Novel Combination of the Biophysical, Nutritional, and Nutraceutical Properties in Subtropical Pigmented Maize Hybrids
Abstract
:1. Introduction
2. Results
2.1. Biophysical Properties
2.2. Nutritional Properties
2.3. Nutraceutical Properties
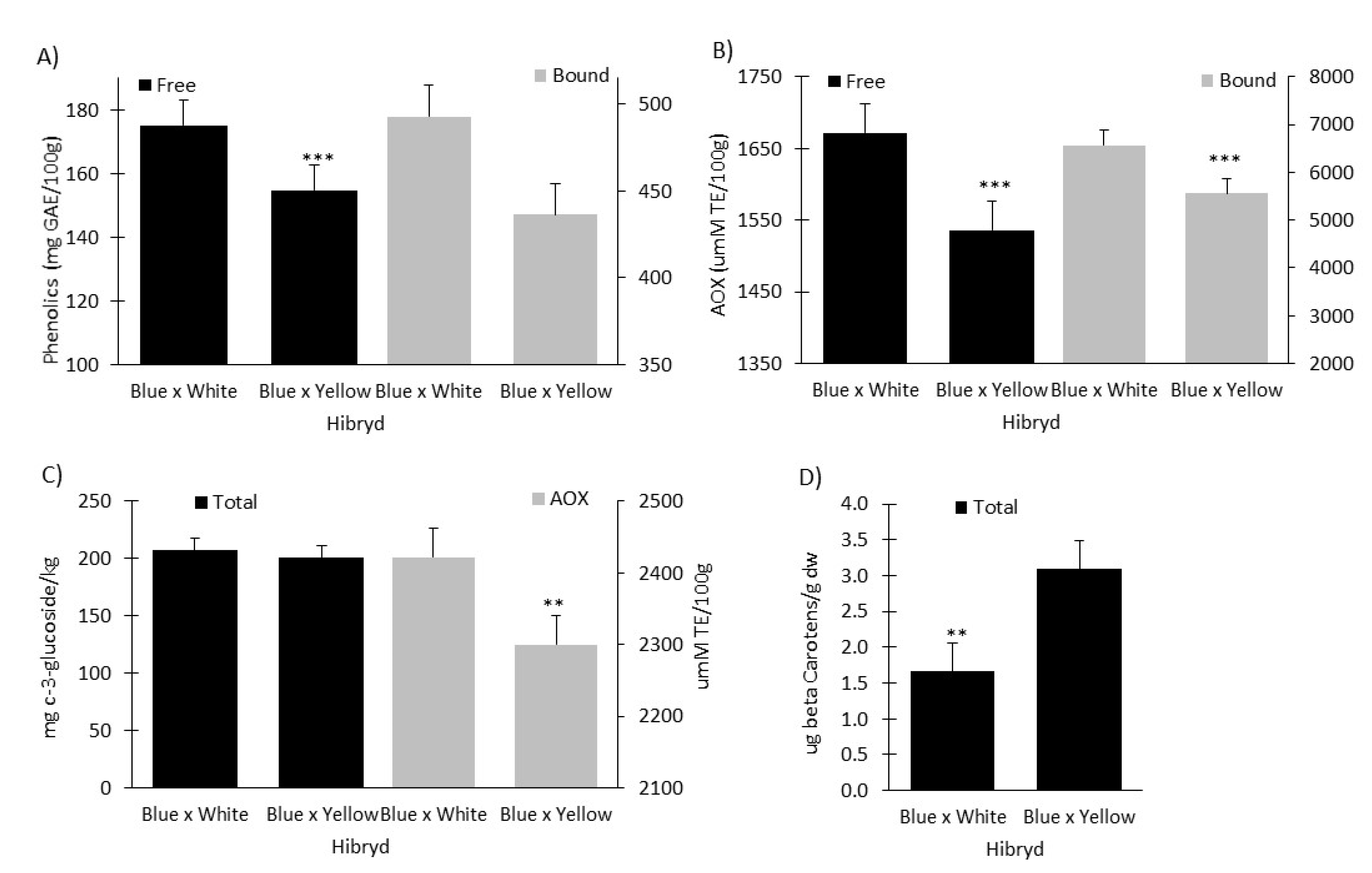
2.4. Free and Bound Phenolic Acids and Antioxidant Capacity
2.5. Anthocyanin Content and Antioxidant Capacity
2.6. Total Carotenoid Content of Vitamaiz Hybrids
2.7. Comparison between Crosses
2.8. Association Analysis
3. Discussion
3.1. Physical Properties of Novel Hybrids
3.2. Nutraceuticals in Pigmented Maize
3.3. Effects of Genetic Background and Origin
3.4. Physical, Nutrimental, and Nutraceutical Associations
4. Materials and Methods
4.1. Maize Germplasm and Hybrid Production
4.2. Measurement of Biophysical Properties
4.3. Nutrimental Analysis
4.4. Quantification of Total Soluble and Bound Phenolic Acids
4.5. Total Anthocyanin Quantification
4.6. Antioxidant Capacity Determination
4.7. Total Carotenoid Content Determination
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Fernie, A.R.; Yan, J. The past, present, and future of maize improvement: Domestication, genomics, and functional genomic routes toward crop enhancement. Plant Commun. 2020, 1, 100010. [Google Scholar] [CrossRef] [PubMed]
- Ureta, C.; González, E.J.; Espinosa, A.; Trueba, A.; Piñeyro-Nelson, A.; Álvarez-Buylla, E.R. Maize yield in Mexico under climate change. Agric. Syst. 2020, 177, 102697. [Google Scholar] [CrossRef]
- Escalante-Aburto, A.; Mariscal-Moreno, R.M.; Santiago-Ramos, D.; Ponce-García, N. An update of different nixtamalization technologies, and its effects on chemical composition and nutritional value of corn tortillas. Food Rev. Int. 2020, 36, 456–498. [Google Scholar] [CrossRef]
- Colombo, R.; Ferron, L.; Papetti, A. Colored Corn: An up-date on metabolites extraction, health implication, and potential use. Molecules 2021, 26, 199. [Google Scholar] [CrossRef]
- Escalante-Aburto, A.; Torres-Chávez, P.I.; Ramírez-Wong, B.; Ponce-García, N. Pigmented maizes: Anthocyanin profile and content. In Handbook of Anthocyanins: Food Sources, Chemical Applications and Health Benefits; Universidad de Monterrey: Monterrey, Mexico, 2014; Volume 7, ISBN 9781633217959. [Google Scholar]
- Hong, H.T.; Netzel, M.E.; O’Hare, T.J. Optimisation of extraction procedure and development of lc–dad–ms methodology for anthocyanin analysis in anthocyanin-pigmented corn kernels. Food Chem. 2020, 319, 126515. [Google Scholar] [CrossRef]
- Eng, K.H.; Azrina, A.; Teng, T.S.; Meng, L.S. Anthocyanidins and Anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Serna-Saldivar, S.O. Cereal Grains: Properties, Processing and Nutritional Attributes; CRC Press: Boca Ratón, FL, USA, 2010. [Google Scholar]
