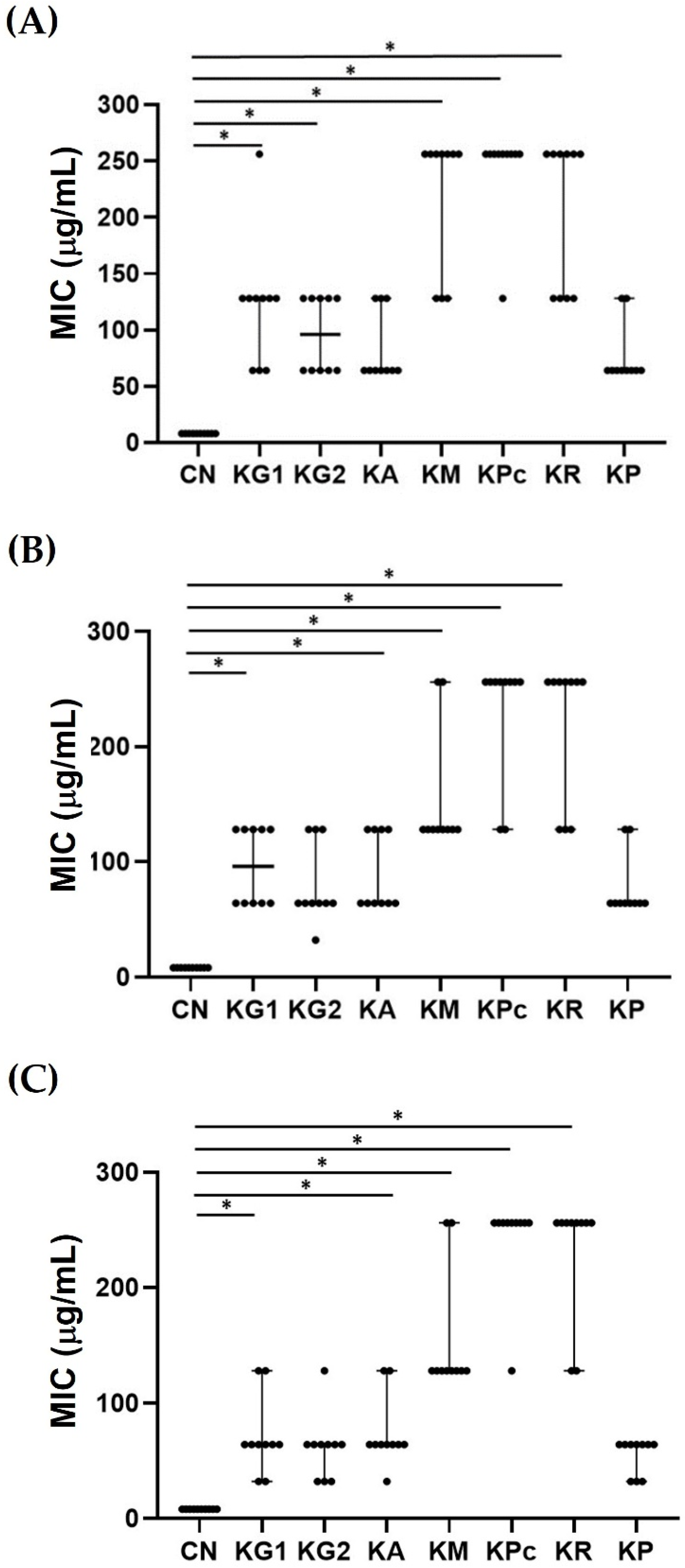
2.1. Minimum Inhibitory Concentrations (MIC) of the Crude Ethanolic Extracts of Kaempferia
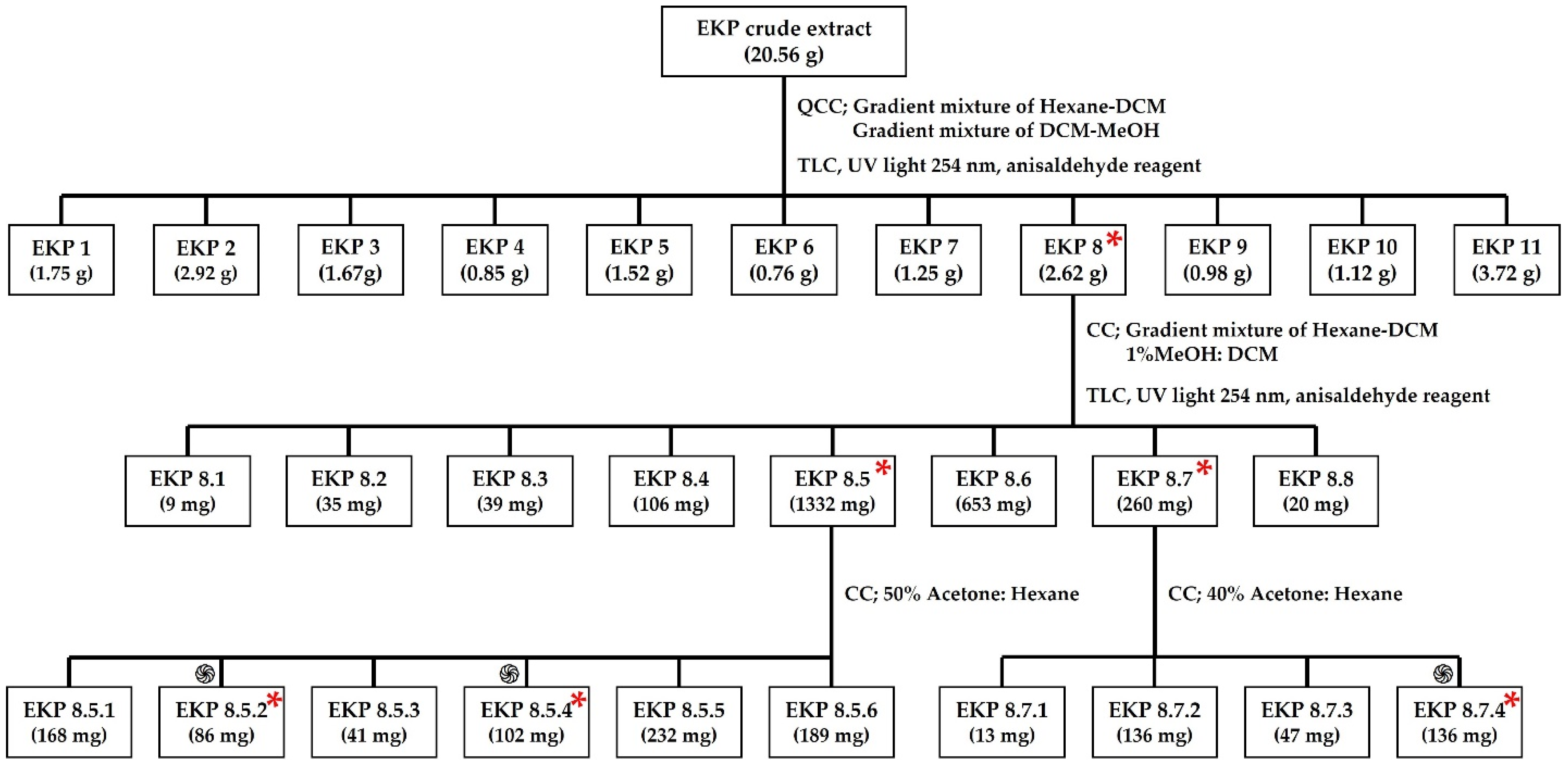
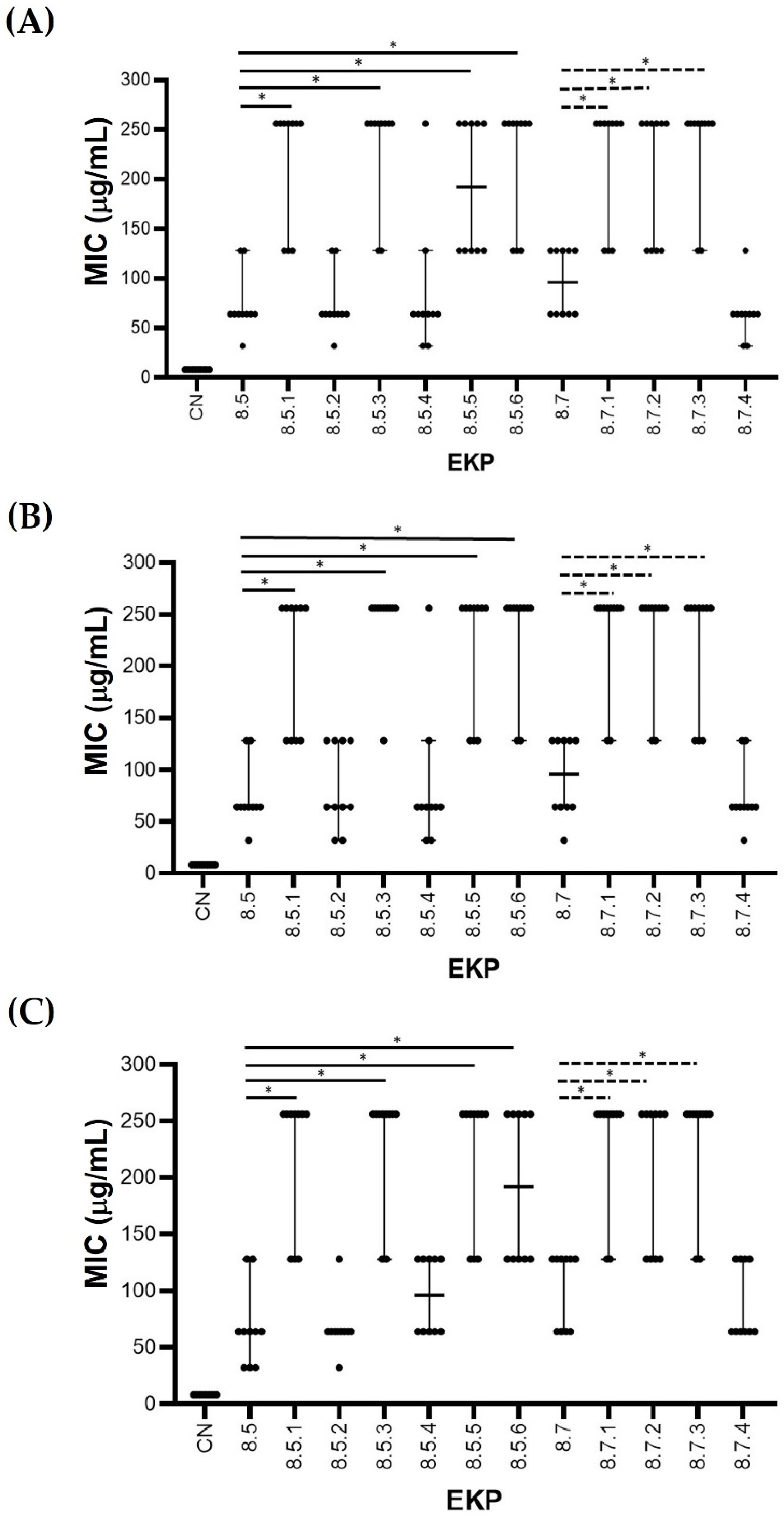
2.2. MIC of the EKPs after Fractionated Extraction and Purification
2.3. Structural Analysis of the Selected Antibacterial EKP Extracts
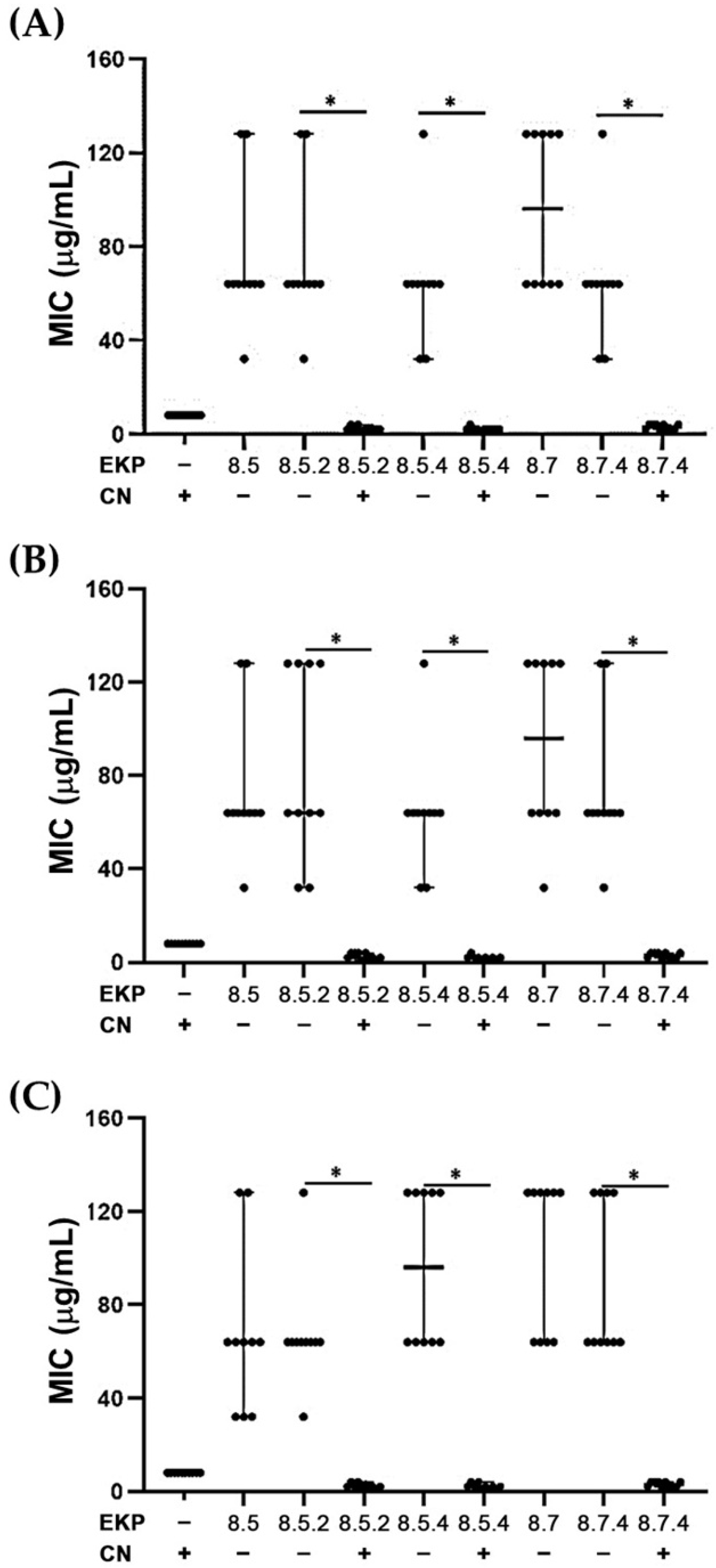
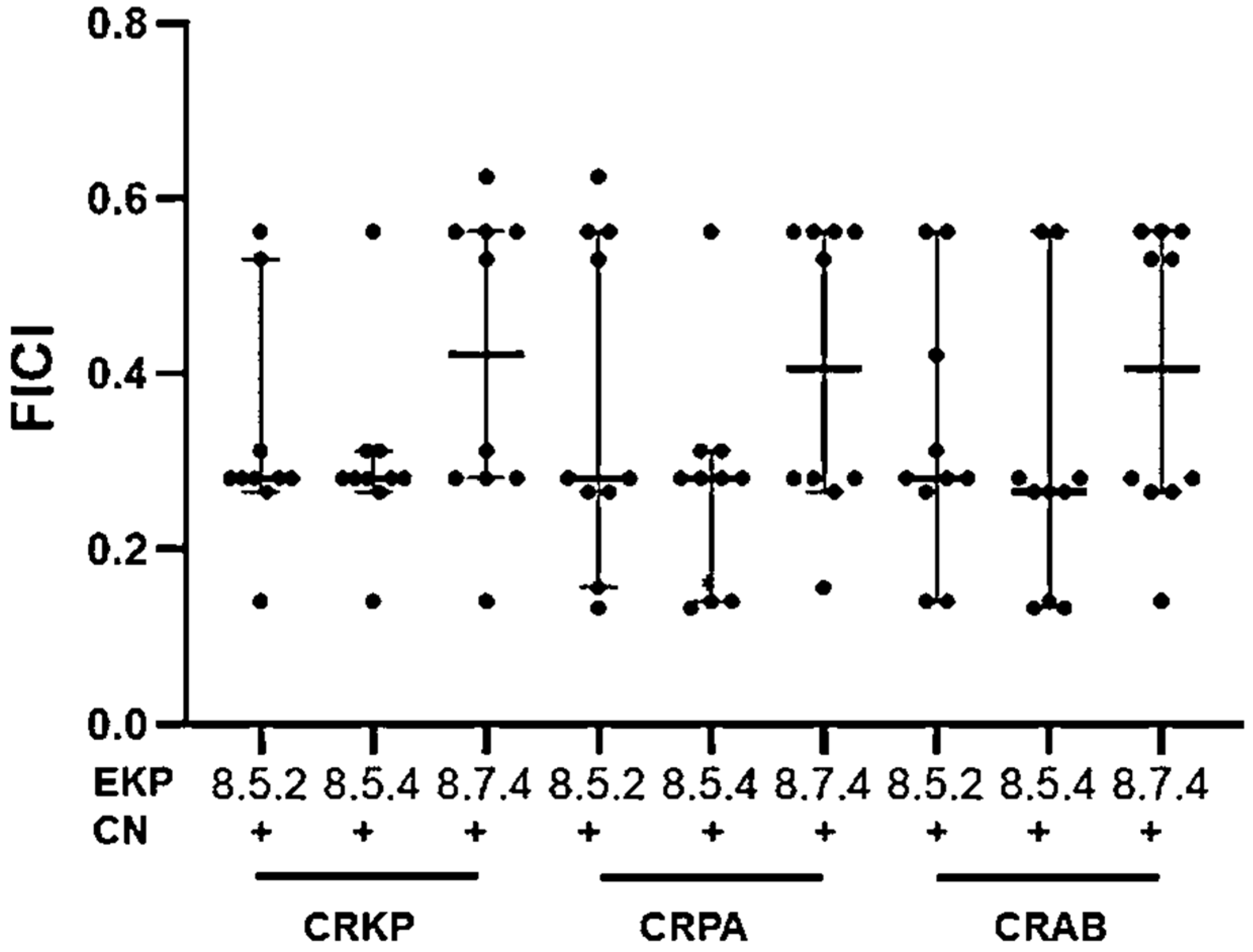
2.4. Synergistic Effects against CRKP, CRPA, and CRAB of Three Selected EKP Extracts and Gentamicin
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Ethanolic Extraction of Kaempferia Rhizomes
4.3. Fractionated Purification of Ethanolic KP Extracts (EKP)
4.4. High-Performance Liquid Chromatograph (HPLC) Analysis of EKP Purified Extracts
4.5. Microorganisms
4.6. Determination of the Minimal Inhibitory Concentration (MIC)
4.7. Determination of Synergistic Effects between each EKP Extract and Gentamicin
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez, J.L. General principles of antibiotic resistance in bacteria. Drug Discov. Today Technol. 2014, 11, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Fazly Bazzaz, B.S.; Amani, A.; Rostami, J.; Vahdati-Mashhadian, N. Combination of anti-tuberculosis drugs with vitamin C or NAC against different Staphylococcus aureus and Mycobacterium tuberculosis strains. Microb. Pathog. 2016, 93, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.A.; Robinson, S.M.; Roy, C.M.; Dennis, M.; Liu, V.; Dorit, R.L. Resistance is futile: The bacteriocin model for addressing the antibiotic resistance challenge. Biochem. Soc. Trans. 2012, 40, 1438–1442. [Google Scholar] [CrossRef] [PubMed]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [PubMed]
- Corvec, S.; Furustrand Tafin, U.; Betrisey, B.; Borens, O.; Trampuz, A. Activities of fosfomycin, tigecycline, colistin, and gentamicin against Extended-Spectrum-β-Lactamase-producing Escherichia coli in a foreign-body infection model. Antimicrob. Agents Chemother. 2013, 57, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, M.; Crandon, J.L.; Nicolau, D.P. The efficacy and safety of antibiotic combination therapy for infections caused by Gram-positive and Gram-negative organisms. Expert Opin. Drug Saf. 2012, 11, 221–233. [Google Scholar] [CrossRef]
