Impact of Web Blight on Photosynthetic Performance of an Elite Common Bean Line in the Western Amazon Region of Colombia
Abstract
1. Introduction
2. Results
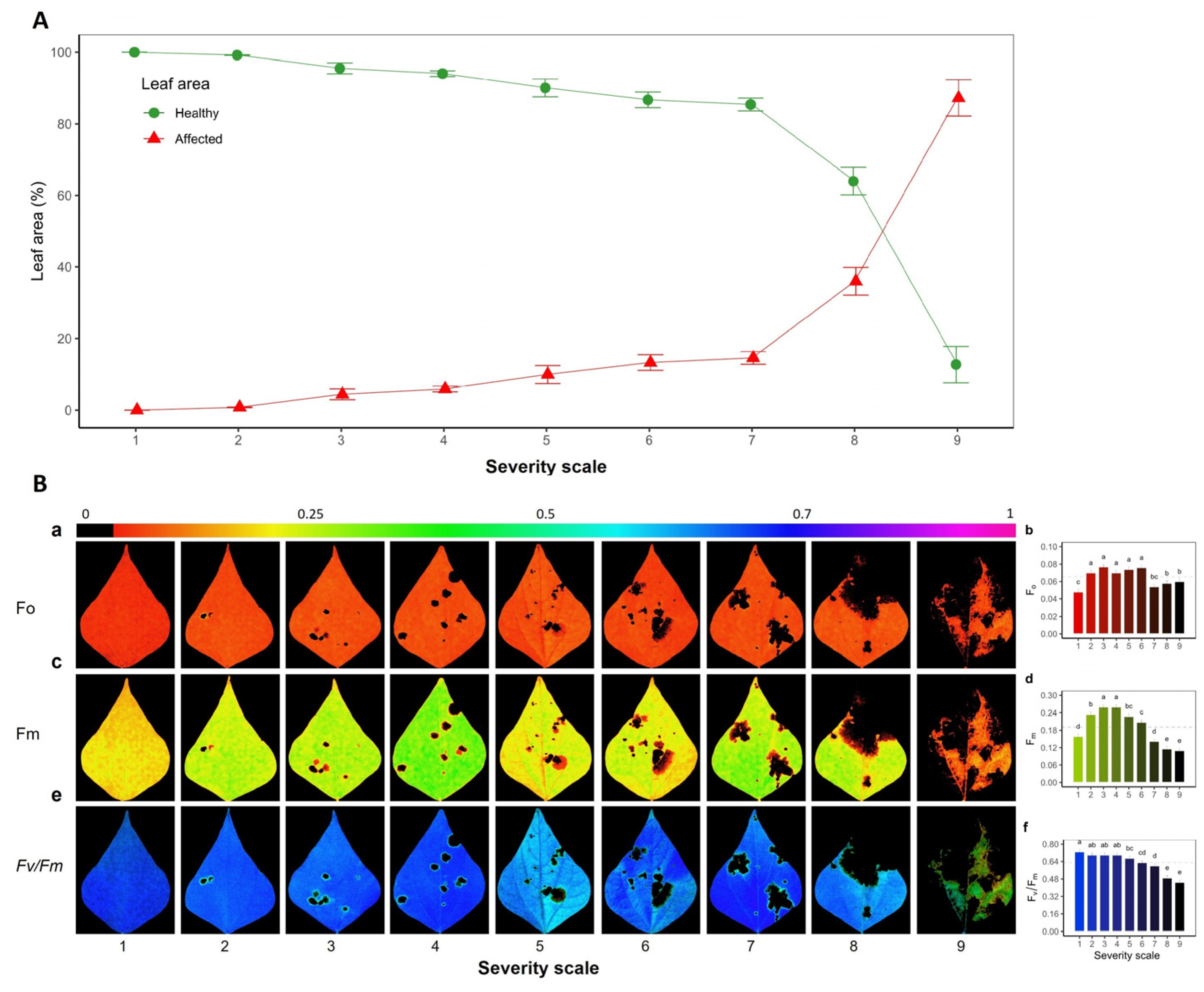
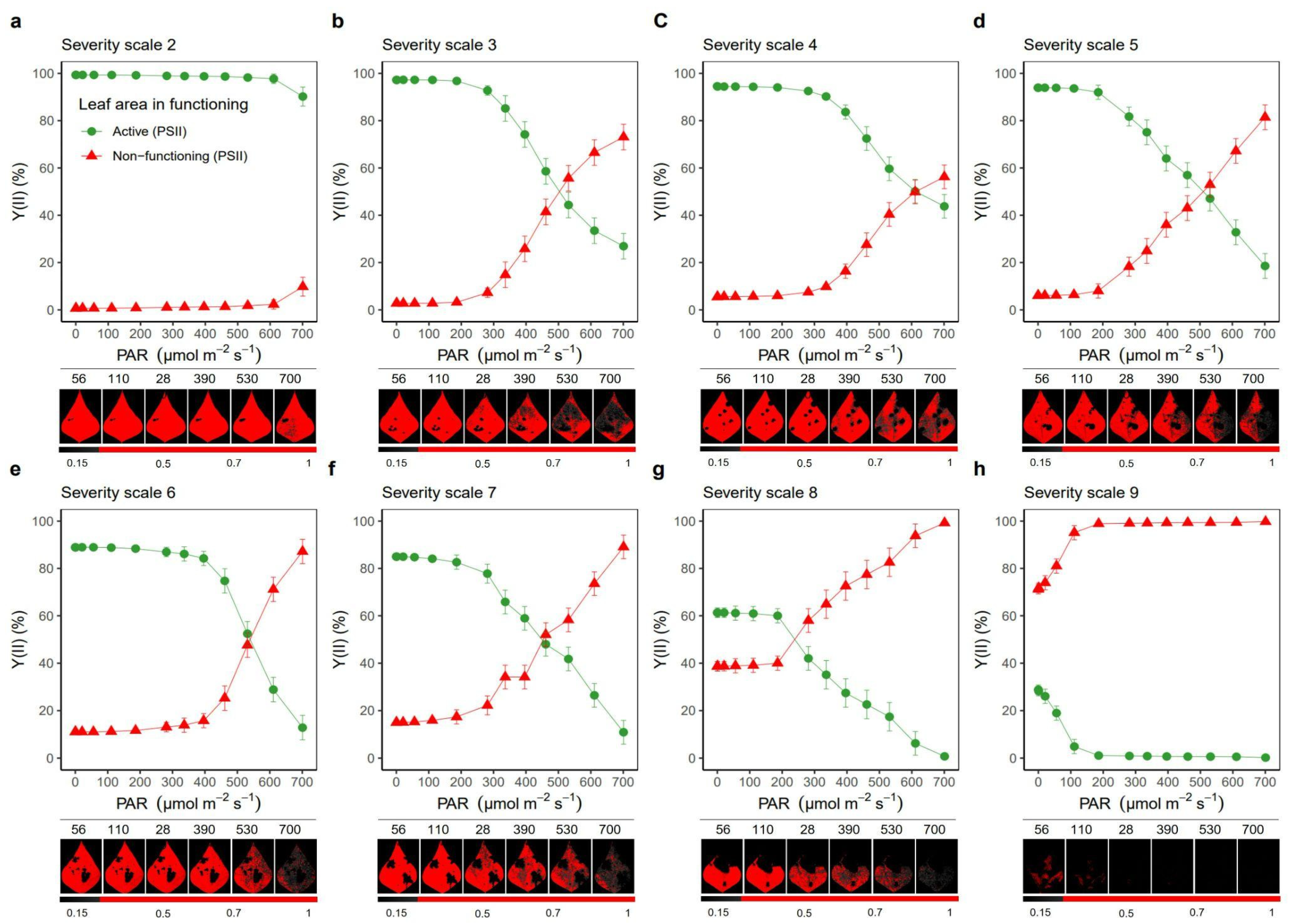
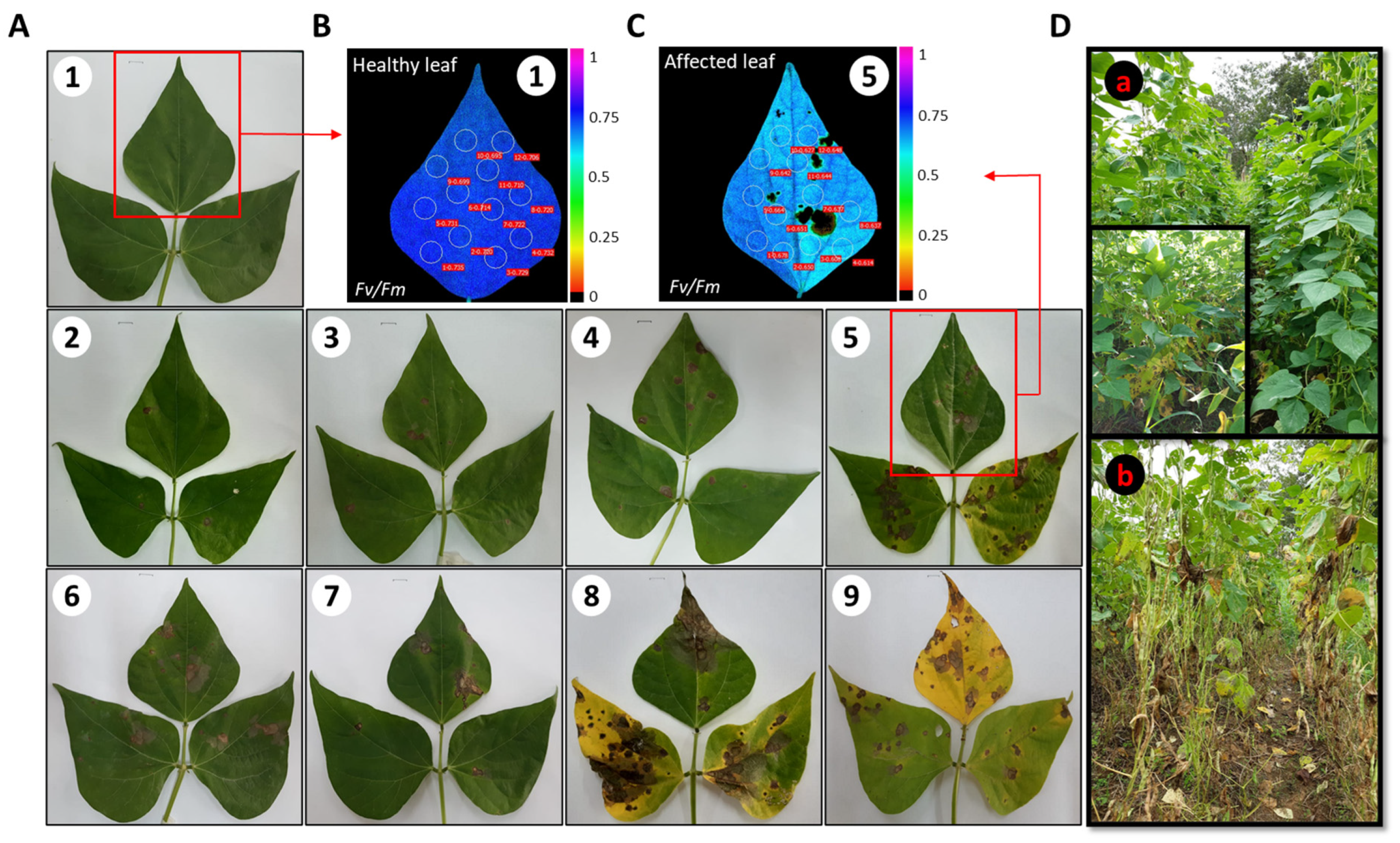
2.1. Severity of Web Blight on Common Bean Leaves
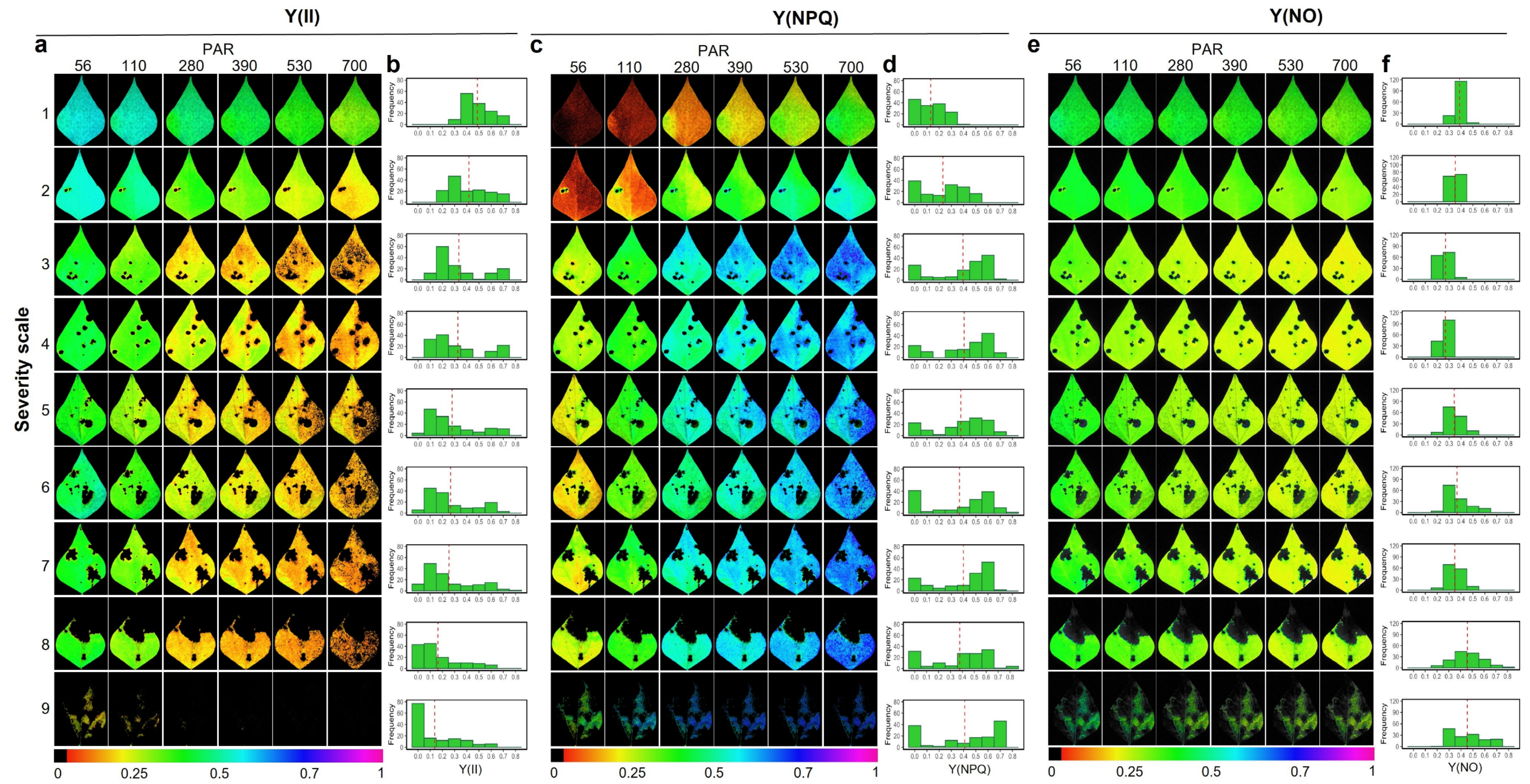
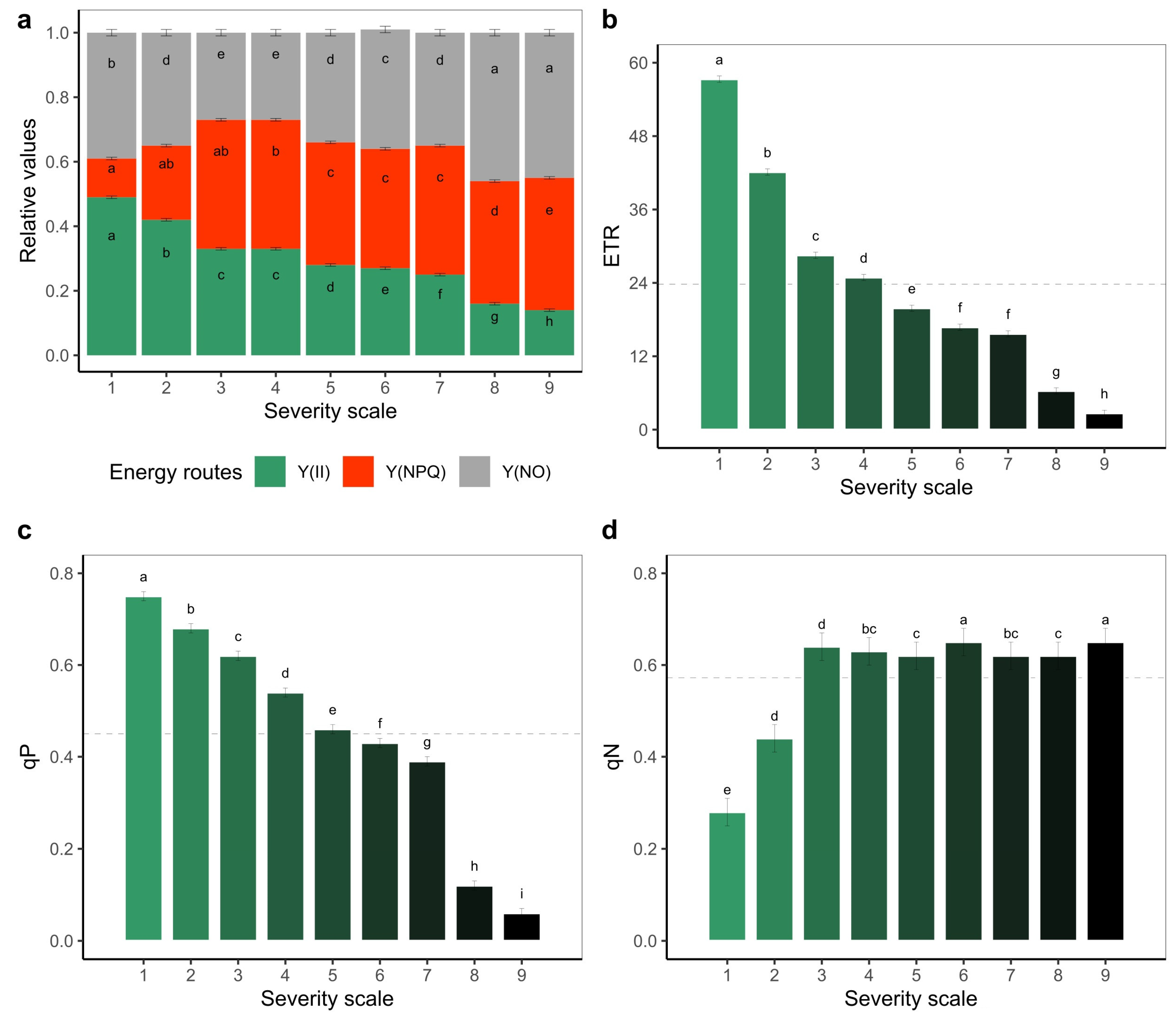
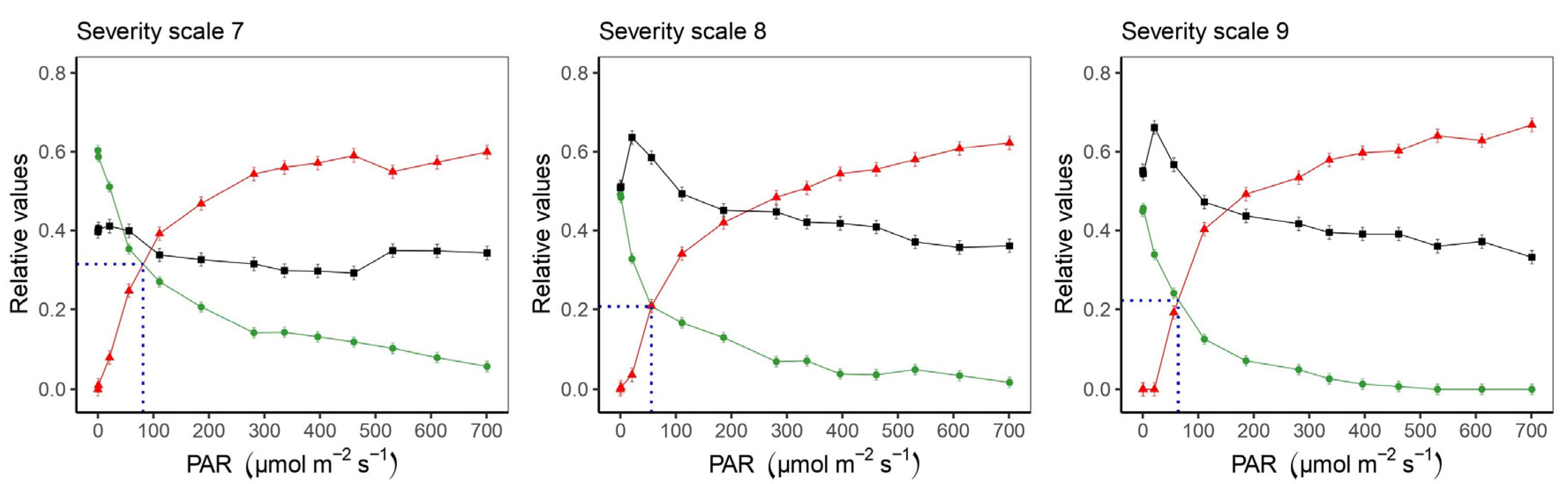
2.2. Response of the Photosynthetic Apparatus to the Incidence of Web Blight
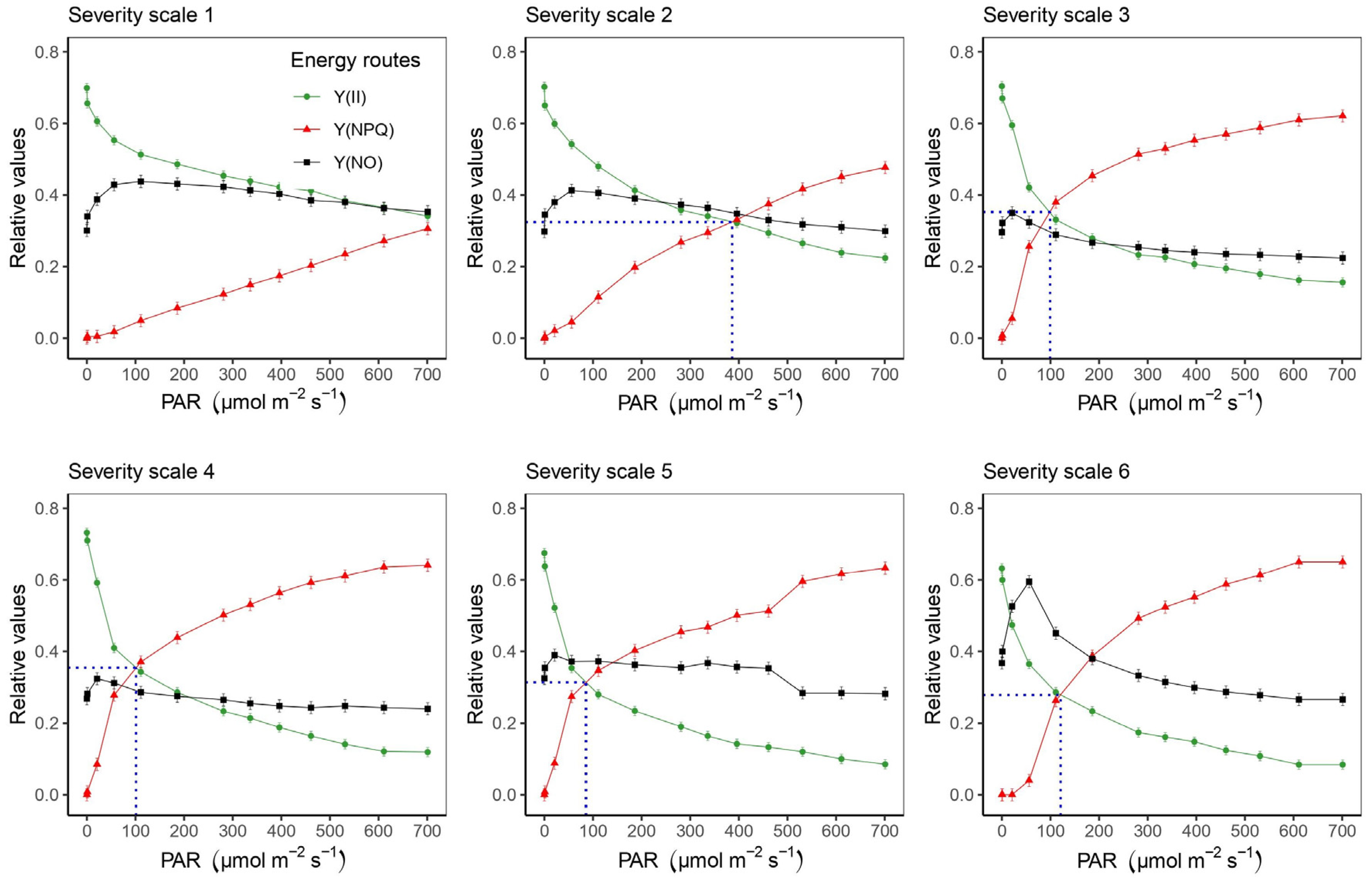
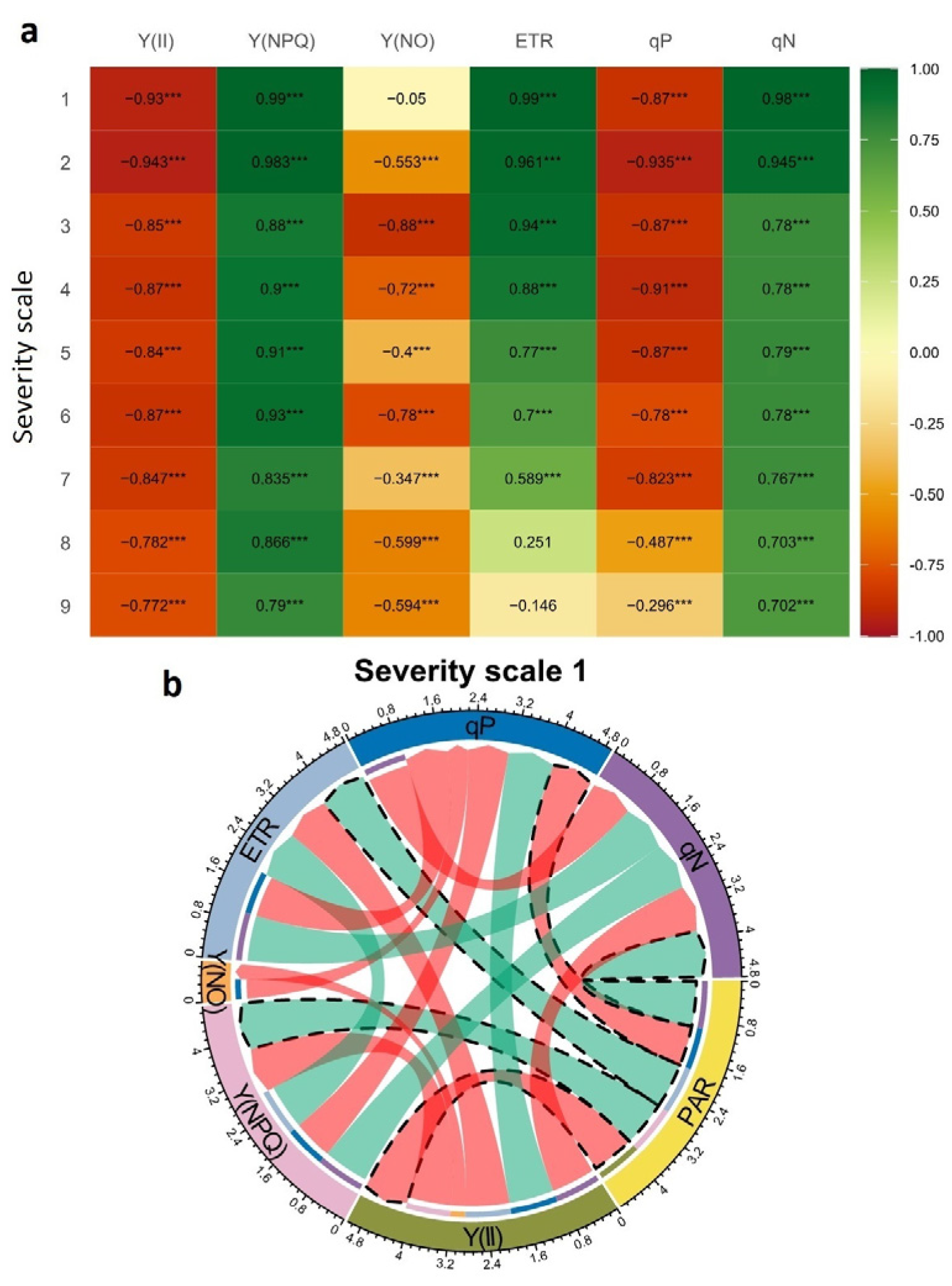
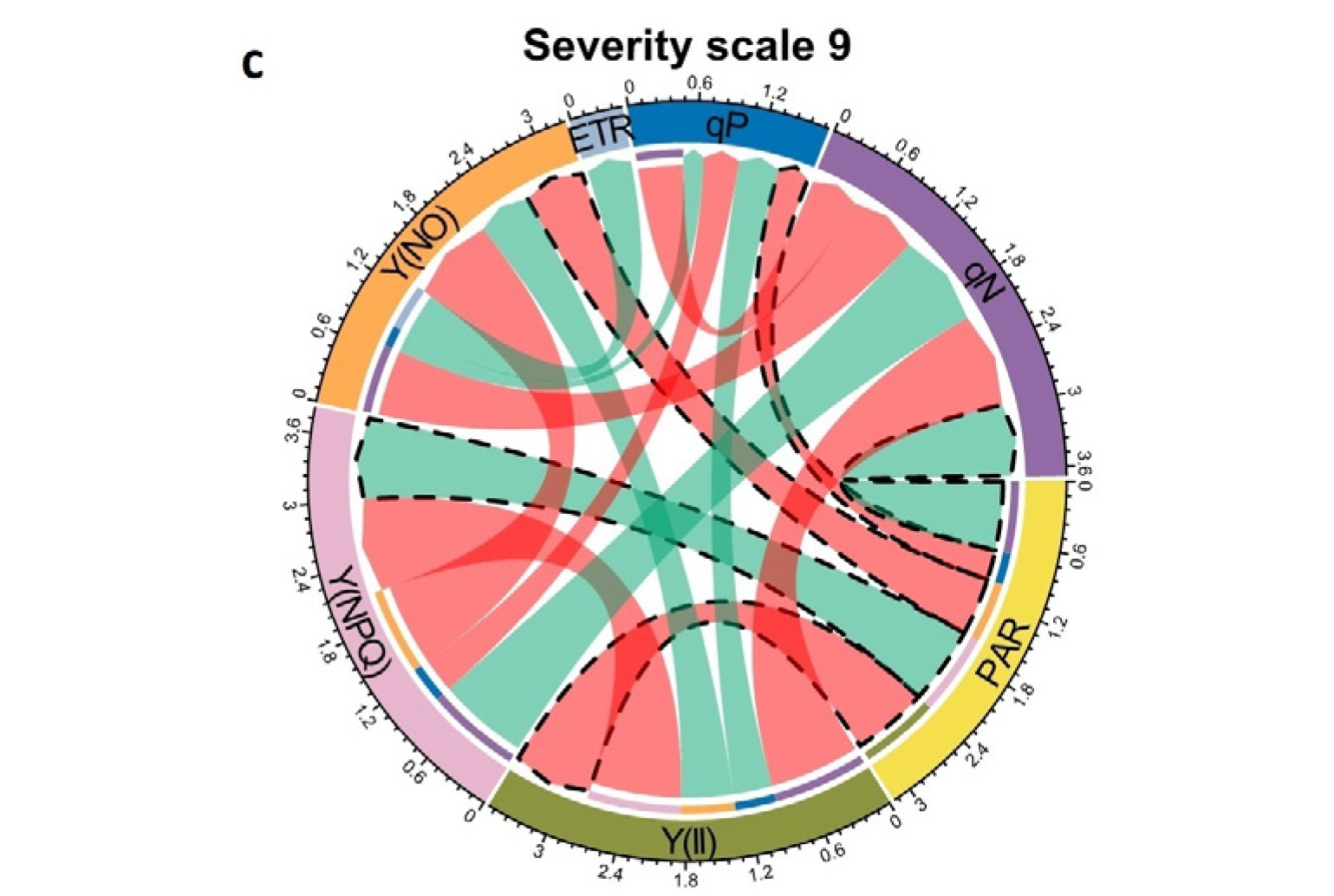
2.3. Correlation Coefficients between the Increase in Photosynthetically Active Radiation (PAR) and Physiological Variables at Different Severity Scales of Web Blight
3. Discussion
3.1. Quantifying of Web Blight Incidence Using Fv/Fm Chlorophyll Fluorescence Imaging
3.2. Response of the Photosynthetic Apparatus When the Severity Level of Web Blight in Leaves Increases
3.3. Relationships between Photosynthetically Active Radiation (PAR) and Photosynthetic Variables at Different Severity Scale Scores of Web Blight
4. Materials and Methods
4.1. Experimental Site and Meteorological Conditions
4.2. Plant Material and Experimental Design
4.3. Visual Assessment of the Degree of Incidence and Severity of Web Blight
4.4. Determination of Chlorophyll Fluorescence (Chla) Parameters in Leaves Affected by Web Blight
4.5. Quantification of the Incidence of Web Blight on the Functioning of the Photosynthetic Apparatus
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Broughton, W.J.; Hernández, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.)—Model food legumes. Plant Soil 2003, 252, 55–128. Available online: https://link.springer.com/article/10.1023/A:1024146710611 (accessed on 29 August 2022). [CrossRef]
- Beebe, S. Common bean breeding in the tropics. Plant Breed. Rev. 2012, 36, 357–426. [Google Scholar]
- Beebe, S.E.; Rao, I.M.; Blair, M.W.; Acosta-Gallegos, J.A. Phenotyping common beans for adaptation to drought. Front. Physiol. 2013, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Polania, J.; Poschenrieder, C.; Rao, I.; Beebe, S. Root traits and their potential links to plant ideotypes to improve drought resistance in common bean. Theor. Exp. Plant Physiol. 2017, 29, 143–154. Available online: https://link.springer.com/article/10.1007/s40626-017-0090-1 (accessed on 29 August 2022). [CrossRef] [PubMed]
