Soil Treatment with Nitric Oxide-Releasing Chitosan Nanoparticles Protects the Root System and Promotes the Growth of Soybean Plants under Copper Stress
Abstract
1. Introduction
2. Results
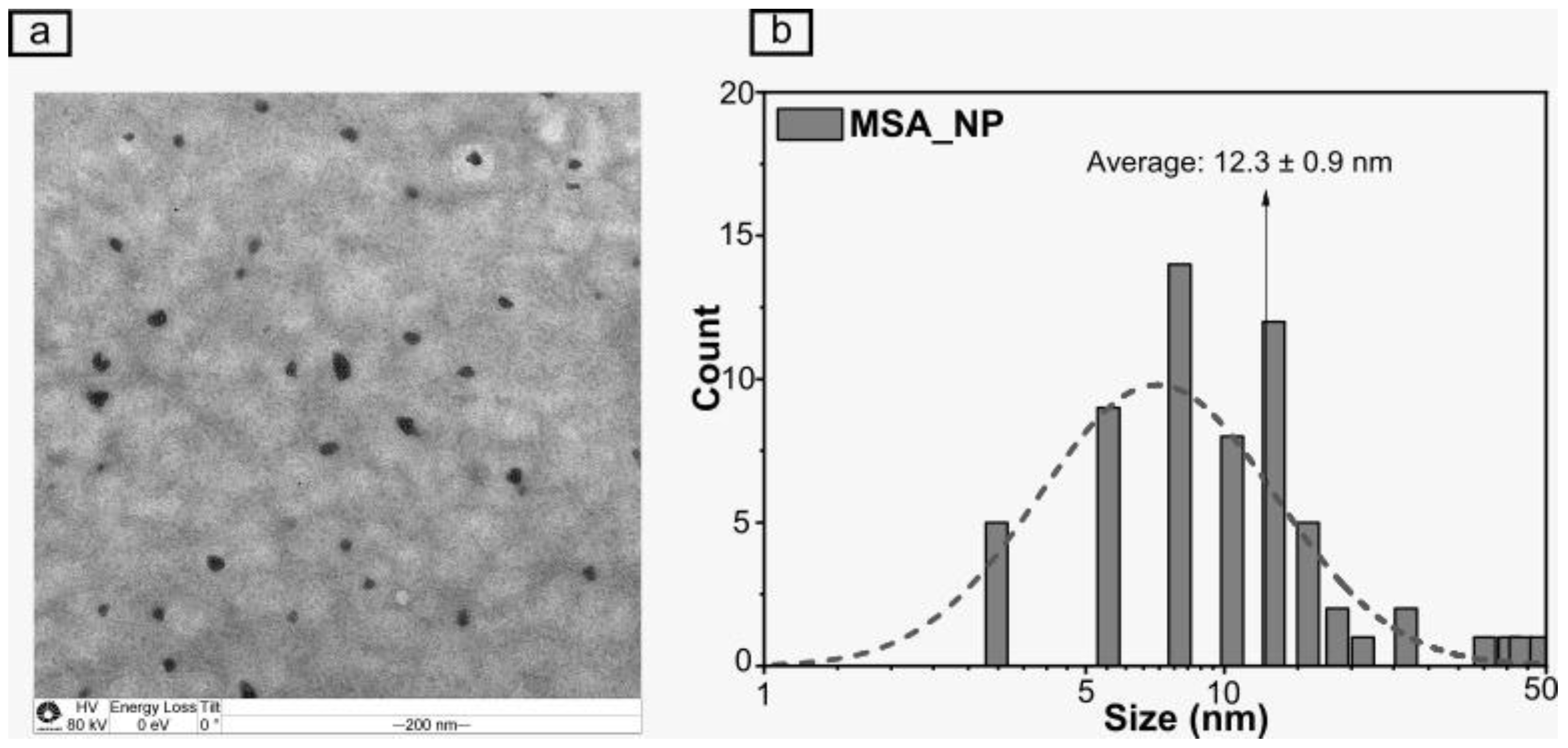