- Kummee, S.; Tewtrakul, S.; Subhadhirasakul, S. Antimicrobial activity of the ethanol extract and compounds from the rhizomes of Kaempferia parviflora. Songklanakarin J. Sci. Technol. 2008, 30, 463–466. [Google Scholar]
- Yenjai, C.; Prasanphen, K.; Daodee, S.; Wongpanich, V.; Kittakoop, P. Bioactive flavonoids from Kaempferia parviflora. Fitoterapia 2004, 75, 89–92. [Google Scholar] [CrossRef]
- Rujjanawate, C.; Kanjanapothi, D.; Amornlerdpison, D.; Pojanagaroon, S. Anti-gastric ulcer effect of Kaempferia parviflora. J. Ethnopharmacol. 2005, 102, 120–122. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Subhadhirasakul, S.; Kummee, S. Anti-allergic activity of compounds from Kaempferia parviflora. J. Ethnopharmacol. 2008, 116, 191–193. [Google Scholar] [CrossRef]
- Potikanond, S.; Sookkhee, S.; Na Takuathung, M.; Mungkornasawakul, P.; Wikan, N.; Smith, D.R.; Nimlamool, W. Kaempferia parviflora Extract Exhibits Anti-cancer Activity against HeLa Cervical Cancer Cells. Front. Pharmacol. 2017, 8, 630. [Google Scholar] [CrossRef] [PubMed]
- Paramee, S.; Sookkhee, S.; Sakonwasun, C.; Na Takuathung, M.; Mungkornasawakul, P.; Nimlamool, W.; Potikanond, S. Anti-cancer effects of Kaempferia parviflora on ovarian cancer SKOV3 cells. BMC Complement. Altern. Med. 2018, 18, 178. [Google Scholar] [CrossRef] [PubMed]
- Suradej, B.; Sookkhee, S.; Panyakaew, J.; Mungkornasawakul, P.; Wikan, N.; Smith, D.R.; Potikanond, S.; Nimlamool, W. Kaempferia parviflora extract inhibits STAT3 activation and interleukin-6 production in HeLa cervical cancer cells. Int. J. Mol. Sci. 2019, 20, 4226. [Google Scholar] [CrossRef] [PubMed]
- Tewtrakul, S.; Subhadhirasakul, S.; Karalai, C.; Ponglimanont, C.; Cheenpracha, S. Anti-inflammatory effects of compounds from Kaempferia parviflora and Boesenbergia pandurata. Food Chem. 2009, 115, 534–538. [Google Scholar] [CrossRef]
- Eng-Chong, T.; Yean-Kee, L.; Chin-Fei, C.; Choon-Han, H.; Sher-Ming, W.; Li-Ping, C.T.; Gen-Teck, F.; Khalid, N.; Abd Rahman, N.; Karsani, S.A.; et al. Boesenbergia rotunda: From ethnomedicine to drug discovery. Evid.-Based Complement. Altern. Med. 2012, 2012, 473637. [Google Scholar] [CrossRef]
