The LC-MS/MS Identification and Analgesic and Wound Healing Activities of Lavandula officinalis Chaix: In Vivo and In Silico Approaches
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Analysis with LC-MS/MS
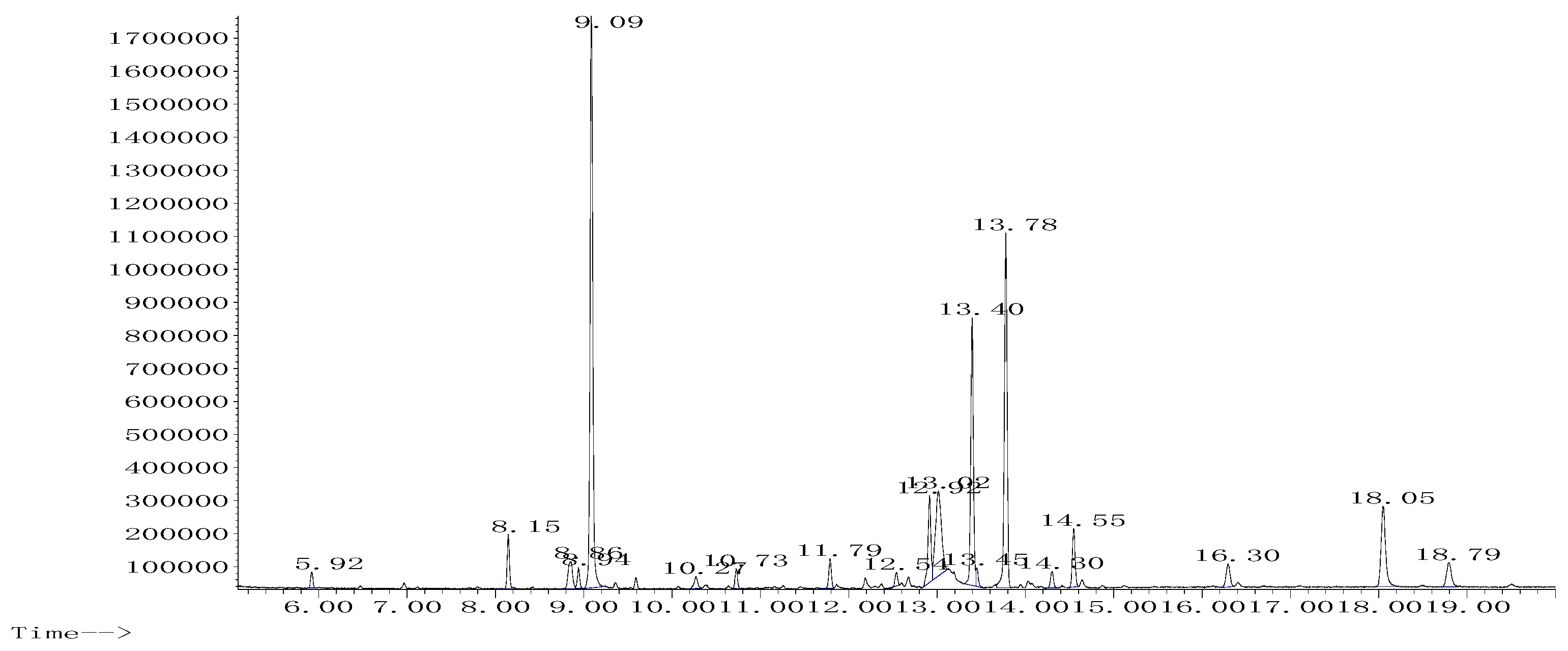
2.2. Phytochemical Analysis with GC-MS
2.3. Analgesic Activity
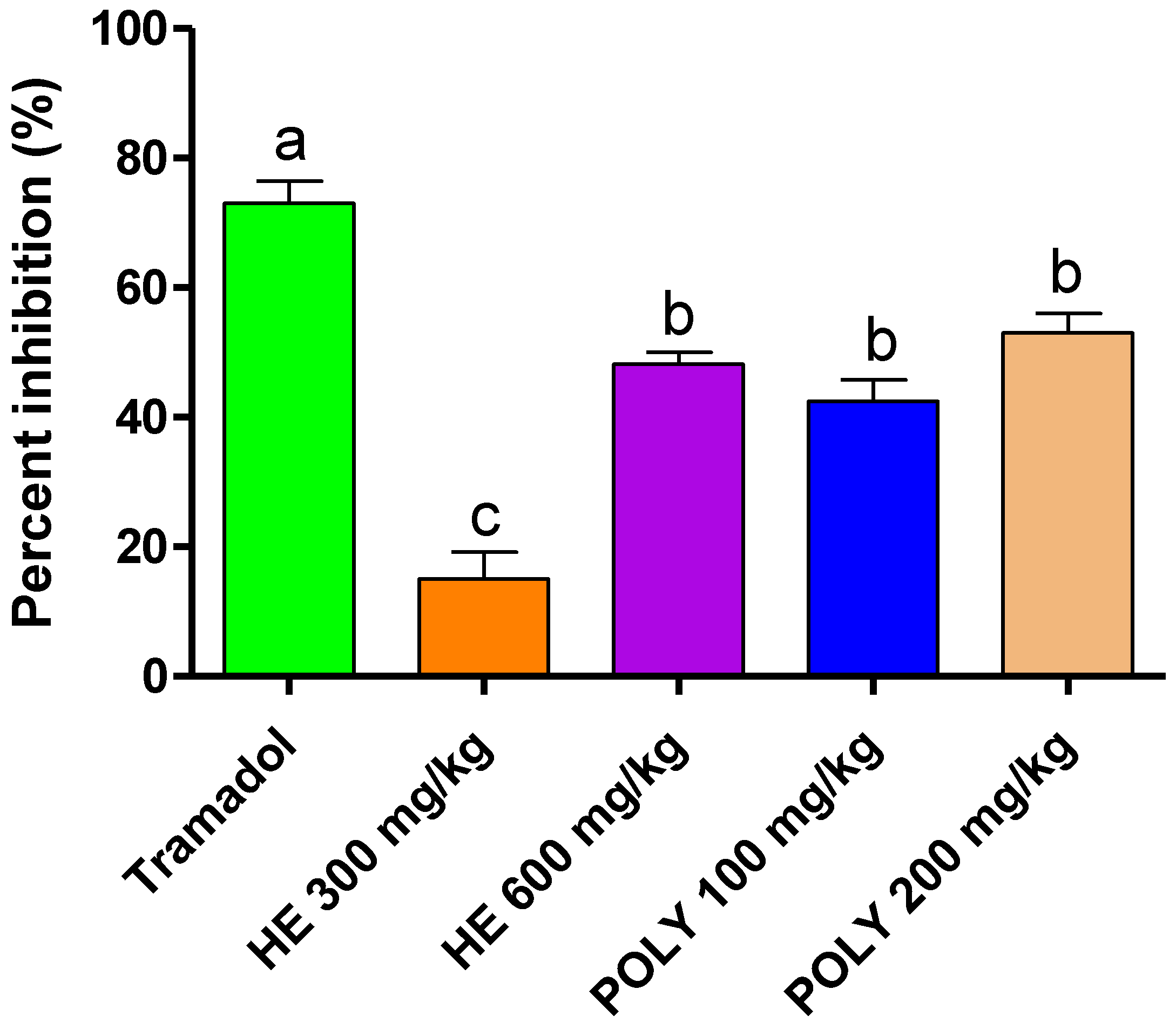
2.3.1. Abdominal Writhes
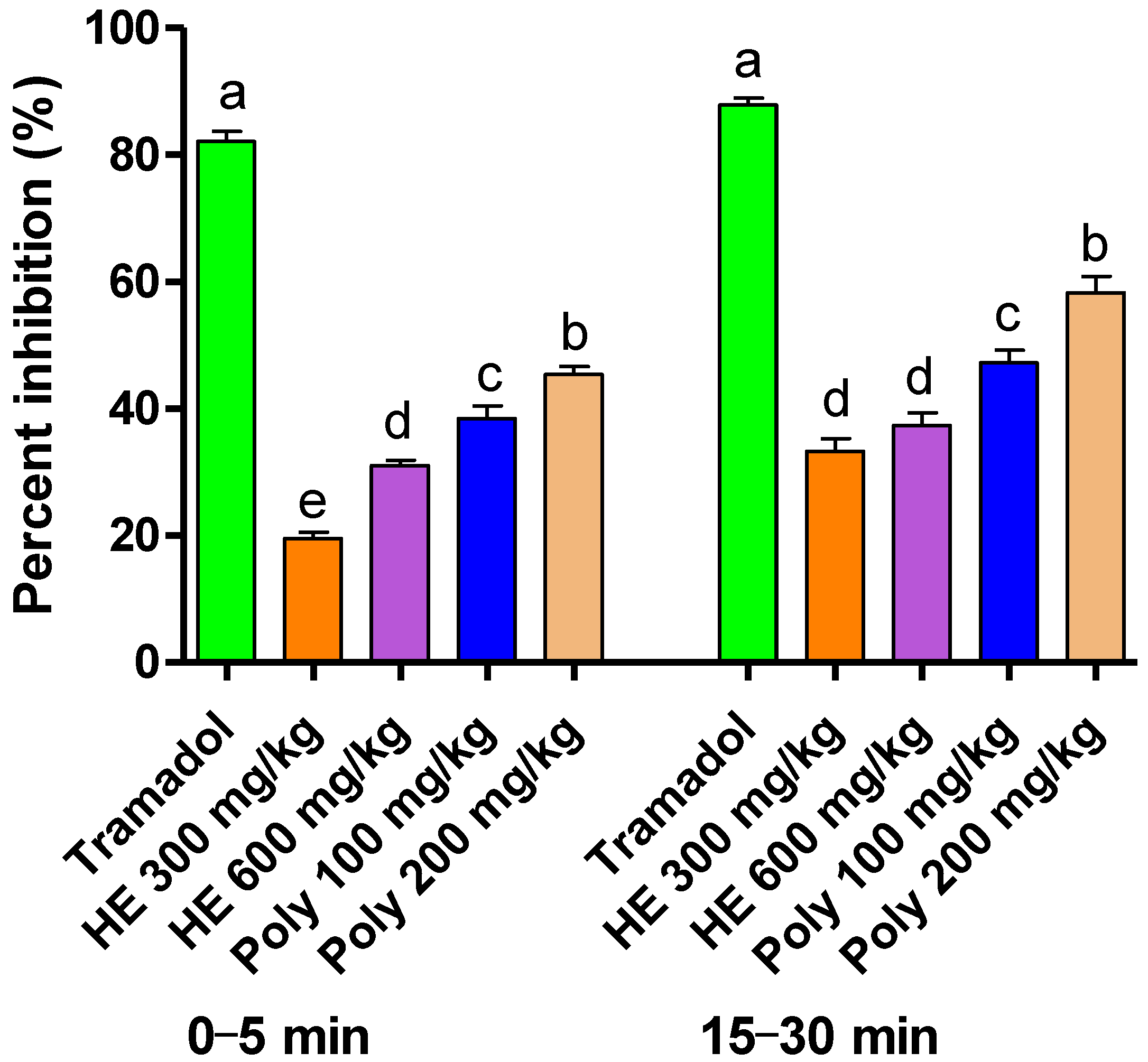
2.3.2. Formalin-Induced Pain
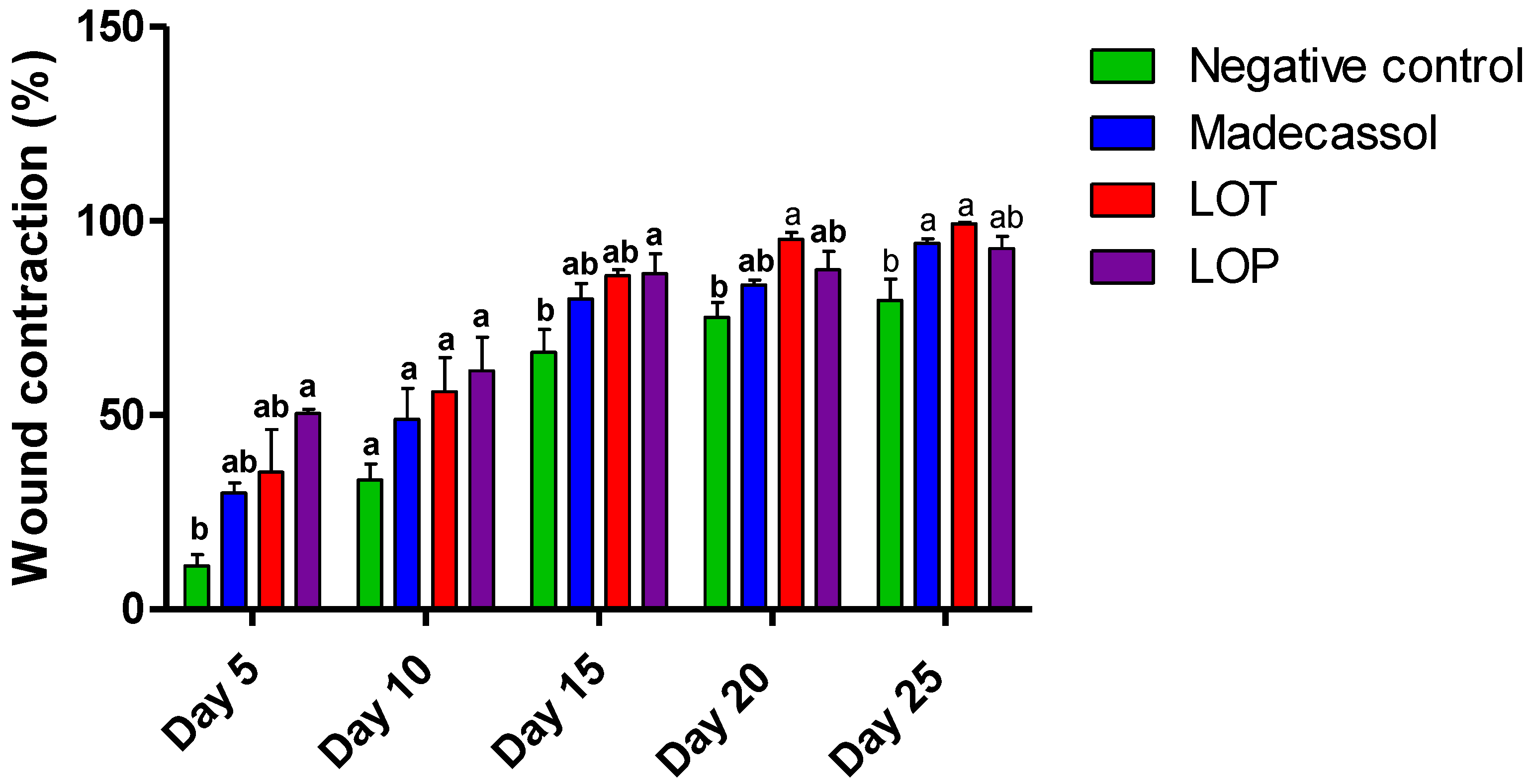
2.4. Wound-Healing Effect
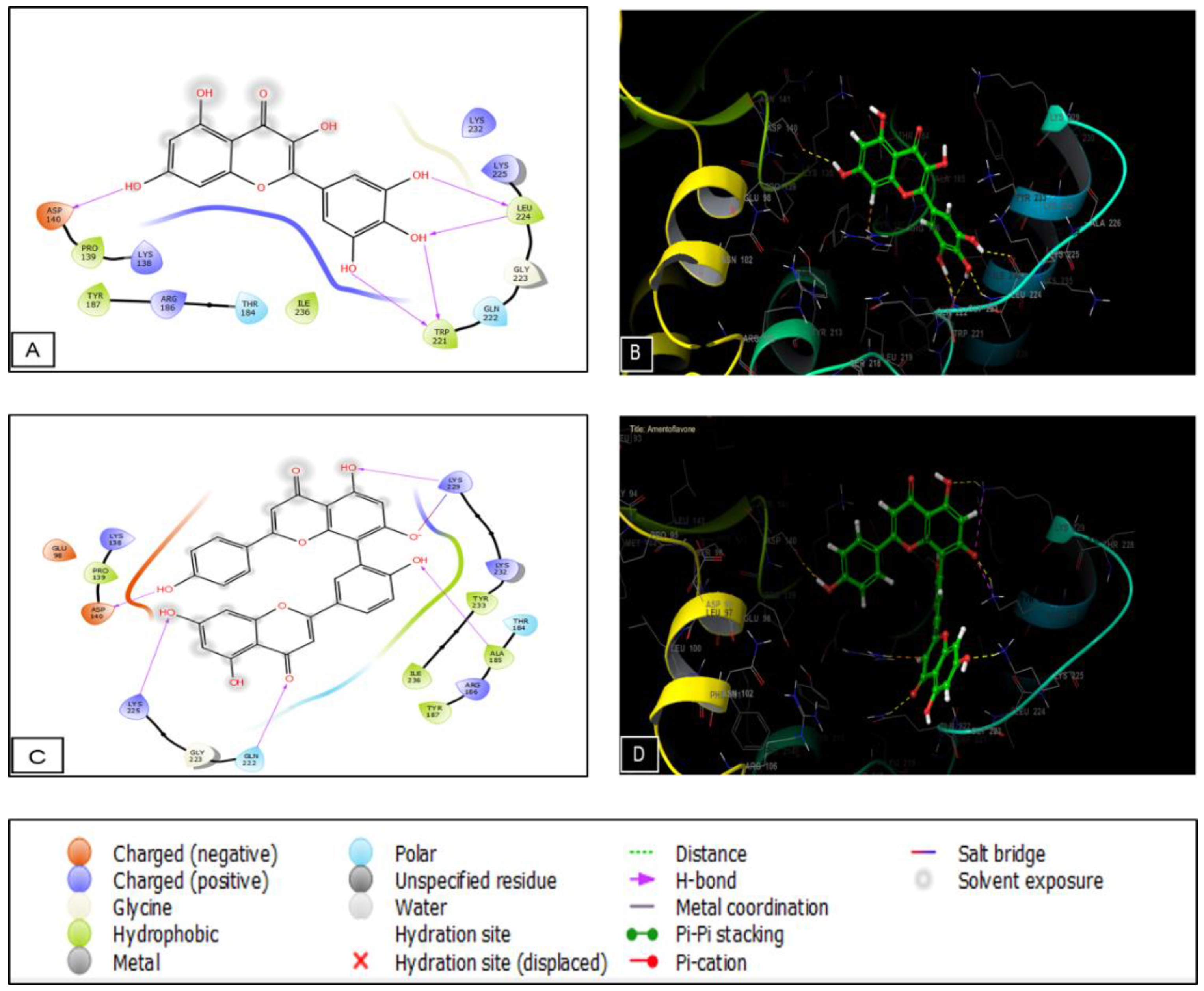
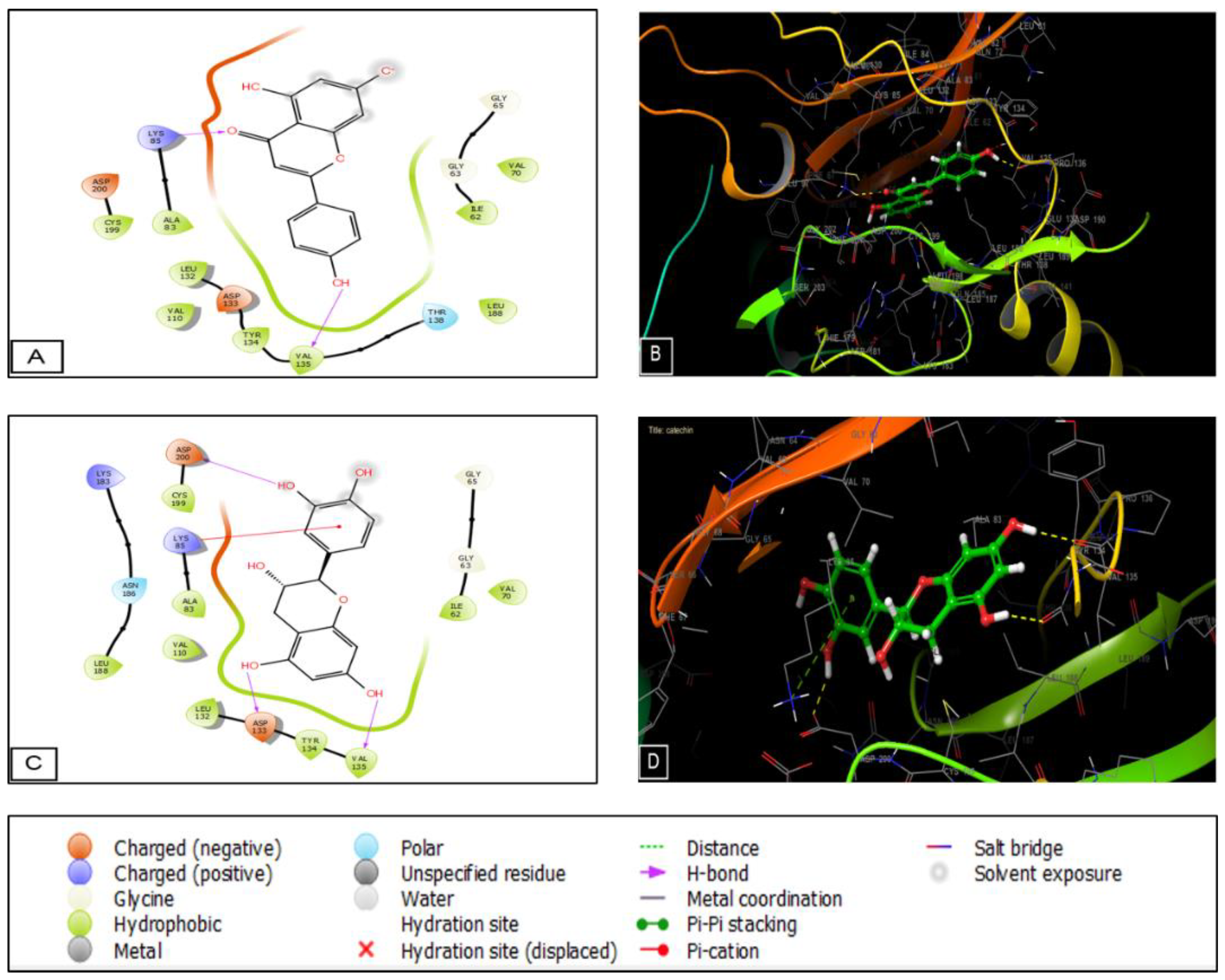
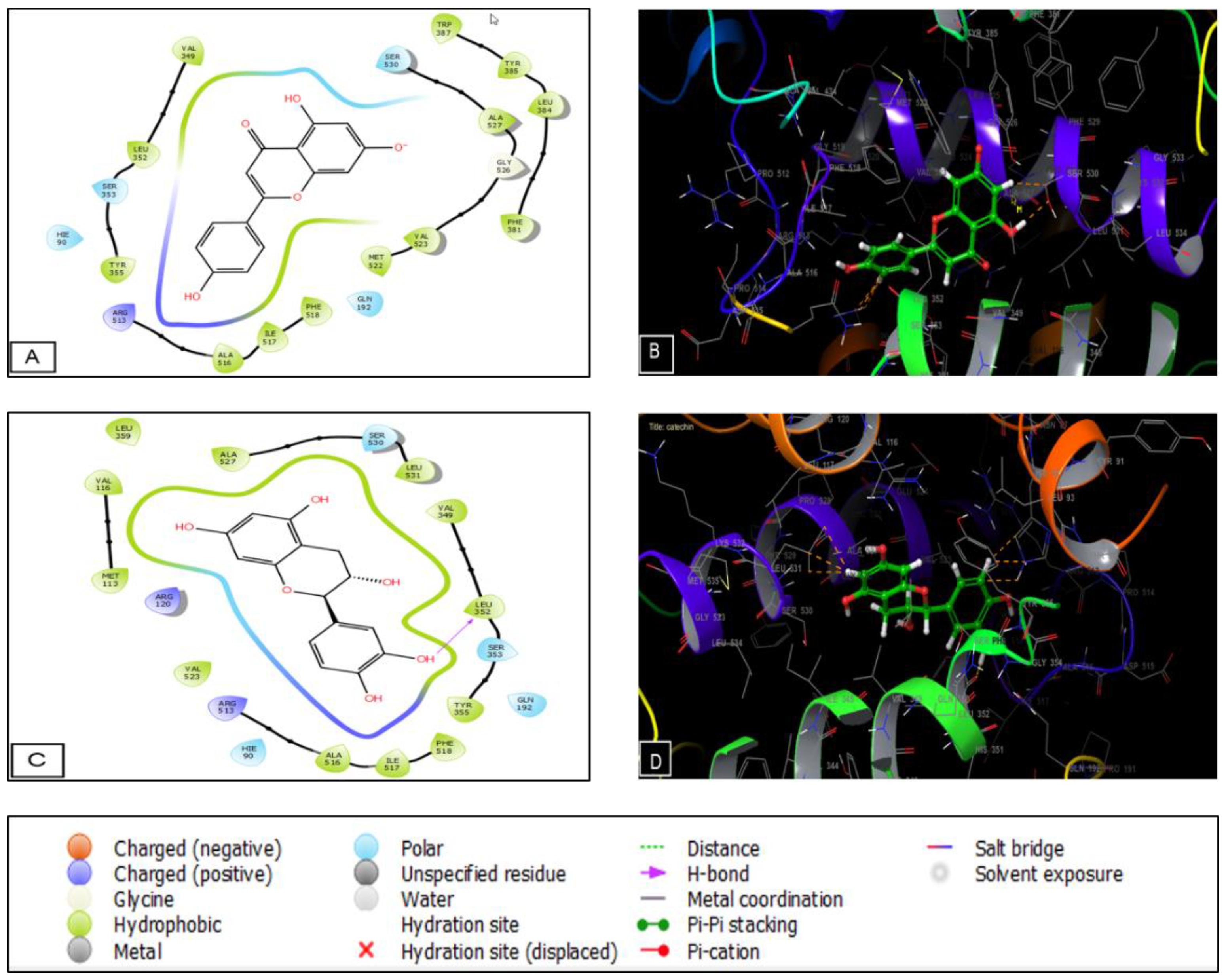
2.5. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Animal Material
4.3. Preparation of the Extracts
4.4. Phytochemical Analysis with LC-MS/MS
4.5. GC-MS Analysis of the Phytochemicals
4.6. Analysis of the Activity
4.6.1. Abdominal Writhes
4.6.2. Formalin-Induced Pain
4.7. Wound-Healing Test
4.7.1. Preparation of the Ointments
4.7.2. Burn-Wound Induction
- WC (%) = Wound contraction percentage
- WS0 = Wound size on the first day
- WSSD = Wound size at each specific day
4.8. Molecular Docking
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rao, M.; Palada, M.; Becker, B. Medicinal and Aromatic Plants in Agroforestry Systems. Agrofor. Syst. 2004, 61, 107–122. [Google Scholar]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of Polyphenols from Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters. In Polyphenol Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 243–259. [Google Scholar]
- Fritsch, J.; Abreu, M.T. The Microbiota and the Immune Response: What Is the Chicken and What Is the Egg? Gastrointest. Endosc. Clin. 2019, 29, 381–393. [Google Scholar] [CrossRef]
- Michels da Silva, D.; Langer, H.; Graf, T. Inflammatory and Molecular Pathways in Heart Failure—Ischemia, HFpEF and Transthyretin Cardiac Amyloidosis. Int. J. Mol. Sci. 2019, 20, 2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.W. Early Drug Discovery and the Rise of Pharmaceutical Chemistry. Drug Test. Anal. 2011, 3, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Chidambara Murthy, K.N.; Reddy, V.K.; Veigas, J.M.; Murthy, U.D. Study on Wound Healing Activity of Punica Granatum Peel. J. Med. Food 2004, 7, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Agra, M.d.F.; Silva, K.N.; Basílio, I.J.L.D.; Freitas, P.F.d.; Barbosa-Filho, J.M. Survey of Medicinal Plants Used in the Region Northeast of Brazil. Rev. Bras. Farmacogn. 2008, 18, 472–508. [Google Scholar] [CrossRef]