2.1. Characterization of the Nanoparticles and Encapsulation Efficiency of MSA
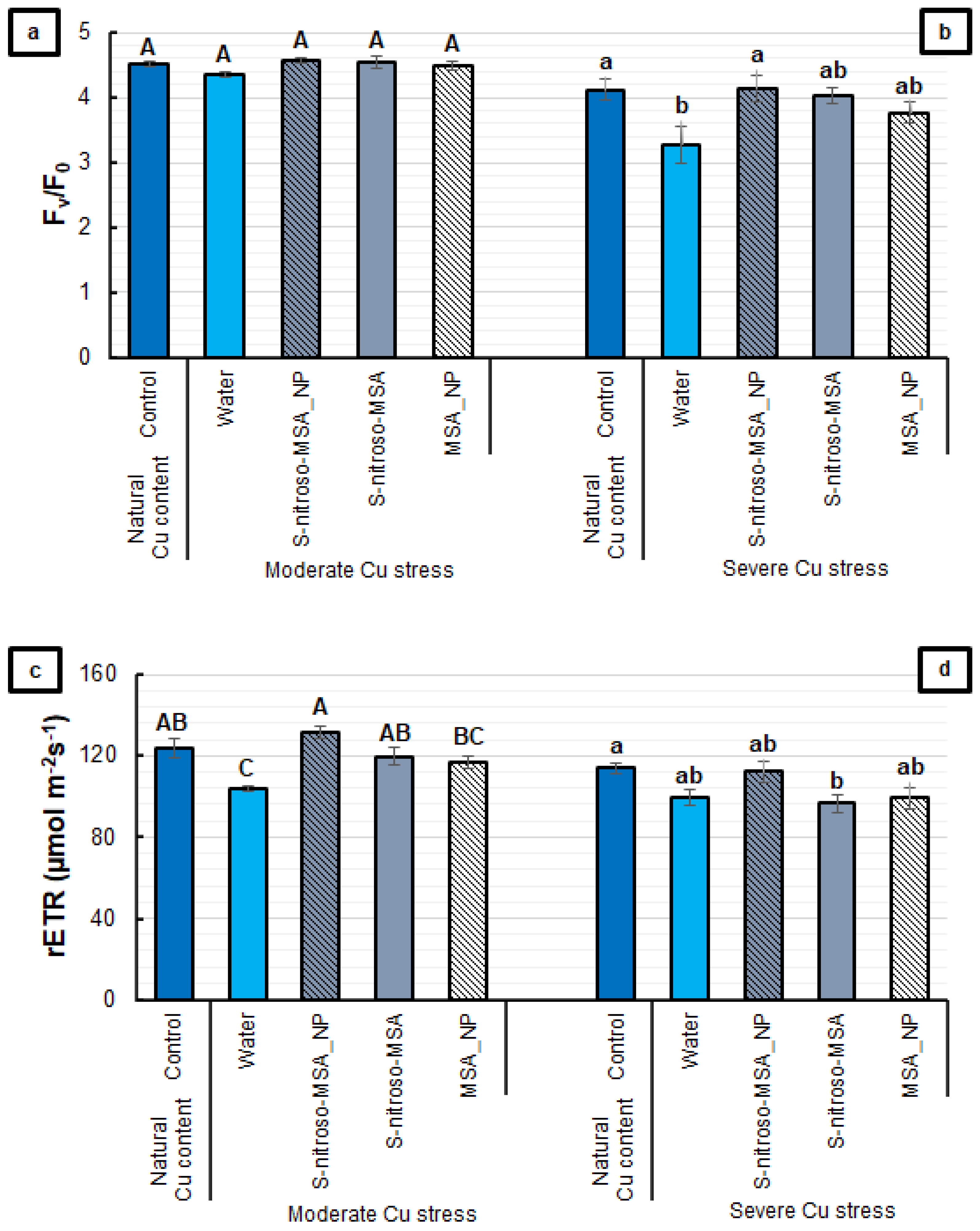
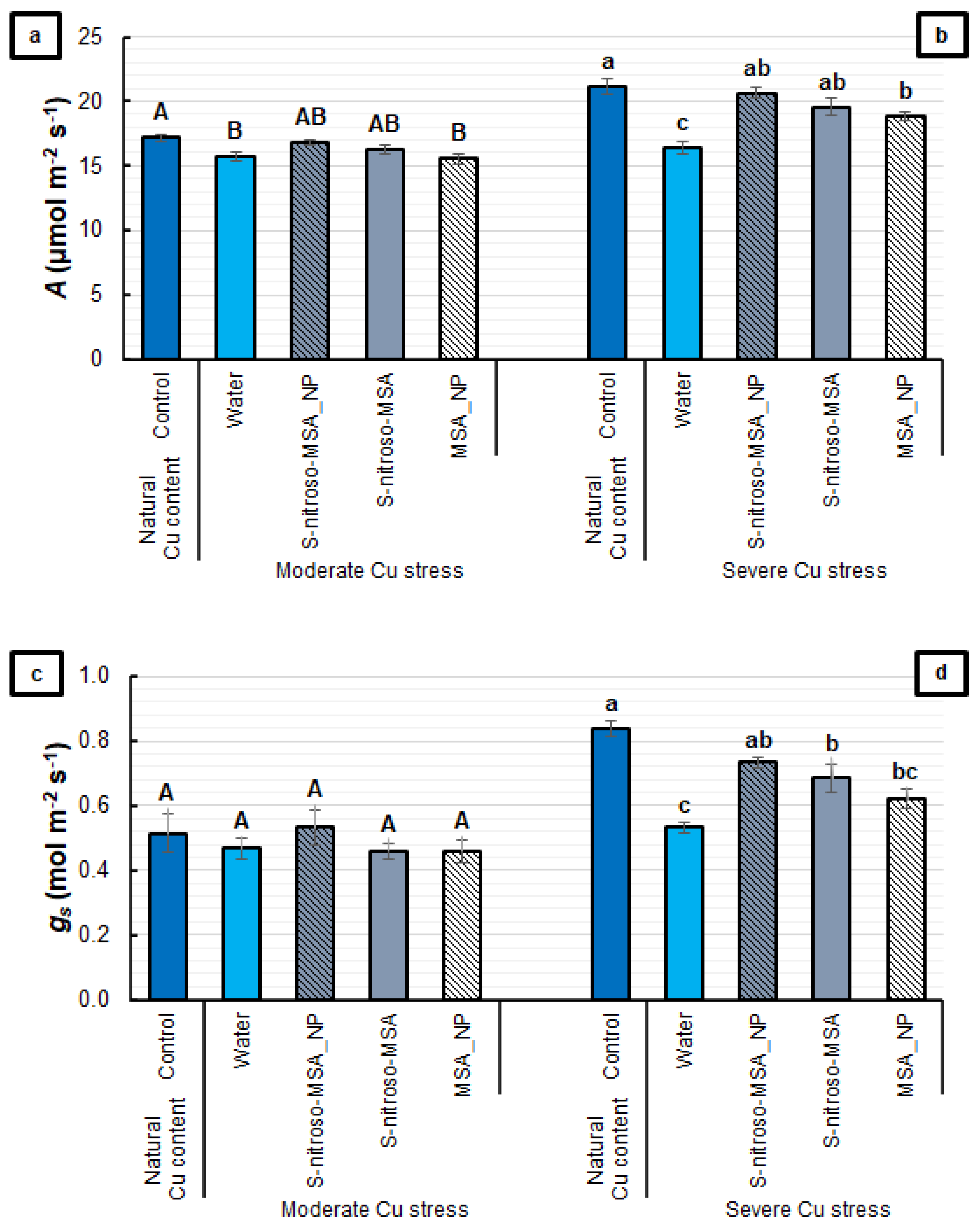
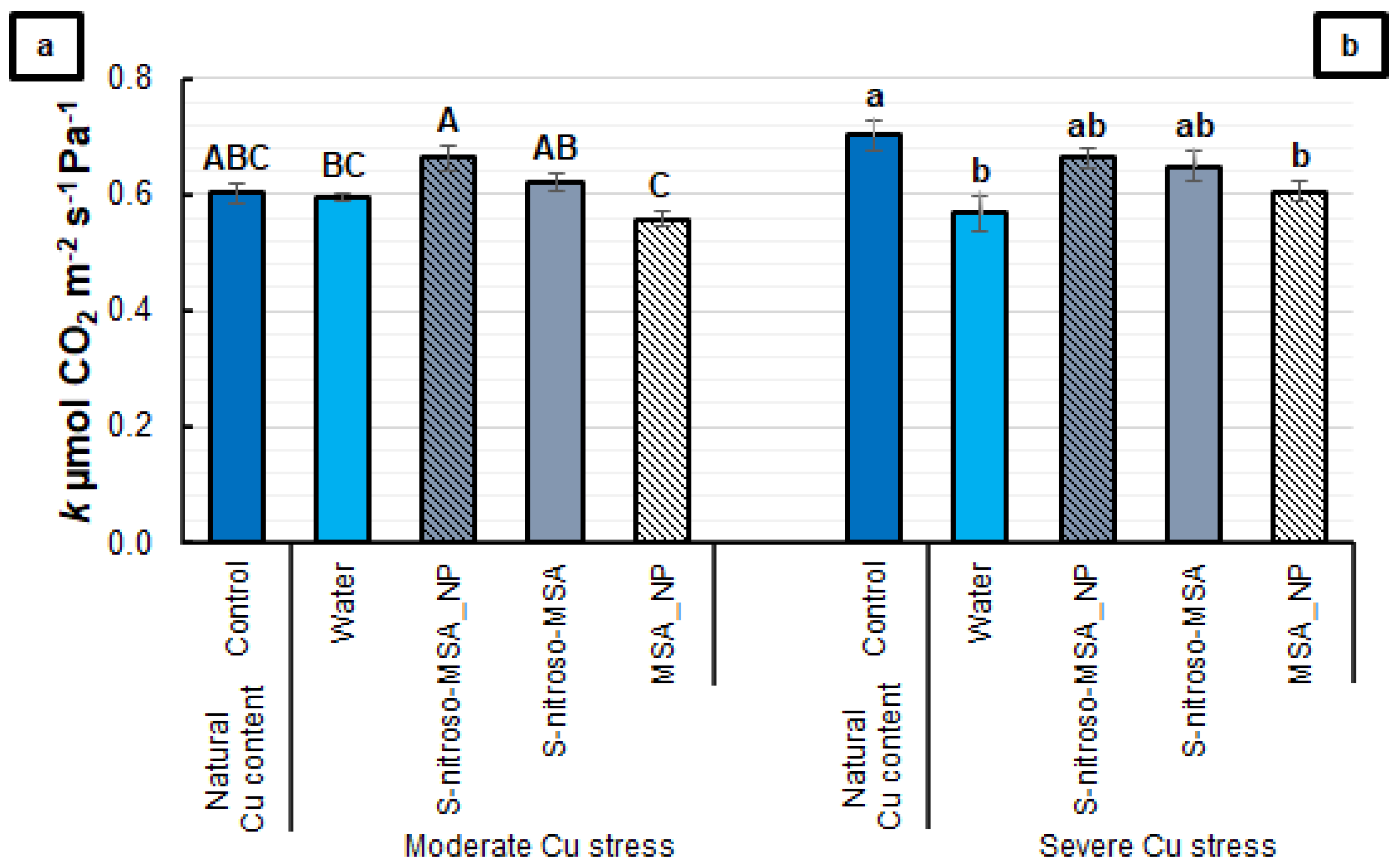