- Zurak, D.; Grbeša, D.; Duvnjak, M.; Kiš, G.; Dimurec, T.M.; Kljak, K. Carotenoid content and bioaccessibility in commercial maize hybrids. Agriculture 2021, 11, 586. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Torres, A.; Fernández-Aulis, F.; Peña, C. Bioactive compounds in pigmented maize. In Corn; Amanullah, F.S., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Urias-Lugo, D.A.; Heredia, J.B.; Serna-Saldivar, S.O.; Muy-Rangel, M.D.; Valdez-Torres, J.B. Total phenolics, total anthocyanins and antioxidant capacity of native and elite blue maize hybrids (Zea Mays L.). CyTA J. Food 2015, 13, 336–339. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Chilungo, S.; Memba, A.; Mponela, P. Biofortification of maize and sweetpotatoes with provitamin a carotenoids and implication on eradicating vitamin A deficiency in developing countries. J. Agric. Food Res. 2020, 2, 100068. [Google Scholar] [CrossRef]
- Rojas, E.B.; Gricelda, M.; Carrillo, V.; Gabriel, N.; Chulím, E.; Hilario, J.; Salgado, H.; Valverde, B.R.; Delgado, G.B. Physicochemical characteristics and quality of the protein of pigmented native maize from morelos in two years of cultivation. Rev. Mex. De Cienc. Agric. 2019, 10, 683–697. [Google Scholar]
- Xie, C.; Weng, J.; Liu, W.; Zou, C.; Hao, Z.; Li, W.; Li, M.; Guo, X.; Zhang, G.; Xu, Y.; et al. Zea Mays (L.) P1 locus for cob glume color identified as a post-domestication selection target with an effect on temperate maize genomes. Crop J. 2013, 1, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Casali, L.; Herrera, J.M.; Rubio, G. Resilient soybean and maize production under a varying climate in the semi-arid and sub-humid chaco. Eur. J. Agron. 2022, 135, 126463. [Google Scholar] [CrossRef]
- Msungu, S.D.; Mushongi, A.A.; Venkataramana, P.B.; Mbega, E.R. Status of carotenoids in elite and landrace maize genotypes: Implications for provitamin A biofortification in Tanzania. Food Res. Int. 2022, 156, 111303. [Google Scholar] [CrossRef]
- Urias-Peraldí, M.; Gutiérrez-Uribe, J.A.; Preciado-Ortiz, R.E.; Cruz-Morales, A.S.; Serna-Saldívar, S.O.; García-Lara, S. Nutraceutical profiles of improved blue maize (Zea Mays) hybrids for subtropical regions. Field Crops Res. 2013, 141, 69–76. [Google Scholar] [CrossRef]
- Preciado-Ortiz, R.E.; Ochoa-Centeno, N.J.; Vázquez-Carrillo, M.G.; Santiago-Ramos, D.; Terrón-Ibarra, A.D. Grain yield, physical and pasting properties, and anthocyanins of non-conventional pigmented corn hybrids for pozole end-use adapted to subtropical regions. Appl. Food Res. 2022, 2, 100180. [Google Scholar] [CrossRef]