- Hossain, M.A.; Wongsrikaew, N.; Yoo, G.-W.; Han, J.; Shin, C.-G. Cytotoxic effects of polymethoxyflavones isolated from Kaempferia parviflora. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 471–476. [Google Scholar] [CrossRef]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Chen, D.; Li, H.; Li, W.; Feng, S.; Deng, D. Kaempferia parviflora and Its Methoxyflavones: Chemistry and Biological Activities. Evid.-Based Complement. Altern. Med. 2018, 2018, 4057456. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Herranz-López, M.; Funes, L.; Borrás-Linares, I.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenylpropanoids and their metabolites are the major compounds responsible for blood-cell protection against oxidative stress after administration of Lippia citriodora in rats. Phytomedicine 2013, 20, 1112–1118. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Ono, S.; Yoshida, N.; Maekawa, D.; Kitakaze, T.; Kobayashi, Y.; Kitano, T.; Fujita, T.; Okuwa-Hayashi, H.; Harada, N.; Nakano, Y.; et al. 5-Hydroxy-7-methoxyflavone derivatives from Kaempferia parviflora induce skeletal muscle hypertrophy. Food Sci. Nutr. 2019, 7, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Somintara, S.; Sripanidkulchai, B.; Pariwatthanakun, C.; Sripanidkulchai, K. Hypolipidemic effect of methoxyflavone-enriched extract of Kaempferia parviflora in cholesterol-induced dyslipidemic rats. Songklanakarin J. Sci. Technol. 2019, 41, 305–310. [Google Scholar] [CrossRef]
- Khameneh, B.; Diab, R.; Ghazvini, K.; Fazly Bazzaz, B.S. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog. 2016, 95, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M.I.; Rahman, M.M.; Gibbons, S.; Piddock, L.J. Medicinal plant extracts with efflux inhibitory activity against Gram-negative bacteria. Int. J. Antimicrob. Agents 2011, 37, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Funes, L.; Laporta, O.; Cerdán-Calero, M.; Micol, V. Effects of verbascoside, a phenylpropanoid glycoside from Lemon verbena, on phospholipid model membranes. Chem. Phys. Lipids 2010, 163, 190–199. [Google Scholar] [CrossRef]
- Stavri, M.; Piddock, L.J.; Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 2007, 59, 1247–1260. [Google Scholar] [CrossRef]
- Coutinho, H.D.; Costa, J.G.; Lima, E.O.; Falcão-Silva, V.S.; Siqueira, J.P., Jr. Herbal therapy associated with antibiotic therapy: Potentiation of the antibiotic activity against methicillin–resistant Staphylococcus aureus by Turnera ulmifolia L. BMC Complement. Altern. Med. 2009, 9, 13. [Google Scholar] [CrossRef]