- Suárez, J.C.; Polanía, J.A.; Contreras, A.T.; Rodríguez, L.; Machado, L.; Ordoñez, C.; Beebe, S.; Rao, I.M. Adaptation of common bean lines to high temperature conditions: Genotypic differences in phenological and agronomic performance. Euphytica 2020, 216, 1–20. [Google Scholar] [CrossRef]
- Diaz, S.; Ariza-Suarez, D.; Ramdeen, R.; Aparicio, J.; Arunachalam, N.; Hernandez, C.; Diaz, H.; Ruiz, H.; Piepho, H.-P.; Raatz, B. Genetic Architecture and Genomic Prediction of Cooking Time in Common Bean (Phaseolus vulgaris L.). Front. Plant Sci. 2021, 11, 2257. [Google Scholar] [CrossRef] [PubMed]
- Diaz, S.; Ariza-Suarez, D.; Izquierdo, P.; Lobaton, J.D.; de la Hoz, J.F.; Acevedo, F.; Duitama, J.; Guerrero, A.F.; Cajiao, C.; Mayor, V.; et al. Genetic mapping for agronomic traits in a MAGIC population of common bean (Phaseolus vulgaris L.) under drought conditions. BMC Genom. 2020, 21, 1–20. Available online: https://bmcgenomics.biomedcentral.com/articles/10.1186/s12864-020-07213-6 (accessed on 29 August 2022). [CrossRef] [PubMed]
- Contreras-Medina, L.M.; Osornio-Rios, R.A.; Torres-Pacheco, I.; Romero-Troncoso, R.d.J.; Guevara-González, R.G.; Millan-Almaraz, J.R. Smart sensor for real-time quantification of common symptoms present in unhealthy plants. Sensors 2012, 12, 784–805. Available online: https://www.mdpi.com/1424-8220/12/1/784/htm (accessed on 7 April 2022). [CrossRef] [PubMed]
- Bassi, D.; Briñez, B.; Rosa, J.S.; Oblessuc, P.R.; De Almeida, C.P.; Nucci, S.M.; da Silva, L.C.D.; Chiorato, A.F.; Vianello, R.P.; Camargo, L.E.A.; et al. Linkage and mapping of quantitative trait loci associated with angular leaf spot and powdery mildew resistance in common beans. Genet. Mol. Biol. 2017, 40, 109–122. Available online: http://www.scielo.br/j/gmb/a/R6qcbkCDtTFQPKYJYRwQ6rk/?lang=en&format=html (accessed on 7 April 2022). [CrossRef] [PubMed]
- Bock, C.H.; Parker, P.E.; Cook, A.Z.; Gottwald, T.R. Characteristics of the perception of different severity measures of citrus canker and the relationships between the various symptom types. Plant Dis. 2008, 92, 927–939. Available online: https://apsjournals.apsnet.org/doi/abs/10.1094/PDIS-92-6-0927 (accessed on 7 April 2022). [CrossRef] [PubMed]
- van Schoonhoven, A.; Pastor Corrales, M.A. Sistema Estándar para la Evaluación de Germoplasma de Fríjol; Centro Internacional de Agricultura Tropical: Cali, Colombia, 1987; 56p. [Google Scholar]
- Godoy-Lutz, G.; Kuninaga, S.; Steadman, J.R.; Powers, K. Phylogenetic analysis of Rhizoctonia solani subgroups associated with web blight symptoms on common bean based on ITS-5.8S rDNA. J. Gen. Plant Pathol. 2008, 74, 32–40. Available online: https://link.springer.com/article/10.1007/s10327-007-0060-6 (accessed on 29 August 2022). [CrossRef]