- Serna-Saldivar, S.O. Understanding the functionality and manufacturing of nixtamalized maize products. J. Cereal Sci. 2021, 99, 103205. [Google Scholar] [CrossRef]
- Uriarte-Aceves, P.M.; Cuevas-Rodríguez, E.O.; Gutiérrez-Dorado, R.; Mora-Rochín, S.; Reyes-Moreno, C.; Puangpraphant, S.; Milan-Carrillo, J. Physical, compositional, and wet-milling characteristics of Mexican blue maize (Zea Mays L.) landrace. Cereal Chem. 2015, 92, 491–496. [Google Scholar] [CrossRef]
- Secretaria de Comercio y Fomento Industrial NMX-FF-034/1-SCFI-2002. Available online: https://sitios1.dif.gob.mx/alimentacion/docs/NMX-FF-034-1-SCFI-2002_MAIZ_blanco.pdf (accessed on 9 October 2022).
- Vázquez-Carrillo, G.; García-Lara, S.; Salinas-Moreno, Y.; Bergvinson, D.J.; Palacios-Rojas, N. Grain and tortilla quality in landraces and improved maize grown in the highlands of Mexico. Plant Foods Hum. Nutr. 2011, 66, 203–208. [Google Scholar] [CrossRef]
- Mora-Rochin, S.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S.O.; Sánchez-Peña, P.; Reyes-Moreno, C.; Milán-Carrillo, J. Phenolic content and antioxidant activity of tortillas produced from pigmented maize processed by conventional nixtamalization or extrusion cooking. J. Cereal Sci. 2010, 52, 502–508. [Google Scholar] [CrossRef]
- Urias-Peraldí, M. Phytochemical and Nutraceutical Profiles of Blue Maize (Zea mays) Hybrids Evaluated in Two Locations. Master Thesis, Tecnologico de Monterrey, Monterrey, Mexico, 2010. [Google Scholar]
- Lopez-Martinez, L.X.; Oliart-Ros, R.M.; Valerio-Alfaro, G.; Lee, C.H.; Parkin, K.L.; Garcia, H.S. Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexicanmaize. LWT Food Sci. Technol. 2009, 42, 1187–1192. [Google Scholar] [CrossRef]
- Del Pozo-Insfran, D.; Brenes, C.H.; Serna Saldivar, S.O.; Talcott, S.T. Polyphenolic and antioxidant content of white and blue corn (Zea Mays L.) Products. Food Res. Int. 2006, 39, 696–703. [Google Scholar] [CrossRef]
- Harrigan, G.G.; Stork, L.A.G.; Riordan, S.G.; Reynolds, T.L.; Ridley, W.P.; Masucci, J.D.; MacIsaac, S.; Halls, S.C.; Orth, R.; Smith, R.G.; et al. Impact of genetics and environment on nutritional and metabolite components of maize grain. J. Agric. Food Chem. 2007, 55, 6177–6185. [Google Scholar] [CrossRef]
- Suriano, S.; Balconi, C.; Valoti, P.; Redaelli, R. Comparison of total polyphenols, profile anthocyanins, color analysis, carotenoids and tocols in pigmented maize. LWT 2021, 144, 111257. [Google Scholar] [CrossRef]
- Kuhnen, S.; Menel Lemos, P.M.; Campestrini, L.H.; Ogliari, J.B.; Dias, P.F.; Maraschin, M. Carotenoid and anthocyanin contents of grains of Brazilianmaize landraces. J. Sci. Food Agric. 2011, 91, 1548–1553. [Google Scholar] [CrossRef] [PubMed]