- Biswas, T.K.; Mukherjee, B. Plant Medicines of Indian Origin for Wound Healing Activity: A Review. Int. J. Low. Extrem. Wounds 2003, 2, 25–39. [Google Scholar] [CrossRef]
- Naghdi, F.; Gholamnezhad, Z.; Boskabady, M.H.; Bakhshesh, M. Muscarinic Receptors, Nitric Oxide Formation and Cyclooxygenase Pathway Involved in Tracheal Smooth Muscle Relaxant Effect of Hydro-Ethanolic Extract of Lavandula angustifolia Flowers. Biomed. Pharmacother. 2018, 102, 1221–1228. [Google Scholar] [CrossRef]
- Cardia, G.; Silva-Filho, S.; Silva, E.; Uchida, N.; Cavalcante, H.; Cassarotti, L.L.; Salvadego, V.E.C.; Spironello, R.A.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of Lavender (Lavandula angustifolia) Essential Oil on Acute Inflammatory Response. Evid. Based Complement. Altern. Med. 2018, 2018, 1413940. [Google Scholar] [CrossRef] [Green Version]
- Husseini, Y.; Sahraei, H.; Meftahi, G.H.; Dargahian, M.; Mohammadi, A.; Hatef, B.; Zardooz, H.; Ranjbaran, M.; Hosseini, S.B.; Alibeig, H.; et al. Analgesic and Anti-Inflammatory Activities of Hydro-Alcoholic Extract of Lavandula officinalis in Mice: Possible Involvement of the Cyclooxygenase Type 1 and 2 Enzymes. Rev. Bras. Farmacogn. 2016, 26, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Reshad, R.A.I.; Alam, S.; Raihan, H.B.; Meem, K.N.; Rahman, F.; Zahid, F.; Rafid, M.d.I.; Rahman, S.M.O.; Omit, S.; Ali, M.d.H. In Silico Investigations on Curcuminoids from Curcuma Longa as Positive Regulators of the Wnt/β-Catenin Signaling Pathway in Wound Healing. Egypt. J. Med. Hum. Genet. 2021, 22, 1–16. [Google Scholar] [CrossRef]
- Peng, J.; Ramesh, G.; Sun, L.; Dong, Z. Impaired Wound Healing in Hypoxic Renal Tubular Cells: Roles of Hypoxia-Inducible Factor-1 and Glycogen Synthase Kinase 3β/β-Catenin Signaling. J. Pharmacol. Exp. Ther. 2012, 340, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.L.; Gu, L.J.; Liu, L.; Wang, C.Y.; Sun, B.S.; Li, Z.; Sung, C.K. Effect of Wnt Signaling Pathway on Wound Healing. Biochem. Biophys. Res. Commun. 2009, 378, 149–151. [Google Scholar] [CrossRef]