- Beaver, J.; Rosas, J.; Myers, J.; Acosta, J.; Kelly, J.; Nchimbi-Msolla, S.; Misangu, R.; Bokosi, J.; Temple, S.; Arnaud-Santana, E.; et al. Contributions of the Bean/Cowpea CRSP to cultivar and germplasm development in common bean. Field Crops Res. 2003, 82, 87–102. [Google Scholar] [CrossRef]
- Beaver, J.S.; Zapata, M.; Alameda, M.; Porch, T.G.; Rosas, J.C. Registration of PR0401-259 and PR0650-31 Dry bean germplasm lines. J. Plant Regist. 2012, 6, 81–84. Available online: https://onlinelibrary.wiley.com/doi/full/10.3198/jpr2011.05.0283crg (accessed on 29 August 2022). [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1364-3703.2011.00783.x (accessed on 29 August 2022). [CrossRef] [PubMed]
- Das, K.; Prasanna, R.; Saxena, A.K. Rhizobia: A potential biocontrol agent for soilborne fungal pathogens. Folia Microbiol. 2017, 62, 425–435. Available online: https://link.springer.com/article/10.1007/s12223-017-0513-z (accessed on 29 August 2022). [CrossRef] [PubMed]
- Schmidt, T.M.; Thomé, A.H.E.; Sperotto, R.A.; Granada, C.E. Effect of rhizobia inoculation on the development of soil-borne pathogens infecting common bean plants. Eur. J. Plant Pathol. 2019, 153, 687–694. Available online: https://link.springer.com/article/10.1007/s10658-018-1600-y (accessed on 29 August 2022). [CrossRef]
- Huber, D.M.; Haneklaus, S. Managing nutrition to control plant disease. Landbauforsch Volkenrode 2007, 57, 313–320. [Google Scholar]
- Mayo-Prieto, S.; Rodríguez-González, Á.; Lorenzana, A.; Gutiérrez, S.; Casquero, P.A. Influence of substrates in the development of bean and in pathogenicity of rhizoctonia solani jg kühn. Agronomy 2020, 10, 707. [Google Scholar] [CrossRef]
- Kheyri, F.; Taheri, P. The role of biological and chemical inducers in activating bean defense responses against Rhizoctonia solani. Physiol. Mol. Plant Pathol. 2021, 116, 101718. [Google Scholar] [CrossRef]
- Beaver, J.S.; Martínez Figueroa, H.; Godoy Lutz, G.; Estévez de Jensen, C.; Porch, T.G.; Rosas, J.C. Breeding for resistance and integrated management of web blight in common bean. Crop Sci. 2022, 62, 20–35. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/csc2.20658 (accessed on 28 August 2022). [CrossRef]
- Costa-Coelho, G.R.; Café Filho, A.C.; Lobo, M. A comparison of web blight epidemics on common bean cultivars with different growth habits. Crop Prot. 2014, 55, 16–20. [Google Scholar] [CrossRef]