- De La Parra, C.; Serna Saldivar, S.O.; Liu, R.H. Effect of Processing on the phytochemical profiles and antioxidant activity of corn for production of masa, tortillas, and tortilla chips. J. Agric. Food Chem. 2007, 55, 4177–4183. [Google Scholar] [CrossRef] [PubMed]
- Kurilich, A.C.; Juvik, J.A. Quantification of carotenoid and tocopherol antioxidants in Zea Mays. J. Agric. Food Chem. 1999, 47, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Akintunde, A.; Olaoye, G.; Olakojo, S.A. Assessment of diversity among tropical and subtropical maize inbreds based on morphological traits and carotenoid content. J. Exp. Agric. Int. 2019, 30, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mahan, A.L.; Murray, S.C.; Rooney, L.W.; Crosby, K.M. Combining ability for total phenols and secondary traits in a diverse set of colored (red, blue, and purple) maize. Crop Sci. 2013, 53, 1248–1255. [Google Scholar] [CrossRef]
- Duncan, B.; Leyva-Guerrero, E.; Werk, T.; Stojšin, D.; Baltazar, B.M.; García-Lara, S.; Zavala-López, M.; De la Fuente-Martínez, J.M.; Meng, C. Assessment of potential impacts associated with gene flow from transgenic hybrids to mexican maize landraces. Transgenic Res. 2019, 28, 509–523. [Google Scholar] [CrossRef] [Green Version]
- García-Lara, S.; Bergvinson, D.J. Phytochemical and nutraceutical changes during recurrent selection for storage pest resistance in tropical maize. Crop Sci. 2014, 54, 2423–2432. [Google Scholar] [CrossRef]
- Marques, G.; Rencoret, J.; Gutiérrez, A.; Del Río, J.C. lipophilic compounds from maize fiber and rice husk residues–an abundant and inexpensive source of valuable phytochemicals. Ind. Crops Prod. 2020, 146, 112203. [Google Scholar] [CrossRef]
- Montero-Vargas, J.M.; Ortíz-Islas, S.; Ramírez-Sánchez, O.; García-Lara, S.; Winkler, R. Prediction of the antioxidant capacity of maize (Zea Mays) hybrids using mass fingerprinting and data mining. Food Biosci. 2020, 37, 100647. [Google Scholar] [CrossRef]
- Salinas-Moreno, Y.; Pérez-Alonso, J.J.; Vázquez-Carrillo, G.; Aragón-Cuevas, F.; Velázquez-Cardelas, G.A. Antocianinas y actividad antioxidante en maíces (Zea Mays L.) de las razas chalqueño, elotes cónicos y bolita. Agrociencia 2012, 46, 693–706. [Google Scholar]
- Adalberto, I.B.T.E.; Ruiz, C. Vitamaiz: Caracterización agronómica y bioquímica de líneas de maíz convertidas a azul. Exploraciones Intercambios Rel. Entre Diseño Tecnol. 2016, 29, 57–79. [Google Scholar] [CrossRef]
- Monneveux, P.; Sanchez, C.; Tiessen, A. future progress in drought tolerance in maize needs new secondary traits and cross combinations. J. Agric. Sci. 2008, 146, 287–300. [Google Scholar] [CrossRef] [Green Version]
- Instituto Nacional de Estadística y Geografía INEGI. Available online: https://www.inegi.org.mx/ (accessed on 22 August 2022).