- Araico, A.; Terencio, M.C.; Alcaraz, M.J.; Domínguez, J.N.; León, C.; Ferrándiz, M.L. Evaluation of the Anti-Inflammatory and Analgesic Activity of Me-UCH9, a Dual Cyclooxygenase-2/5-Lipoxygenase Inhibitor. Life Sci. 2007, 80, 2108–2117. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Zielins, E.R.; Brett, E.A.; Luan, A.; Hu, M.S.; Walmsley, G.G.; Paik, K.; Senarath-Yapa, K.; Atashroo, D.A.; Wearda, T.; Lorenz, H.P. Emerging Drugs for the Treatment of Wound Healing. Expert Opin. Emerg. Drugs 2015, 20, 235–246. [Google Scholar] [CrossRef]
- Dumitru, M.G. Phytochemical Screening, Total Hydroxycinnamic Acids, Total Phenolic and Antioxidant Activity of Lavandula angustifolia Mill. GSC Biol. Pharm. Sci. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Shafaghat, A.; Salimi, F.; Amani-Hooshyar, V. Phytochemical and Antimicrobial Activities of Lavandula officinalis Leaves and Stems against Some Pathogenic Microorganisms. J. Med. Plants Res. 2012, 6, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Shaza, M.A. Phytochemical Screening of Petroselinum Crispum (Mill.) Fuss and in Vitro Evaluation of Its Antimicrobial Activity against Some Uropathogens. Arab. J. Med. Aromat. Plants 2016, 2, 86–98. [Google Scholar]
- Al-Howiriny, T.A.; Al-Sohaibani, M.O.; El-Tahir, K.H.; Rafatullah, S. Preliminary Evaluation of the Anti-Inflammatory and Anti-Hepatotoxic Activities of ‘Parsley’ Petroselinum Crispum in Rats. J. Nat. Remedies 2003, 3, 54–62. [Google Scholar]
- Kashmar, A.M.; Naser, E.H. Comparison between Two Extraction Methods on Total Extract with Primary Investigation of Phytochemical Compounds of Some Medicinal Plants Used in Treatment of Urinary Tract Disease. Karbala J. Pharm. Sci. 2017, 22, 29–36. [Google Scholar]
- Wokoun, U.; Hellriegel, M.; Emons, G.; Gründker, C. Co-Treatment of Breast Cancer Cells with Pharmacologic Doses of 2-Deoxy-D-Glucose and Metformin: Starving Tumors. Oncol. Rep. 2017, 37, 2418–2424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Li, L.; Kim, S.-H.; Hagerman, A.E.; Lü, J. Anti-Cancer, Anti-Diabetic and Other Pharmacologic and Biological Activities of Penta-Galloyl-Glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Park, N.-Y.; Kho, Y.; Ji, K. Effects of 4-Hydroxyphenyl 4-Isoprooxyphenylsulfone (BPSIP) Exposure on Reproduction and Endocrine System of Zebrafish. Environ. Sci. Technol. 2018, 52, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.I.; Hussain, F.; Saqueeb, N.; Abdur Rahman, S.M. Synthesis and Evaluation of Pharmacological Activities of Some 3-O-Benzyl-4-C-(Hydroxymethyl)-1,2-O-Isopropylidene-α-D-Ribofuranose Derivatives as Potential Anti-Inflammatory Agents and Analgesics. Res. Pharm. Sci. 2020, 15, 209–217. [Google Scholar] [CrossRef]