- Panyakaew, J.; Chalom, S.; Sookkhee, S.; Saiai, A.; Chandet, N.; Meepowpan, P.; Thavornyutikarn, P.; Mungkornasawakul, P. Kaempferia sp. extracts as UV protecting and antioxidant agents in sunscreen. J. Herbs Spices Med. Plants 2021, 27, 37–56. [Google Scholar] [CrossRef]
- Institute, C.a.L.S. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Sookkhee, S.; Manonuek, S.; Apichartpiyakul, C.; Sakonwasun, C. Antibacterial, sperm immobilization and spermicidal activities of bacteriocins produced by vaginal lactobacilli. CMU J. Nat. Sci. 2022, 21, e2022015. [Google Scholar] [CrossRef]
- White, R.L.; Burgess, D.S.; Manduru, M.; Bosso, J.A. Comparison of three different in vitro methods of detecting synergy: Time-kill, checkerboard, and E test. Antimicrob. Agents Chemother. 1996, 40, 1914–1918. [Google Scholar] [CrossRef]
- Orhan, G.; Bayram, A.; Zer, Y.; Balci, I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J. Clin. Microbiol. 2005, 43, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Au-Koeth, L.M.; Au-DiFranco-Fisher, J.M.; Au-McCurdy, S. A Reference Broth Microdilution Method for Dalbavancin In Vitro Susceptibility Testing of Bacteria that Grow Aerobically. JoVE 2015, 103, e53028. [Google Scholar] [CrossRef]
- Santos, R.C.; Kushima, H.; Rodrigues, C.M.; Sannomiya, M.; Rocha, L.R.; Bauab, T.M.; Tamashiro, J.; Vilegas, W.; Hiruma-Lima, C.A. Byrsonima intermedia A. Juss.: Gastric and duodenal anti-ulcer, antimicrobial and antidiarrheal effects in experimental rodent models. J. Ethnopharmacol. 2012, 140, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Du, X.; Chen, Y.; Fu, Y.; Wang, H.; Yu, Y. In vitro activity of sulbactam in combination with imipenem, meropenem, panipenem or cefoperazone against clinical isolates of Acinetobacter baumannii. Int. J. Antimicrob. Agents 2013, 41, 400–401. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
| Position | EKP8.5.2 | EKP8.5.4 | EKP8.7.4 |
|---|---|---|---|
| δH (mult., J (Hz)) | δH (mult., J (Hz)) | δH (mult., J (Hz)) | |
| - | - | - | |
| 3 | - | - | 6.62 (s) |
| 4 | - | - | - |
| 5 | - | - | |
| 6 | 6.34 (d, 2.3) | 6.34 (d, 2.3) | 6.39 (d, 2.3) |
| 7 | - | - | - |
| 8 | 6.51 (d, 2.3) | 6.50 (d, 2.3) | 6.58 (d, 2.3) |
| 9 | - | - | - |
| 10 | - | - | - |