- AACCI Approved Methods of the AACCI, 10th ed.; 55-10 method; American Association of Cereal Chemists: St. Paul, MN, USA, 2009.
- Peralta-Veran, L.; Espinosa-Leal, C.; Escalante-Aburto, A.; Preciado-Ortiz, R.E.; Puente-Garza, C.A.; Serna-Saldivar, S.O.; García-Lara, S. Effects of pozole broth production on phenolic acids and antioxidant activity of specialty maize landraces. J. Cereal Sci. 2022, 107, 103543. [Google Scholar] [CrossRef]
- Zavala-López, M.; García-Lara, S. An improved microscale method for extraction of phenolic acids from maize. Plant Methods 2017, 13, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Abdel-Aal, E.-S.M.; Hucl, P.; Rabalski, I. Compositional and antioxidant properties of anthocyanin-rich products prepared from purple wheat. Food Chem. 2018, 254, 13–19. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Hallauer, A.R.; Carena, M.J.; Filho, J.B.M. Selection: Experimental results. In Quantitative Genetics in Maize Breeding; Springer: New York, NY, USA, 2010; pp. 291–382. ISBN 978-1-4419-0766-0. [Google Scholar]
| ID | Hybrid Cross | Genetic Source BC Parent | Yield Ton ha−1 | Test Weight kg/hL | TKW 1 g | FI % | Color | Kernel Dimensions (mm) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| b | L | Thickness | Width | Length | |||||||
| 1 | Vm451 × Vm311 | CML451 (Y) | 8.3 | 78.5 | 400.1 | 3.0 | 10.9 | 42.6 | 3.5 | 9.1 | 12.6 |
| 2 | Vm496 × Vm311 | CML496 (Y) | 7.9 | 79.1 | 330.9 | 4.8 | 10.4 | 36.1 | 3.4 | 8.7 | 11.5 |
| 3 | Vm496 × Vm451 | CML451 (Y) | 8.1 | 79.8 | 328.3 | 1.7 | 13.5 | 51.7 | 3.0 | 9.0 | 11.1 |
| 4 | Vm492 × Vm451 | CML451 (Y) | 7.5 | 82.0 | 355.2 | 5.7 | 7.7 | 38.8 | 4.2 | 8.7 | 10.5 |
| 5 | MzDtp222 × Vm311 | CML311 (W) | 6.9 | 79.3 | 274.5 | 3.5 | 13.1 | 56.1 | 2.8 | 8.5 | 10.6 |
| 6 | MzDtp222 × Vm321 | CML321 (W) | 5.6 | 78.9 | 269.7 | 8.5 | 10.4 | 49.3 | 3.1 | 8.7 | 10.4 |
| 7 | MzDtp222 × Vm451 | CML451 (Y) | 8.2 | 80.0 | 336.7 | 4.3 | 13.7 | 47.0 | 3.2 | 8.7 | 11.4 |
| 8 | MzDtp411 × Vm311 | CML311 (W) | 6.7 | 80.4 | 333.3 | 2.2 | 10.5 | 47.7 | 3.4 | 8.9 | 11.1 |
| 9 | MzDtp411 × Vm321 | CML321 (W) | 6.0 | 78.3 | 302.8 | 11.3 | 10.7 | 45.4 | 3.1 | 9.2 | 10.7 |
| 10 | MzDtp413 × Vm451 | CML451 (Y) | 8.4 | 80.0 | 342.8 | 4.2 | 11.3 | 41.3 | 3.4 | 9.0 | 11.2 |