- Ciotta, L.; Stracquadanio, M.; Pagano, I.; Carbonaro, A.; Palumbo, M.; Gulino, F. Effects of Myo-Inositol Supplementation on Oocyte’s Quality in PCOS Patients: A Double Blind Trial. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 509–514. [Google Scholar]
- Condorelli, R.A.; La Vignera, S.; Mongioì, L.M.; Vitale, S.G.; Laganà, A.S.; Cimino, L.; Calogero, A.E. Myo-Inositol as a Male Fertility Molecule: Speed Them Up! Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 30–35. [Google Scholar] [PubMed]
- Slavov, A.M.; Karneva, K.B.; Vasileva, I.N.; Denev, P.N.; Denkova, R.S.; Shikov, V.T.; Manolova, M.N.; Lazarova, Y.L.; Ivanova, V.N. Valorization of Lavender Waste—Obtaining and Characteristics of Polyphenol Rich Extracts. Food Sci. Appl. Biotechnol. 2018, 1, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Lesage-Meessen, L.; Bou, M.; Ginies, C.; Chevret, D.; Navarro, D.; Drula, E.; Bonnin, E.; Del Río, J.C.; Odinot, E.; Bisotto, A. Lavender-and Lavandin-Distilled Straws: An Untapped Feedstock with Great Potential for the Production of High-Added Value Compounds and Fungal Enzymes. Biotechnol. Biofuels 2018, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ablikim, G.; Bobakulov, K.; Li, J.; Yadikar, N.; Aisa, H.A. Two New Glucoside Derivatives of Truxinic and Cinnamic Acids from Lavandula angustifolia Mill. Nat. Prod. Res. 2019, 35, 2526–2534. [Google Scholar] [CrossRef]
- Areias, F.M.; Valentão, P.; Andrade, P.B.; Moreira, M.M.; Amaral, J.; Seabra, R.M. Hplc/Dad Analysis of Phenolic Compounds from Lavender and Its Application to Quality Control. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 2563–2572. [Google Scholar] [CrossRef]
- Slighoua, M.; Mahdi, I.; Amrati, F.e.-z.; Boucetta, N.; Cristo, F.D.; Boukhira, S.; Hamsas, A.E.y.e.; Tattou, M.I.; Grafov, A.; Bari, A.; et al. Pharmacological Effects of Lavandula officinalis Chaix and Its Polyphenols: Focus on Their in Vivo Estrogenic and Anti-Inflammatory Properties. South Afr. J. Bot. 2022, 146, 354–364. [Google Scholar] [CrossRef]