| 1′ | - | - | - |
| 2′ | 8.06 (m) | 7.70 (d, 7.5) | 7.84 (m) |
| 3′ | 7.47 (m) | - | 6.90 (d, 8.8) |
| 4′ | 7.47 (m) | - | - |
| 5′ | 7.47 (m) | 6.98 (d, 8.8) | 6.90 (d, 8.8) |
| 6′ | 8.06 (m) | 7.70 (d, 7.3) | 7.84 (m) |
| 3-OCH3 | 3.96 (s) | 3.96 (s) | - |
| 5-OCH3 | 3.89 (s) | 3.96 (s) | 3.97 (s) |
| 7-OCH3 | 3.88 (s) | 3.96 (s) | 3.93 (s) |
| 3′-OCH3 | - | 3.90 (s) | - |
| 4′-OCH3 | - | 3.87 (s) | 3.90 (s) |
| CRKP | MIC (μg/mL) | FIC of | FICI of | Interpretation | |||
|---|---|---|---|---|---|---|---|
| CN | EKP 8.5.2 |
CN+
EKP 8.5.2 | CN | EKP 8.5.2 |
CN+
EKP 8.5.2 | ||
| CMU001 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU002 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU003 | 8 | 64 | 1 | 0.125 | 0.016 | 0.141 | Synergism |
| CMU004 | 8 | 32 | 2 | 0.25 | 0.063 | 0.313 | Synergism |
| CMU005 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU006 | 8 | 128 | 4 | 0.5 | 0.031 | 0.531 | Partial synergy |
| CMU007 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU008 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU009 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Partial synergy |
| CMU010 | 8 | 128 | 2 | 0.25 | 0.016 | 0.266 | Synergism |
| CRKP | MIC (μg/mL) | FIC of | FICI of | Interpretation | |||
|---|---|---|---|---|---|---|---|
| CN | EKP 8.5.4 |
CN+
EKP 8.5.4 | CN | EKP 8.5.4 |
CN+
EKP 8.5.4 | ||
| CMU001 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU002 | 8 | 32 | 2 | 0.25 | 0.063 | 0.313 | Synergism |
| CMU003 | 8 | 64 | 1 | 0.125 | 0.016 | 0.141 | Synergism |
| CMU004 | 8 | 32 | 2 | 0.25 | 0.063 | 0.313 | Synergism |
| CMU005 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU006 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Partial synergy |
| CMU007 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU008 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU009 | 8 | 128 | 2 | 0.25 | 0.016 | 0.266 | Synergism |
| CMU010 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CRKP | MIC (μg/mL) | FIC of | FICI of | Interpretation | |||
|---|---|---|---|---|---|---|---|
| CN | EKP 8.7.4 |
CN+
EKP 8.7.4 | CN | EKP 8.7.4 |
CN+
EKP 8.7.4 | ||
| CMU001 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Partial synergy |
| CMU002 | 8 | 32 | 4 | 0.5 | 0.125 | 0.625 | Partial synergy |