| 11 | MzDtp831 × Vm321 | CML321 (W) | 7.3 | 77.3 | 321.8 | 16.5 | 6.5 | 39.4 | 3.6 | 9.5 | 11.1 |
| 12 | MzDtp831 × Vm451 | CML451 (Y) | 8.2 | 78.8 | 347.4 | 18.0 | 12.3 | 47.1 | 3.8 | 9.0 | 11.3 |
| 13 | MzDtpY1212 × Vm451 | CML451 (Y) | 9.2 | 79.6 | 403.7 | 3.8 | 14.4 | 49.8 | 3.8 | 9.5 | 11.9 |
| 14 | MzLPSF86 × Vm311 | CML449 (W) | 7.2 | 78.5 | 340.5 | 10.5 | 9.1 | 41.0 | 3.0 | 9.5 | 11.3 |
| 15 | MzLPSF86 × Vm321 | CML321 (W) | 7.1 | 78.5 | 304.8 | 7.2 | 11.0 | 47.2 | 2.5 | 9.2 | 10.7 |
| 16 | MzLPSF86 × Vm451 | CML451 (Y) | 8.1 | 80.3 | 350.2 | 3.3 | 9.1 | 39.7 | 3.5 | 8.8 | 11.5 |
| 17 | VmLPSF103 × Vm451 | CML451 (Y) | 7.4 | 82.3 | 313.1 | 0.5 | 6.1 | 32.7 | 3.1 | 8.1 | 11.3 |
| 18 | Vm254 × Vm311 | CML311 (W) | 6.6 | 77.7 | 300.6 | 9.0 | 11.6 | 53.0 | 2.8 | 8.9 | 11.2 |
| 19 | Vm254 × Vm321 | CML321 (W) | 6.0 | 78.8 | 310.9 | 6.2 | 6.9 | 39.5 | 2.9 | 9.2 | 11.2 |
| 20 | Vm321 × Vm311 | CML321 (W) | 7.1 | 78.3 | 288.9 | 7.5 | 12.0 | 49.2 | 3.3 | 8.9 | 10.7 |
| 21 | Vm492 × Vm311 | CML311 (W) | 6.0 | 79.2 | 310.7 | 10.8 | 10.7 | 44.6 | 3.3 | 8.6 | 10.6 |
| 22 | Vm492 × Vm321 | CML321 (W) | 7.3 | 77.4 | 282.3 | 21.5 | 9.1 | 42.8 | 2.8 | 8.8 | 10.5 |
| 23 | Vm492 × Vm451 | CML451 (Y) | 7.2 | 80.9 | 372.7 | 5.3 | 8.5 | 37.1 | 4.3 | 8.5 | 11.3 |
| 24 | Vm311 × Vm321 | CML311 (W) | 6.4 | 76.4 | 299.6 | 17.0 | 7.6 | 39.9 | 3.0 | 8.9 | 10.8 |
| 25 | Vm311 × Vm451 | CML451 (Y) | 7.3 | 79.1 | 334.3 | 13.8 | 14.0 | 50.9 | 4.1 | 8.8 | 10.4 |
| 26 | Vm490 × Vm311 | CML311 (W) | 6.4 | 80.4 | 312.0 | 2.3 | 10.0 | 47.7 | 3.4 | 8.9 | 10.8 |
| 27 | Vm490 × Vm321 | CML321 (W) | 6.6 | 79.5 | 300.6 | 7.0 | 7.6 | 38.1 | 3.1 | 9.1 | 10.5 |
| 28 | Vm327 × Vm321 | CML496 (Y) | 7.1 | 76.1 | 309.7 | 34.7 | 12.1 | 43.5 | 3.1 | 9.0 | 10.3 |
| 29 | Vm327 × Vm451 | CML451 (Y) | 9.0 | 79.8 | 363.0 | 8.3 | 12.8 | 38.4 | 3.8 | 8.4 | 11.1 |
| 30 | Vm338 × Vm311 | CML338 (Y) | 7.3 | 75.5 | 318.7 | 16.3 | 15.7 | 50.3 | 3.0 | 9.4 | 11.2 |
| Mean by Cross (C) | Blue × White | 6.6 | 79.4 | 347.1 | 8.5 | 11.5 | 43.1 | 3.54 | 8.9 | 11.2 | |
| Blue × Yellow | 7.9 | 78.5 | 303.5 | 9.4 | 9.8 | 45.4 | 3.07 | 9.0 | 10.8 | ||
| Cross (C) | ns | ** | *** | ns | ** | ns | *** | ns | ns | ||
| Genotype (G) | *** | *** | *** | *** | *** | *** | *** | *** | *** | ||