- Stanciu, G.; Aonofriesei, F.; Lupsor, S.; Popescu, A.; Sirbu, R. Study of Phenolic Compounds and Antimicrobial Activity of Lavandula angustifolia l. Flowers Macerates. Rev. Chime 2019, 70, 1800–1804. [Google Scholar] [CrossRef]
- Baranwal, A.; Aggarwal, P.; Rai, A.; Kumar, N. Pharmacological Actions and Underlying Mechanisms of Catechin: A Review. Mini Rev. Med. Chem. 2022, 22, 821–833. [Google Scholar] [CrossRef]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14, 1934578X19874174. [Google Scholar] [CrossRef]
- Gupta, G.; Siddiqui, M.A.; Khan, M.M.; Ajmal, M.; Ahsan, R.; Rahaman, M.A.; Ahmad, M.A.; Arshad, M.; Khushtar, M. Current Pharmacological Trends on Myricetin. Drug Res. 2020, 70, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Wang, K.; Zheng, S.; Wang, Y.; Ren, Q.; Li, H.; Ding, L.; Li, W.; Zhang, L. Antibacterial Effect of Caffeic Acid Phenethyl Ester on Cariogenic Bacteria and Streptococcus mutans Biofilms. Antimicrob. Agents Chemother. 2020, 64, e00251-20. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.-T.; Xiao, L.; Zhu, L.; Wang, Q.; Yan, T. Anti-Inflammatory Effects of Apigenin in Lipopolysaccharide-Induced Inflammatory in Acute Lung Injury by Suppressing COX-2 and NF-KB Pathway. Inflammation 2014, 37, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- De Fátima Arrigoni-Blank, M.; Dmitrieva, E.G.; Franzotti, E.M.; Antoniolli, A.R.; Andrade, M.R.; Marchioro, M. Anti-Inflammatory and Analgesic Activity of Peperomia pellucida (L.) HBK (Piperaceae). J. Ethnopharmacol. 2004, 91, 215–218. [Google Scholar] [CrossRef]
- Lucarini, R.; Bernardes, W.A.; Ferreira, D.S.; Tozatti, M.G.; Furtado, R.; Bastos, J.K.; Pauletti, P.M.; Januário, A.H.; Silva, M.L.A.e.; Cunha, W.R. In Vivo Analgesic and Anti-Inflammatory Activities of Rosmarinus officinalis Aqueous Extracts, Rosmarinic Acid and Its Acetyl Ester Derivative. Pharm. Biol. 2013, 51, 1087–1090. [Google Scholar] [CrossRef] [Green Version]