| CMU003 | 8 | 64 | 1 | 0.125 | 0.016 | 0.141 | Synergism |
| CMU004 | 8 | 32 | 2 | 0.25 | 0.063 | 0.313 | Synergism |
| CMU005 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Partial synergy |
| CMU006 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Partial synergy |
| CMU007 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU008 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU009 | 8 | 128 | 4 | 0.5 | 0.031 | 0.531 | Partial synergy |
| CMU010 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CRPA | MIC (μg/mL) | FIC of | FICI of | Interpretation | |||
|---|---|---|---|---|---|---|---|
| CN | EKP 8.5.2 |
CN+
EKP 8.5.2 | CN | EKP 8.5.2 |
CN+
EKP 8.5.2 | ||
| CMU001 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU002 | 8 | 32 | 1 | 0.125 | 0.031 | 0.156 | Synergism |
| CMU003 | 8 | 32 | 4 | 0.5 | 0.125 | 0.625 | Partial synergy |
| CMU004 | 8 | 128 | 2 | 0.25 | 0.016 | 0.266 | Synergism |
| CMU005 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Partial synergy |
| CMU006 | 8 | 128 | 1 | 0.125 | 0.008 | 0.133 | Synergism |
| CMU007 | 8 | 128 | 2 | 0.25 | 0.016 | 0.266 | Synergism |
| CMU008 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Partial synergy |
| CMU009 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU010 | 8 | 128 | 4 | 0.5 | 0.031 | 0.531 | Partial synergy |
| CRPA | MIC (μg/mL) | FIC of | FICI of | Interpretation | |||
|---|---|---|---|---|---|---|---|
| CN | EKP 8.5.4 |
CN+
EKP 8.5.4 | CN | EKP 8.5.4 |
CN+
EKP 8.5.4 | ||
| CMU001 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU002 | 8 | 64 | 1 | 0.125 | 0.016 | 0.141 | Synergism |
| CMU003 | 8 | 32 | 2 | 0.25 | 0.063 | 0.313 | Synergism |
| CMU004 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU005 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU006 | 8 | 64 | 1 | 0.125 | 0.016 | 0.141 | Synergism |
| CMU007 | 8 | 128 | 1 | 0.125 | 0.008 | 0.133 | Synergism |
| CMU008 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Partial synergy |
| CMU009 | 8 | 32 | 2 | 0.25 | 0.063 | 0.313 | Synergism |
| CMU010 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CRPA | MIC (μg/mL) | FIC of | FICI of | Interpretation | |||
|---|---|---|---|---|---|---|---|
| CN | EKP 8.7.4 |
CN+
EKP 8.7.4 | CN | EKP 8.7.4 |
CN+
EKP 8.7.4 | ||
| CMU001 | 8 | 128 | 4 | 0.5 | 0.031 | 0.531 | Partial synergy |