- De Almeida, C.P.; Paulino, J.F.D.C.; Barbosa, C.C.F.; De Moraes Cunha Goncalves, G.; Fritsche-Neto, R.; Carbonell, S.A.M.; Chiorato, A.F.; Benchimol-Reis, L.L. Genome-wide association mapping reveals race-specific SNP markers associated with anthracnose resistance in carioca common beans. PLoS ONE 2021, 16, e0251745. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0251745 (accessed on 7 April 2022). [CrossRef]
- Tung, J.; Goodwin, P.H.; Hsiang, T. Chlorophyll fluorescence for quantification of fungal foliar infection and assessment of the effectiveness of an induced systemic resistance activator. Eur. J. Plant Pathol. 2013, 136, 301–315. Available online: https://link.springer.com/article/10.1007/s10658-012-0164-5 (accessed on 7 April 2022). [CrossRef]
- Poland, J.A.; Nelson, R.J. In the eye of the beholder: The effect of rater variability and different rating scales on QTL mapping. Phytopathology 2011, 101, 290–298. Available online: https://apsjournals.apsnet.org/doi/10.1094/PHYTO-03-10-0087 (accessed on 29 August 2022). [CrossRef] [PubMed]
- Capucho, A.S.; Zambolim, L.; Duarte, H.S.S.; Vaz, G.R.O. Development and validation of a standard area diagram set to estimate severity of leaf rust in Coffea arabica and C. canephora. Plant Pathol. 2011, 60, 1144–1150. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-3059.2011.02472.x (accessed on 28 August 2022). [CrossRef]
- Rousseau, C.; Belin, E.; Bove, E.; Rousseau, D.; Fabre, F.; Berruyer, R.; Guillaumès, J.; Manceau, C.; Jacques, M.-A.; Boureau, T. High throughput quantitative phenotyping of plant resistance using chlorophyll fluorescence image analysis. Plant Methods 2013, 9, 1–13. Available online: https://link.springer.com/articles/10.1186/1746-4811-9-17 (accessed on 7 April 2022). [CrossRef] [PubMed]
- Bannihatti, R.K.; Sinha, P.; Raju, D.; Das, S.; Mandal, S.N.; Raje, R.S.; Viswanathan, C.; Kumar, S.; Gaikwad, K.; Aggarwal, R. Image based high throughput phenotyping for Fusarium wilt resistance in pigeon pea (Cajanus cajan). Phytoparasitica 2022, 50, 1075–1090. Available online: https://link.springer.com/article/10.1007/s12600-022-00993-5 (accessed on 28 August 2022). [CrossRef]
- Linn, A.I.; Zeller, A.K.; Pfündel, E.E.; Gerhards, R. Features and applications of a field imaging chlorophyll fluorometer to measure stress in agricultural plants. Precis. Agric. 2021, 22, 947–963. Available online: https://link.springer.com/article/10.1007/s11119-020-09767-7 (accessed on 29 August 2022). [CrossRef]
- Suárez, J.C.; Vanegas, J.I.; Contreras, A.T.; Anzola, J.A.; Urban, M.O.; Beebe, S.E.; Rao, I.M. Chlorophyll fluorescence imaging as a tool for evaluating disease resistance of common bean lines in the Western Amazon Region of Colombia. Plants 2022, 11, 1371. Available online: https://www.mdpi.com/2223-7747/11/10/1371/htm (accessed on 29 August 2022). [CrossRef] [PubMed]