- Palanichamy, S.; Nagarajan, S. Analgesic Activity of Cassia Alata Leaf Extract and Kaempferol 3-O-Sophoroside. J. Ethnopharmacol. 1990, 29, 73–78. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, X.; Gui, X.; Chen, L.; Huang, B. Natural Flavonoids as Promising Analgesic Candidates: A Systematic Review. Chem. Biodivers. 2016, 13, 1427–1440. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A Comprehensive Review on Its Biological Potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; de Luna-Bertos, E.; Ramos-Torrecillas, J.; Illescas-Montesa, R.; Costela-Ruiz, V.J.; García-Martínez, O. Potential Effects of Phenolic Compounds That Can Be Found in Olive Oil on Wound Healing. Foods 2021, 10, 1642. [Google Scholar] [CrossRef]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid. Based Complement. Alternat. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuah, X.Q.; Okechukwu, P.N.; Amini, F.; Teo, S.S. Eicosane, Pentadecane and Palmitic Acid: The Effects in in Vitro Wound Healing Studies. Asian Pac. J. Trop. Biomed. 2018, 8, 490. [Google Scholar]
- Özay, Y.; Güzel, S.; Yumrutaş, Ö.; Pehlivanoğlu, B.; Erdoğdu, İ.H.; Yildirim, Z.; Türk, B.A.; Darcan, S. Wound Healing Effect of Kaempferol in Diabetic and Nondiabetic Rats. J. Surg. Res. 2019, 233, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Witte, M.B.; Barbul, A. Arginine Physiology and Its Implication for Wound Healing. Wound Repair Regen. 2003, 11, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I.; Akkol, E.K.; Keles, H.; Yesilada, E.; Sarker, S.D. Exploration of the Wound Healing Potential of Helichrysum graveolens (Bieb.) Sweet: Isolation of Apigenin as an Active Component. J. Ethnopharmacol. 2013, 149, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, S.E.; Ebrahimi, S.N.; Salehi, P.; Moridi Farimani, M.; Hamburger, M.; Jabbarzadeh, E. Wound Healing Potential of Chlorogenic Acid and Myricetin-3-O-β-Rhamnoside Isolated from Parrotia persica. Molecules 2017, 22, 1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyati, S.; Ranga, R.; Ross, B.D.; Rehemtulla, A.; Bhojani, M.S. Molecular Imaging of Glycogen Synthase Kinase-3β and Casein Kinase-1α Kinases. Anal. Biochem. 2010, 405, 246–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, M.; Mehedi, M.; Akter, M.; Sajon, S.R.; Mazumder, K.; Rana, M. In Vivo and in Silico Evaluation of Analgesic Activity of Lippia alba. Clin. Phytosci. 2019, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Latitude to GPS Coordinates of Imouzzer Kandar, Morocco. Latitude: 33.7247 Longitude: −5.0060. Available online: https://latitude.to/articles-by-country/ma/morocco/205555/imouzzer-kandar (accessed on 14 October 2020).