| CMU002 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU003 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Partial synergy |
| CMU004 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU005 | 8 | 128 | 2 | 0.25 | 0.016 | 0.266 | Synergism |
| CMU006 | 8 | 32 | 1 | 0.125 | 0.031 | 0.156 | Synergism |
| CMU007 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Synergism |
| CMU008 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Partial synergy |
| CMU009 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Partial synergy |
| CMU010 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CRAB | MIC (μg/mL) | FIC of | FICI of | Interpretation | |||
|---|---|---|---|---|---|---|---|
| CN | EKP 8.5.2 |
CN+
EKP 8.5.2 | CN | EKP 8.5.2 |
CN+
EKP 8.5.2 | ||
| CMU001 | 8 | 128 | 2 | 0.25 | 0.016 | 0.266 | Synergism |
| CMU002 | 8 | 64 | 3 | 0.375 | 0.047 | 0.422 | Synergism |
| CMU003 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU004 | 8 | 64 | 1 | 0.125 | 0.016 | 0.141 | Synergism |
| CMU005 | 8 | 64 | 1 | 0.125 | 0.016 | 0.141 | Synergism |
| CMU006 | 8 | 32 | 2 | 0.25 | 0.063 | 0.313 | Synergism |
| CMU007 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU008 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Partial synergy |
| CMU009 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Partial synergy |
| CMU010 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CRAB | MIC (μg/mL) | FIC of | FICI of | Interpretation | |||
|---|---|---|---|---|---|---|---|
| CN | EKP 8.5.4 |
CN+
EKP 8.5.4 | CN | EKP 8.5.4 |
CN+
EKP 8.5.4 | ||
| CMU001 | 8 | 128 | 2 | 0.25 | 0.016 | 0.266 | Synergism |
| CMU002 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Partial synergy |
| CMU003 | 8 | 64 | 1 | 0.125 | 0.016 | 0.141 | Synergism |
| CMU004 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU005 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Partial synergy |
| CMU006 | 8 | 128 | 1 | 0.125 | 0.008 | 0.133 | Synergism |
| CMU007 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU008 | 8 | 128 | 1 | 0.125 | 0.008 | 0.133 | Synergism |
| CMU009 | 8 | 128 | 2 | 0.25 | 0.016 | 0.266 | Synergism |
| CMU010 | 8 | 128 | 2 | 0.25 | 0.016 | 0.266 | Synergism |
| CRAB | MIC (μg/mL) | FIC of | FICI of | Interpretation | |||
|---|---|---|---|---|---|---|---|