- Bilgin, D.D.; Zavala, J.A.; Zhu, J.; Clough, S.J.; Ort, D.R.; Delucia, E.H. Biotic stress globally downregulates photosynthesis genes. Plant Cell Environ. 2010, 33, 1597–1613. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-3040.2010.02167.x (accessed on 7 April 2022). [CrossRef] [PubMed]
- Hu, H.; Sheng, L.; Zhang, G.; Gu, Q.; Zheng, K. Influence of bacterial leaf blight on the photosynthetic characteristics of resistant and susceptible rice. J. Phytopathol. 2018, 166, 547–554. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/jph.12716 (accessed on 7 April 2022). [CrossRef]
- Matouš, K.; Benediktyová, Z.; Berger, S.; Roitsch, T.; Nedbal, L. Case study of combinatorial imaging: What protocol and what chlorophyll fluorescence image to use when visualizing infection of Arabidopsis thaliana by Pseudomonas syringae? Photosynth. Res. 2006, 90, 243–253. Available online: https://link.springer.com/article/10.1007/s11120-006-9120-6 (accessed on 29 August 2022). [CrossRef] [PubMed]
- Mahlein, A.K.; Oerke, E.C.; Steiner, U.; Dehne, H.W. Recent advances in sensing plant diseases for precision crop protection. Eur. J. Plant Pathol. 2012, 133, 197–209. Available online: https://link.springer.com/article/10.1007/s10658-011-9878-z (accessed on 7 April 2022). [CrossRef]
- Rios, J.A.; Aucique-Pérez, C.E.; Debona, D.; Cruz Neto, L.B.M.; Rios, V.S.; Rodrigues, F.A. Changes in leaf gas exchange, chlorophyll a fluorescence and antioxidant metabolism within wheat leaves infected by Bipolaris sorokiniana. Ann. Appl. Biol. 2017, 170, 189–203. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/aab.12328 (accessed on 7 April 2022). [CrossRef]
- Zlatev, Z.; Petrova, Z.; Penchev, E. Changes in the chlorophyll fluorescence of common winter wheat depending on the weed infestation and the date of application of a set of herbicides. Agric. Sci. 2016, 8, 75–83. [Google Scholar] [CrossRef][Green Version]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. Available online: https://www.annualreviews.org/doi/abs/10.1146/annurev.arplant.59.032607.092759 (accessed on 7 April 2022). [CrossRef]
- Rolfe, S.A.; Scholes, J.D. Chlorophyll fluorescence imaging of plant-pathogen interactions. Protoplasma 2010, 247, 163–175. Available online: https://link.springer.com/article/10.1007/s00709-010-0203-z (accessed on 29 August 2022). [CrossRef]
- Scholes, J.D.; Rolfe, S.A. Chlorophyll fluorescence imaging as tool for understanding the impact of fungal diseases on plant performance: A phenomics perspective. Funct. Plant Biol. 2009, 36, 880–892. Available online: https://www.publish.csiro.au/fp/FP09145 (accessed on 29 August 2022). [CrossRef]