- Slighoua, M.; Mahdi, I.; Amrati, F.e.-z.; Di Cristo, F.; Amaghnouje, A.; Grafov, A.; Boucetta, N.; Bari, A.; Bousta, D. Assessment of in Vivo Estrogenic and Anti-Inflammatory Activities of the Hydro-Ethanolic Extract and Polyphenolic Fraction of Parsley (Petroselinum sativum Hoffm.). J. Ethnopharmacol. 2021, 265, 113290. [Google Scholar] [CrossRef] [PubMed]
- Wafa, G.; Amadou, D.; Larbi, K.M.; Héla, E.F.O. Larvicidal Activity, Phytochemical Composition, and Antioxidant Properties of Different Parts of Five Populations of Ricinus communis L. Ind. Crops Prod. 2014, 56, 43–51. [Google Scholar] [CrossRef]
- Ho, C.T. Antioxidative effect of polyphenol extract prepared from various Chinese teas. Prev. Med. 1992, 21, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Amaghnouje, A.; Mechchate, H.; Es-safi, I.; Boukhira, S.; Aliqahtani, A.S.; M. Noman, O.; Nasr, F.A.; Conte, R.; Calarco, A.; Bousta, D. Subacute Assessment of the Toxicity and Antidepressant-like Effects of Origanum majorana L. Polyphenols in Swiss Albino Mice. Molecules 2020, 25, 5653. [Google Scholar] [CrossRef] [PubMed]
- Kabran, G.R.; Mamyrbekova-Bekro, J.A.; Pirat, J.-L.; Bekro, Y.-A.; Sommerer, N.; Verbaere, A.; Meudec, E. Identification de composés phénoliques extraits de deux plantes de la pharmacopée ivoirienne */Identification of phenolic compounds from two plants of ivorian pharmacopeia *. J. Soc. Ouest Afr. Chim. 2014, 38, 57–63. [Google Scholar]
- Farzaei, M.H.; Abbasabadi, Z.; Ardekani, M.R.S.; Rahimi, R.; Farzaei, F. Parsley: A Review of Ethnopharmacology, Phytochemistry and Biological Activities. J. Tradit. Chin. Med. 2013, 33, 815–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajhashemi, V.; Ghannadi, A.; Sharif, B. Anti-Inflammatory and Analgesic Properties of the Leaf Extracts and Essential Oil of Lavandula angustifolia Mill. J. Ethnopharmacol. 2003, 89, 67–71. [Google Scholar] [CrossRef]
- Manglik, A.; Lin, H.; Aryal, D.K.; McCorvy, J.D.; Dengler, D.; Corder, G.; Levit, A.; Kling, R.C.; Bernat, V.; Hübner, H. Structure-Based Discovery of Opioid Analgesics with Reduced Side Effects. Nature 2016, 537, 185–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M.; Bahramsoltani, R.; Abdolghaffari, A.H.; Rahimi, R.; Esfandyari, M.; Baeeri, M.; Hassanzadeh, G.; Abdollahi, M.; Farzaei, M.H. Efficacy of Topical Application of Standardized Extract of Tragopogon Graminifolius in the Healing Process of Experimental Burn Wounds. J. Tradit. Complement. Med. 2019, 9, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Soud, M.A.; Ennaji, H.; Kumar, A.; Alfhili, M.A.; Bari, A.; Ahamed, M.; Chebaibi, M.; Bourhia, M.; Khallouki, F.; Alghamdi, K.M. Antioxidant, Anti-Proliferative Activity and Chemical Fingerprinting of Centaurea Calcitrapa against Breast Cancer Cells and Molecular Docking of Caspase-3. Antioxidants 2022, 11, 1514. [Google Scholar] [CrossRef] [PubMed]
- Lafraxo, S.; El Moussaoui, A.; A Bin Jardan, Y.; El Barnossi, A.; Chebaibi, M.; Baammi, S.; Ait Akka, A.; Chebbac, K.; Akhazzane, M.; Chelouati, T. GC-MS Profiling, In Vitro Antioxidant, Antimicrobial, and In Silico NADPH Oxidase Inhibition Studies of Essential Oil of Juniperus Thurifera Bark. Evid. Based Complement. Alternat. Med. 2022, 2022. [Google Scholar] [CrossRef] [PubMed]


| Molecules | Formula | Retention Time (min) | Molecular Adduct [M − H]− | Area under the Curve |
|---|---|---|---|---|
| Trans-ferulic acid | C10H10O4 | 1.311 | 193.00 | 60945002 |
| Ursolic acid | C30H48O3 | 1.164 | 455.00 | 4652510 |
| Apigenin | C15H10O5 | 1.116 | 269.00 | 3867802 |
| Amentoflavone | C30H18O10 | 1.030 | 537.00 | 5516717 |
| Caffeic acid | C9H8O4 | 1.141 | 179.00 | 115973902 |
| Ferulic acid | C10H10O4 | 1.313 | 193.00 | 65420034 |
| Catechin | C15H14O6 | 0.829 | 289.00 | 15052775 |
| Myricetin | C15H10O8 | 1.430 | 317.00 | 254366279 |
| Peak | Name | R.T (min) | Area % |
|---|---|---|---|
| 1 | Propanoic acid, 2-[(trimethylsilyl)oxy] | 5.924 | 0.686 |
| 2 | Cinnamic acid, o-methoxy-, trimethylsilyl ester | 8.148 | 2.233 |
| 3 | Benzoic acid trimethylsilyl ester | 8.855 | 2.182 |
| 4 | Anthracene, 5,6-dihydro | 8.943 | 0.926 |
| 5 | Triazolo[e]benzofurazan | 9.088 | 29.319 |