| Genotype LSD (0.05) | 2.1 | 0.3 | 7.9 | 3.4 | 4.1 | 10.2 | 0.2 | 0.3 | 0.4 | ||
| Environment (E) | ns | ns | ns | ns | ns | ns | ns | ns | ns | ||
| G×E | ns | *** | *** | * | *** | *** | *** | ns | ns | ||
| Heritability | 0.71 | 0.76 | 0.88 | 0.45 | 0.01 | 0.01 | 0.26 | 0.12 | 0.16 | ||
| ID | Hybrid Cross | Genetic Source BC Parent | Protein | Oil | CHOs 1 | Fiber | Ash |
|---|---|---|---|---|---|---|---|
| g/100 g of Dry Weight | |||||||
| 1 | Vm451 × Vm311 | CML451 (Y) | 10.0 | 3.9 | 60.3 | 1.3 | 1.1 |
| 2 | Vm496 × Vm311 | CML496 (Y) | 9.4 | 4.2 | 61.3 | 0.9 | 1.1 |
| 3 | Vm496 × Vm451 | CML451 (Y) | 9.7 | 4.4 | 59.7 | 1.1 | 1.0 |
| 4 | Vm492 × Vm451 | CML451 (Y) | 10.4 | 4.0 | 59.6 | 1.4 | 1.1 |
| 5 | MzDtp222 × Vm311 | CML311 (W) | 10.3 | 4.2 | 61.2 | 0.6 | 1.1 |
| 6 | MzDtp222 × Vm321 | CML321 (W) | 9.6 | 4.3 | 61.1 | 0.9 | 1.0 |
| 7 | MzDtp222 × Vm451 | CML451 (Y) | 9.4 | 4.3 | 60.2 | 1.1 | 1.0 |
| 8 | MzDtp411 × Vm311 | CML311 (W) | 9.8 | 4.5 | 60.3 | 0.8 | 1.1 |
| 9 | MzDtp411 × Vm321 | CML321 (W) | 9.5 | 4.6 | 60.3 | 1.0 | 1.0 |
| 10 | MzDtp413 × Vm451 | CML451 (Y) | 10.1 | 4.2 | 59.1 | 1.4 | 1.0 |
| 11 | MzDtp831 × Vm321 | CML321 (W) | 9.4 | 4.4 | 60.4 | 1.0 | 1.0 |
| 12 | MzDtp831 × Vm451 | CML451 (Y) | 10.0 | 4.3 | 59.7 | 1.3 | 1.1 |
| 13 | MzDtpY1212 × Vm451 | CML451 (Y) | 10.5 | 3.9 | 59.4 | 1.3 | 1.1 |
| 14 | MzLPSF86 × Vm311 | CML449 (W) | 9.4 | 4.3 | 61.6 | 0.8 | 1.1 |
| 15 | MzLPSF86 × Vm321 | CML321 (W) | 9.6 | 4.5 | 60.1 | 1.0 | 1.0 |
| 16 | MzLPSF86 × Vm451 | CML451 (Y) | 10.0 | 4.1 | 60.1 | 1.1 | 1.1 |
| 17 | VmLPSF103 × Vm451 | CML451 (Y) | 9.4 | 4.2 | 60.6 | 1.0 | 1.1 |
| 18 | Vm254 × Vm311 | CML311 (W) | 9.0 | 4.1 | 61.5 | 1.1 | 1.1 |
| 19 | Vm254 × Vm321 | CML321 (W) | 9.3 | 4.4 | 60.8 | 1.0 | 1.1 |
| 20 | Vm321 × Vm311 | CML321 (W) | 9.6 | 4.3 | 61.0 | 1.0 | 1.0 |
| 21 | Vm492 × Vm311 | CML311 (W) | 9.6 | 4.4 | 61.7 | 0.6 | 1.1 |
| 22 | Vm492 × Vm321 | CML321 (W) | 9.8 | 4.5 | 61.0 | 0.7 | 1.1 |
| 23 | Vm492 × Vm451 | CML451 (Y) | 9.9 | 4.1 | 60.6 | 1.0 | 1.1 |
| 24 | Vm311 × Vm321 | CML311 (W) | 9.4 | 4.1 | 62.0 | 0.9 | 1.0 |
| 25 | Vm311 × Vm451 | CML451 (Y) | 10.3 | 4.0 | 60.4 | 1.2 | 1.1 |
| 26 | Vm490 × Vm311 | CML311 (W) | 9.2 | 4.8 | 60.4 | 0.8 | 1.1 |