- Gorbe, E.; Calatayud, A. Applications of chlorophyll fluorescence imaging technique in horticultural research: A review. Sci. Hortic. 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. Available online: https://link.springer.com/article/10.1023/B:PRES.0000015391.99477.0d (accessed on 28 September 2021). [CrossRef]
- Moustakas, M.; Calatayud, Á.; Guidi, L. Editorial: Chlorophyll fluorescence imaging analysis in biotic and abiotic stress. Front. Plant Sci. 2021, 12, 615. [Google Scholar] [CrossRef] [PubMed]
- Chaerle, L.; Leinonen, I.; Jones, H.G.; Van Der Straeten, D. Monitoring and screening plant populations with combined thermal and chlorophyll fluorescence imaging. J. Exp. Bot. 2007, 58, 773–784. Available online: https://academic.oup.com/jxb/article/58/4/773/430442 (accessed on 7 April 2022). [CrossRef]
- Naumann, J.C.; Young, D.R.; Anderson, J.E. Leaf chlorophyll fluorescence, reflectance, and physiological response to freshwater and saltwater flooding in the evergreen shrub, Myrica cerifera. Environ. Exp. Bot. 2008, 63, 402–409. [Google Scholar] [CrossRef]
- Sánchez-Moreiras, A.M.; Graña, E.; Reigosa, M.J.; Araniti, F. Imaging of chlorophyll a fluorescence in natural compound-induced stress detection. Front. Plant Sci. 2020, 11, 583590. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Buschmann, C.; Lichtenthaler, H.K. Leaf chlorophyll fluorescence corrected for re-absorption by means of absorption and reflectance measurements. J. Plant Physiol. 1998, 152, 283–296. [Google Scholar] [CrossRef]
- Kuckenberg, J.; Tartachnyk, I.; Noga, G. Temporal and spatial changes of chlorophyll fluorescence as a basis for early and precise detection of leaf rust and powdery mildew infections in wheat leaves. Precis. Agric. 2009, 10, 34–44. Available online: https://link.springer.com/article/10.1007/s11119-008-9082-0 (accessed on 29 August 2022). [CrossRef]
- Yordanov, I.; Tsonev, T.; Goltsev, V.; Kruleva, L.; Velikova, V. Interactive effect of water deficit and high temperature on photosynthesis of sunflower and maize plants 1. Changes in parameters of chlorophyll fluorescence induction kinetics and fluorescence quenching. Photosynthetica 1997, 33, 391–402. Available online: https://www.researchgate.net/profile/Vasilij-Goltsev/publication/230737106_Interactive_effect_of_water_deficit_and_high_temperature_on_photosynthesis_of_sunflower_and_maize_plants_1_Changes_in_parameters_of_chlorophyll_fluorescence_induction_kinetics_and_ (accessed on 7 April 2022).