| 6 | Acrylic acid, 2-phenylethyl ester | 10.268 | 0.786 |
| 7 | Malic acid, tris(trimethylsilyl) ester | 10.730 | 0.888 |
| 8 | Glycolic acid-d2-o-(trimethylsilyl) | 11.790 | 1.364 |
| 9 | D-Glucitol, 1,1-di-C-octyl-2,3,4,6-tetra-O-trimethylsilyl | 12.541 | 0.602 |
| 10 | Arabinofuranose, 1,2,3,5-tetrakis-O-(trimethylsilyl) | 12.914 | 4.598 |
| 11 | D-Ribofuranose, 1,2,3,5-tetrakis-O-(trimethylsilyl) | 13.015 | 9.566 |
| 12 | D-Glucose, 2,3,4,5,6-pentakis-O-(trimethylsilyl) | 13.396 | 13.396 |
| 13 | Mannose, 2,3,4,5,6-pentakis-O-(trimethylsilyl) | 13.453 | 0.791 |
| 14 | P-hydroxyphenyl | 13.778 | 18.219 |
| 15 | Talose, 2,3,4,5,6-pentakis-O-(trimethylsilyl) | 14.300 | 0.889 |
| 16 | Synephrine (trimethylsilyl derivative) | 14.549 | 2.976 |
| 17 | Myo-Inositol, 1,2,3,4,5,6-hexakis-O-(trimethylsilyl) | 16.295 | 1.690 |
| 18 | Alpha-D-Galactofuranose, 1,2,3,5,6-pentakis-O-(trimethylsilyl) | 18.050 | 6.678 |
| 19 | Erythritol per-tms Butane, 1,2,3,4-tetrakis[(trimethylsilyl)oxy] | 18.793 | 2.211 |
| Total | 100 |
| Treatment | Dose (mg/kg) | Number of Writhes |
|---|---|---|
| Control | 65.00 ± 2.88 a | |
| Tramadol | 10 | 17.33 ± 1.45 d |
| Hydro-ethanolic extract | 300 | 55.00 ± 2.88 a |
| 600 | 33.67 ± 1.85 bc | |
| Polyphenols | 100 | 37.67 ± 2.18 bc |
| 200 | 30.33 ± 0.66 c |
| Treatment | Dose (mg/kg) | Licking Time (s) | |
|---|---|---|---|
| First Phase (0–5 min) | Second Phase (15–30 min) | ||
| Control | 58.00 ± 0.50 a | 30.33 ± 2.50 a | |
| Tramadol | 10 | 10.33 ± 0.80 d | 4.33 ± 1.70 d |
| Hydro-ethanolic extract | 300 | 46.67 ± 0.33 ab | 20.33 ± 0.66 b |
| 600 | 40.00 ± 0.57 b | 19.00 ± 0.57 b | |
| Polyphenols | 100 | 35.67 ± 0.88 b | 16.00 ± 0.57 bc |
| 200 | 31.67 ± 0.88 bc | 12.67 ± 0.88 c | |
| Wound Size in cm2 | ||||||
|---|---|---|---|---|---|---|
| Treatments | Day 1 | Day 5 | Day 10 | Day 15 | Day 20 | Day 25 |
| Control | 1.91 ± 0.15 a | 1.70 ± 0.18 a | 1.26 ± 0.03 a | 0.62 ± 0.05 a | 0.46 ± 0.03 a | 0.37 ± 0.06 a |
| Madecassol® (1%) | 2.27 ± 0.12 a | 1.59 ± 0.09 a | 1.14 ± 0.11 a | 0.46 ± 0.10 a | 0.37 ± 0.04 a | 0.13 ± 0.03 b |
| Hydro-ethanolic extract (10%) | 2.83 ± 0.25 a | 1.22 ± 0.12 a | 1.01 ± 0.10 a | 0.33 ± 0.02 a | 0.10 ± 0.03 b | 0.01 ± 0.01 b |
| Polyphénols (10%) | 2.95 ± 0.28 a | 1.29 ± 0.25 a | 0.95 ± 0.11 a | 0.37 ± 0.14 a | 0.30 ± 0.10 a | 0.16 ± 0.06 ab |
| CK1 Receptor (PDB: 6GZD) | GSK3-β Receptor (PDB: 1Q5K) | Cyclooxygenase-2 Receptor (PDB: 6COX) | ||||
|---|---|---|---|---|---|---|
| Glide Gscore (Kcal/mol) | Glide Energy (Kcal/mol) | Glide Gscore (Kcal/mol) | Glide Energy (Kcal/mol) | Glide Gscore (Kcal/mol) | Glide Energy (Kcal/mol) | |
| Myricetin | −5.34 | −37.995 | −6.622 | −42.144 | −6.909 | −28.13 |
| Amentoflavone | −5.186 | −43.924 | −7.335 | −52.265 | - | - |
| Caffeic acid | −4.897 | −25.316 | −6.298 | −27.62 | −6.511 | −29.447 |
| Apigenin | −4.869 | −29.071 | −7.652 | −36.061 | −7.526 | −34.259 |
| Catechin | −4.62 | −30.297 | −7.386 | −41.143 | −7.209 | −39.399 |
| Ferulic acid | −4.596 | −26.173 | −6.669 | −25.647 | −6.156 | −28.906 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slighoua, M.; Chebaibi, M.; Mahdi, I.; Amrati, F.E.-z.; Conte, R.; Cordero, M.A.W.; Alotaibi, A.; Saghrouchni, H.; Agour, A.; Zair, T.; et al. The LC-MS/MS Identification and Analgesic and Wound Healing Activities of Lavandula officinalis Chaix: In Vivo and In Silico Approaches. Plants 2022, 11, 3222. https://doi.org/10.3390/plants11233222
Slighoua M, Chebaibi M, Mahdi I, Amrati FE-z, Conte R, Cordero MAW, Alotaibi A, Saghrouchni H, Agour A, Zair T, et al. The LC-MS/MS Identification and Analgesic and Wound Healing Activities of Lavandula officinalis Chaix: In Vivo and In Silico Approaches. Plants. 2022; 11(23):3222. https://doi.org/10.3390/plants11233222
Chicago/Turabian StyleSlighoua, Meryem, Mohamed Chebaibi, Ismail Mahdi, Fatima Ez-zahra Amrati, Raffaele Conte, Mary Anne W. Cordero, Amal Alotaibi, Hamza Saghrouchni, Abdelkrim Agour, Touria Zair, and et al. 2022. "The LC-MS/MS Identification and Analgesic and Wound Healing Activities of Lavandula officinalis Chaix: In Vivo and In Silico Approaches" Plants 11, no. 23: 3222. https://doi.org/10.3390/plants11233222