| CN | EKP 8.7.4 |
CN+
EKP 8.7.4 | CN | EKP 8.7.4 |
CN+
EKP 8.7.4 | ||
| CMU001 | 8 | 128 | 4 | 0.5 | 0.031 | 0.531 | Partial synergy |
| CMU002 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Partial synergy |
| CMU003 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU004 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Partial synergy |
| CMU005 | 8 | 64 | 2 | 0.25 | 0.031 | 0.281 | Synergism |
| CMU006 | 8 | 128 | 2 | 0.25 | 0.016 | 0.266 | Synergism |
| CMU007 | 8 | 64 | 1 | 0.125 | 0.016 | 0.141 | Synergism |
| CMU008 | 8 | 128 | 2 | 0.25 | 0.016 | 0.266 | Synergism |
| CMU009 | 8 | 64 | 4 | 0.5 | 0.063 | 0.563 | Partial synergy |
| CMU010 | 8 | 128 | 4 | 0.5 | 0.031 | 0.531 | Partial synergy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sookkhee, S.; Sakonwasun, C.; Mungkornasawakul, P.; Khamnoi, P.; Wikan, N.; Nimlamool, W. Synergistic Effects of Some Methoxyflavones Extracted from Rhizome of Kaempferia parviflora Combined with Gentamicin against Carbapenem-Resistant Strains of Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii. Plants 2022, 11, 3128. https://doi.org/10.3390/plants11223128
Sookkhee S, Sakonwasun C, Mungkornasawakul P, Khamnoi P, Wikan N, Nimlamool W. Synergistic Effects of Some Methoxyflavones Extracted from Rhizome of Kaempferia parviflora Combined with Gentamicin against Carbapenem-Resistant Strains of Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii. Plants. 2022; 11(22):3128. https://doi.org/10.3390/plants11223128
Chicago/Turabian StyleSookkhee, Siriwoot, Choompone Sakonwasun, Pitchaya Mungkornasawakul, Phadungkiat Khamnoi, Nitwara Wikan, and Wutigri Nimlamool. 2022. "Synergistic Effects of Some Methoxyflavones Extracted from Rhizome of Kaempferia parviflora Combined with Gentamicin against Carbapenem-Resistant Strains of Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii" Plants 11, no. 22: 3128. https://doi.org/10.3390/plants11223128
APA StyleSookkhee, S., Sakonwasun, C., Mungkornasawakul, P., Khamnoi, P., Wikan, N., & Nimlamool, W. (2022). Synergistic Effects of Some Methoxyflavones Extracted from Rhizome of Kaempferia parviflora Combined with Gentamicin against Carbapenem-Resistant Strains of Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii. Plants, 11(22), 3128. https://doi.org/10.3390/plants11223128