- Zhou, J.; Yang, L.; Hao, F.; You, Y. Photosynthesis and chlorophyll-fluorescence of Magnolia grandiflora seedlings under low temperature stress. Acta Bot. Boreali-Occident. Sin. 2009, 29, 136–142. [Google Scholar]
- Janka, E.; Körner, O.; Rosenqvist, E.; Ottosen, C.O. High temperature stress monitoring and detection using chlorophyll a fluorescence and infrared thermography in chrysanthemum (Dendranthema grandiflora). Plant Physiol. Biochem. 2013, 67, 87–94. [Google Scholar] [CrossRef]
- Cen, H.; Weng, H.; Yao, J.; He, M.; Lv, J.; Hua, S.; Li, H.-Y.; He, Y. Chlorophyll fluorescence imaging uncovers photosynthetic fingerprint of citrus Huanglongbing. Front. Plant Sci. 2017, 8, 1509. [Google Scholar] [CrossRef]
- Govindjee. Chlorophyll a Fluorescence: A Bit of Basics and History. In Chlorophyll a Fluorescence; Springer: Dordrecht, The Netherlands, 2007; pp. 1–41. Available online: https://link.springer.com/chapter/10.1007/978-1-4020-3218-9_1 (accessed on 29 August 2022).
- Babani, F.; Lichtenthaler, H.K. Light-induced and age-dependent development of chloroplasts in etiolated barley leaves as visualized by determination of photosynthetic pigments, CO2 assimilation rates and different kinds of chlorophyll fluorescence ratios. J. Plant Physiol. 1996, 148, 555–566. [Google Scholar] [CrossRef]
- Roháček, K.; Soukupová, J.; Barták, M. Chlorophyll fluorescence: A wonderful tool to study plant physiology and plant stress. In Plant Cell Compartments-Selected Topics; Research Signpost: Kerala, India, 2008; pp. 41–104. Available online: http://www.umbr.cas.cz/~rohacek/Publikace/RevChlFsemifin.pdf (accessed on 29 August 2022).
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. Available online: https://link.springer.com/article/10.1007/s11120-016-0318-y (accessed on 7 April 2022). [CrossRef] [PubMed]
- Aucique-Perez, C.E.; Daza, E.S.; Ávila-Diazgranados, R.A.; Romero, H.M. Chlorophyll a fluorescence and leaf temperature are early indicators of oil palm diseases. Sci. Agric. 2020, 77, e20180106. Available online: https://doi.org/10.1590/1678-992X-2018-0106 (accessed on 7 April 2022). [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef]
- Dong, X.; Wang, M.; Ling, N.; Shen, Q.; Guo, S. Potential role of photosynthesis-related factors in banana metabolism and defense against Fusarium. oxysporum f. sp. cubense. Environ. Exp. Bot. 2016, 129, 4–12. [Google Scholar] [CrossRef]
- de Ribou, S.D.B.; Douam, F.; Hamant, O.; Frohlich, M.W.; Negrutiu, I. Plant science and agricultural productivity: Why are we hitting the yield ceiling? Plant Sci. 2013, 210, 159–176. [Google Scholar] [CrossRef]
- Pérez-Bueno, M.L.; Pineda, M.; Barón, M. Phenotyping plant responses to biotic stress by chlorophyll fluorescence imaging. Front. Plant Sci. 2019, 10, 1135. [Google Scholar] [CrossRef] [PubMed]
- Legendre, R.; Basinger, N.T.; van Iersel, M.W. Low-cost chlorophyll fluorescence imaging for stress detection. Sensors 2021, 21, 2055. Available online: https://www.mdpi.com/1424-8220/21/6/2055/htm (accessed on 7 April 2022). [CrossRef] [PubMed]
- Möller, P.A.; Aucique-Pérez, C.E.; Einhardt, A.M.; Lemos, D.C.; Rodrigues, F.Á.; Badel, J.L. Impairment of photosynthetic capacity and hydrogen peroxide removal in castor bean affected by bacterial leaf spot. Physiol. Mol. Plant Pathol. 2020, 111, 101510. [Google Scholar] [CrossRef]
- Casierra-Posada, F. Fotoinhibición: Respuesta fisiológica de los vegetales al estrés por exceso de luz. Rev. Colomb. Cienc. Hortícolas. 2011, 1, 114–123. [Google Scholar] [CrossRef]
- Ruban, A.V. Evolution under the sun: Optimizing light harvesting in photosynthesis. J. Exp. Bot. 2015, 66, 7–23. Available online: https://academic.oup.com/jxb/article/66/1/7/2893399 (accessed on 29 August 2022). [CrossRef]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. Available online: https://academic.oup.com/plphys/article/170/4/1903/6114162 (accessed on 29 August 2022). [CrossRef] [PubMed]
- Berger, S.; Papadopoulos, M.; Schreiber, U.; Kaiser, W.; Roitsch, T. Complex regulation of gene expression, photosynthesis and sugar levels by pathogen infection in tomato. Physiol. Plant 2004, 122, 419–428. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1399-3054.2004.00433.x (accessed on 28 August 2022). [CrossRef]
- Horton, P.; Wentworth, M.; Ruban, A. Control of the light harvesting function of chloroplast membranes: The LHCII-aggregation model for non-photochemical quenching. FEBS Lett. 2005, 579, 4201–4206. [Google Scholar] [CrossRef] [PubMed]
- Pineda, M.; Soukupová, J.; Matouš, K.; Nedbal, L.; Barón, M. Conventional and combinatorial chlorophyll fluorescence imaging of tobamovirus-infected plants. Photosynthetica 2008, 46, 441–451. Available online: https://link.springer.com/article/10.1007/s11099-008-0076-y (accessed on 29 August 2022). [CrossRef]
- Chen, J.; Burke, J.J.; Xin, Z. Chlorophyll fluorescence analysis revealed essential roles of FtsH11 protease in regulation of the adaptive responses of photosynthetic systems to high temperature. BMC Plant Biol. 2018, 18, 1–13. Available online: https://bmcplantbiol.biomedcentral.com/articles/10.1186/s12870-018-1228-2 (accessed on 28 August 2022). [CrossRef] [PubMed]
- Balachandran, S.; Hurry, V.M.; Kelley, S.E.; Osmond, C.B.; Robinson, S.A.; Rohozinski, J.; Seaton, G.G.R.; Sims, D.A. Concepts of plant biotic stress. Some insights into the stress physiology of virus-infected plants, from the perspective of photosynthesis. Physiol. Plant 1997, 100, 203–213. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1399-3054.1997.tb04776.x (accessed on 28 August 2022). [CrossRef]
- Oguchi, R.; Terashima, I.; Chow, W.S. The involvement of dual mechanisms of photoinactivation of photosystem II in Capsicum annuum L. plants. Plant Cell Physiol. 2009, 50, 1815–1825. Available online: https://academic.oup.com/pcp/article/50/10/1815/1851165 (accessed on 29 August 2022). [CrossRef] [PubMed]
- Zavafer, A.; Cheah, M.H.; Hillier, W.; Chow, W.S.; Takahashi, S. Photodamage to the oxygen evolving complex of photosystem II by visible light. Sci. Rep. 2015, 5, 1–8. Available online: https://www.nature.com/articles/srep16363 (accessed on 29 August 2022). [CrossRef] [PubMed]
- Rodríguez-Quiel, E.; Montenegro-Alonso, A.P.; Ureta-Reyas, J.C.; Pitty-Suira, N.; González-Guevara, F.; Muñoz-Fuentes, J. Combate biológico de la mustia hilachosa (Thanatephorus cucumeris) en el frijol en Panamá. Agron. Mesoam. 2012, 23, 13. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=5039795&info=resumen&idioma=ENG (accessed on 29 August 2022). [CrossRef][Green Version]