| 27 | Vm490 × Vm321 | CML321 (W) | 9.1 | 4.8 | 60.4 | 0.8 | 1.1 |
| 28 | Vm327 × Vm321 | CML496 (Y) | 9.9 | 3.5 | 60.4 | 1.6 | 1.0 |
| 29 | Vm327 × Vm451 | CML451 (Y) | 10.7 | 3.7 | 59.5 | 1.4 | 1.1 |
| 30 | Vm338 × Vm311 | CML338 (Y) | 10.0 | 4.6 | 60.1 | 1.0 | 1.0 |
| Cross Mean | Blue × White | 10.0 | 4.1 | 60.1 | 1.2 | 1.1 | |
| Blue × Yellow | 9.5 | 4.4 | 60.9 | 0.9 | 1.1 | ||
| Cross (C) | *** | *** | *** | *** | ns | ||
| Genotype (G) | *** | *** | *** | *** | *** | ||
| Genotype LSD (0.05) | 2.1 | 0.3 | 7.9 | 4.1 | |||
| Environment (E) | ns | ns | ns | ns | ns | ||
| G × E | ** | ns | ns | ** | ns | ||
| Heritability | 0.42 | 0.09 | 0.27 | 0.25 | 0.24 | ||
| Trait | FPA | BPA | Nutraceutical | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AOX-FPA | AOX-BPA | AOX-Ant | Carotenoids | |||||||||
| Biochemical | ||||||||||||
| Oil | −0.311 | ** | ||||||||||
| Protein | 0.403 | *** | ||||||||||
| CHOs | −0.547 | *** | −0.260 | * | −0.526 | *** | −0.260 | * | −0.255 | * | ||
| Fiber | 0.422 | *** | 0.262 | * | 0.448 | *** | 0.390 | ** | 0.355 | ** | ||
| Ash | 0.372 | *** | −0.443 | *** | ||||||||
| Physical | ||||||||||||
| TW | 0.480 | *** | 0.387 | ** | 0.447 | *** | 0.432 | *** | ||||
| TKW | 0.395 | ** | 0.358 | ** | −0.316 | ** | ||||||
| FI | −0.277 | * | −0.291 | * | −0.340 | ** | 0.388 | ** | ||||
| Length | 0.415 | *** | 0.358 | ** | 0.366 | ** | 0.488 | *** | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiessen-Favier, A.; Escalante-Aburto, A.; Espinosa-Leal, C.; García-Lara, S. Novel Combination of the Biophysical, Nutritional, and Nutraceutical Properties in Subtropical Pigmented Maize Hybrids. Plants 2022, 11, 3221. https://doi.org/10.3390/plants11233221
Tiessen-Favier A, Escalante-Aburto A, Espinosa-Leal C, García-Lara S. Novel Combination of the Biophysical, Nutritional, and Nutraceutical Properties in Subtropical Pigmented Maize Hybrids. Plants. 2022; 11(23):3221. https://doi.org/10.3390/plants11233221
Chicago/Turabian StyleTiessen-Favier, Axel, Anayansi Escalante-Aburto, Claudia Espinosa-Leal, and Silverio García-Lara. 2022. "Novel Combination of the Biophysical, Nutritional, and Nutraceutical Properties in Subtropical Pigmented Maize Hybrids" Plants 11, no. 23: 3221. https://doi.org/10.3390/plants11233221