- Chávez-García, W.R.; Mera-Vera, F.N.; Portalanza, D.; Garcés-Fiallos, F.R. Temporal progress of web blight in three common bean genotypes on the central coast of Ecuador. Bionatura 2022, 7, 1–10. Available online: https://doi.org/10.21931/RB/2022.07.01.35 (accessed on 28 August 2022). [CrossRef]
- Long, S.P.; Humphries, S.; Falkowski, P.G. Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1994, 45, 633–662. Available online: https://www.annualreviews.org/doi/abs/10.1146/annurev.pp.45.060194.003221 (accessed on 29 August 2022). [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. Available online: https://academic.oup.com/jxb/article/64/13/3983/436509 (accessed on 7 April 2022). [CrossRef]
- Aro, E.M.; Virgin, I.; Andersson, B. Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. BBA-Bioenerg. 1993, 1143, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Gortari, F.; Guiamet, J.J.; Graciano, C. Plant–pathogen interactions: Leaf physiology alterations in poplars infected with rust (Melampsora medusae). Tree Physiol. 2018, 38, 925–935. Available online: https://academic.oup.com/treephys/article/38/6/925/4821271 (accessed on 7 April 2022). [CrossRef] [PubMed]
- Suárez, J.C.; Polanía, J.A.; Bastidas, A.T.C.; Suárez, L.R.; Beebe, S.; Rao, I.M. Agronomical, phenological and physiological performance of common bean lines in the Amazon region of Colombia. Theor. Exp. Plant Physiol. 2018, 30, 303–320. Available online: https://link.springer.com/article/10.1007/s40626-018-0125-2 (accessed on 23 July 2020).
- Suárez, J.C.; Polanía, J.A.; Anzola, J.A.; Contreras, A.T.; Méndez, D.L.; Vanegas, J.I.; Noriega, J.E.; Rodríguez, L.; Urban, M.O.; Beebe, S.; et al. Influence of nitrogen supply on gas exchange, chlorophyll fluorescence and grain yield of breeding lines of common bean evaluated in the Amazon region of Colombia. Acta Physiol. Plant. 2021, 43, 1–15. Available online: https://link.springer.com/article/10.1007/s11738-021-03233-1 (accessed on 24 January 2022). [CrossRef]
- Suárez, J.C.; Anzola, J.A.; Contreras, A.T.; Salas, D.L.; Vanegas, J.I.; Urban, M.O.; Beebe, S.E.; Rao, I.M. Photosynthetic and grain yield responses to intercropping of two common bean lines with maize under two types of fertilizer application in the Colombian Amazon region. Sci. Hortic. 2022, 301, 1–11. [Google Scholar] [CrossRef]
- Suárez, J.C.; Urban, M.O.; Contreras, A.T.; Noriega, J.E.; Deva, C.; Beebe, S.E.; Polanía, J.A.; Casanoves, F.; Rao, I.M. Water use, leaf cooling and carbon assimilation efficiency of heat resistant common beans evaluated in Western Amazonia. Front. Plant Sci. 2021, 12, 644010. Available online: https://europepmc.org/article/med/34912351 (accessed on 29 September 2022). [CrossRef] [PubMed]
- Galvez, G.; Guzman, P.; Castaño, M. La Mustia Hilachosa; Schuwartz, H., Gálvez, G., Eds.; Problemas de producción del frijol Edo; Centro Int.: Cali, Colombia, 1980; pp. 101–110. [Google Scholar]
- Feller, C.; Bleiholder, H.; Buhr, L.; Hack, H.; Heß, M.; Klose, R.; Meier, U.; Stauß, R.; Boom, T.; van den Weber, E. Phänologische entwicklungsstadien von gemüsepflanzen: II. Fruchtgemüse und hülsenfrüchte. Nachrichtenbl. Deut. Pflanzenschutzd. 1995, 47, 217–232. [Google Scholar]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta-Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components-Calculation of qP and Fv’/Fm’ without measuring Fo’. Photosynth. Res. 1997, 54, 135–142. Available online: https://link.springer.com/article/10.1023/A:1005936823310 (accessed on 29 August 2022). [CrossRef]
- Wijekoon, C.P.; Goodwin, P.H.; Hsiang, T. Quantifying fungal infection of plant leaves by digital image analysis using Scion Image software. J. Microbiol. Methods 2008, 74, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, P.H.; Hsiang, T. Quantification of fungal infection of leaves with digital images and Scion Image software. Methods Mol. Biol. 2010, 638, 125–135. Available online: https://link.springer.com/protocol/10.1007/978-1-60761-611-5_9 (accessed on 29 August 2022). [PubMed]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.Z.J. Visualization of a Correlation Matrix. R package version 0.73. Statistician 2017, 230, 11–15. Available online: https://peerj.com/articles/9945/Supplemental_Data_S10.pdf (accessed on 14 November 2021).
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. circlize implements and enhances circular visualization in R. Bioinformatics 2014, 30, 2811–2812. Available online: https://academic.oup.com/bioinformatics/article/30/19/2811/2422259 (accessed on 14 November 2021). [CrossRef] [PubMed]
- R Development Core Team. R: The R Project for Statistical Computing. In Foundation for Statistical Computing; R Development Core Team: Vienna, Austria, 2021; ISBN 3-900051-07-0. Available online: https://www.r-project.org/ (accessed on 7 April 2022).
- Di Rienzo, J.; Casanoves, F.; Balzarini, M.; González, L. InfoStat Versión 2019. Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. 2019. Available online: http://www.infostat.com.ar (accessed on 7 April 2022).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez, J.C.; Vanegas, J.I.; Anzola, J.A.; Contreras, A.T.; Urban, M.O.; Beebe, S.E.; Rao, I.M. Impact of Web Blight on Photosynthetic Performance of an Elite Common Bean Line in the Western Amazon Region of Colombia. Plants 2022, 11, 3238. https://doi.org/10.3390/plants11233238
Suárez JC, Vanegas JI, Anzola JA, Contreras AT, Urban MO, Beebe SE, Rao IM. Impact of Web Blight on Photosynthetic Performance of an Elite Common Bean Line in the Western Amazon Region of Colombia. Plants. 2022; 11(23):3238. https://doi.org/10.3390/plants11233238
Chicago/Turabian StyleSuárez, Juan Carlos, José Iván Vanegas, José Alexander Anzola, Amara Tatiana Contreras, Milan O. Urban, Stephen E. Beebe, and Idupulapati M. Rao. 2022. "Impact of Web Blight on Photosynthetic Performance of an Elite Common Bean Line in the Western Amazon Region of Colombia" Plants 11, no. 23: 3238. https://doi.org/10.3390/plants11233238
APA StyleSuárez, J. C., Vanegas, J. I., Anzola, J. A., Contreras, A. T., Urban, M. O., Beebe, S. E., & Rao, I. M. (2022). Impact of Web Blight on Photosynthetic Performance of an Elite Common Bean Line in the Western Amazon Region of Colombia. Plants, 11(23), 3238. https://doi.org/10.3390/plants11233238