2.2. Morphophysiological Analyses
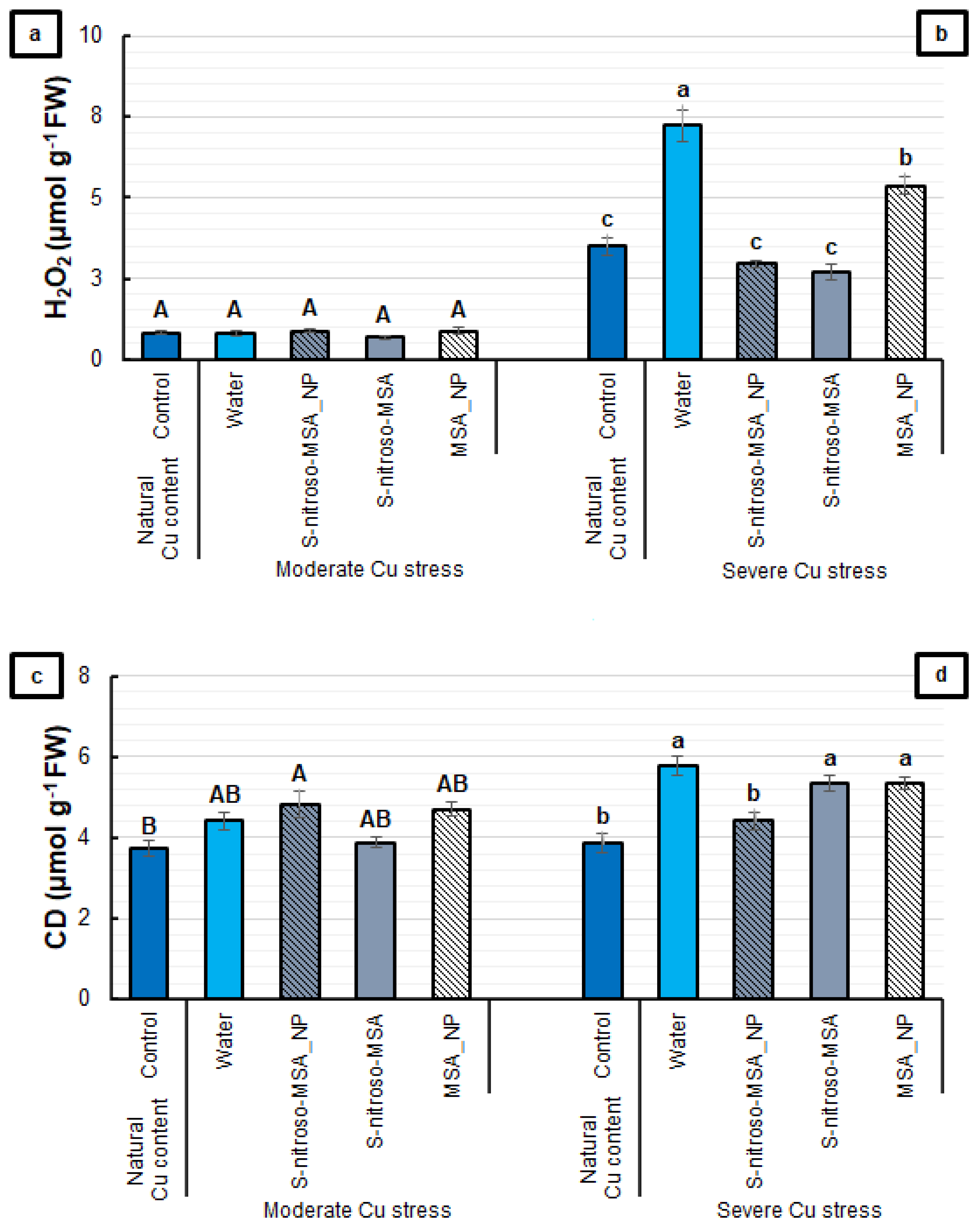
2.3. Biochemical Analyses
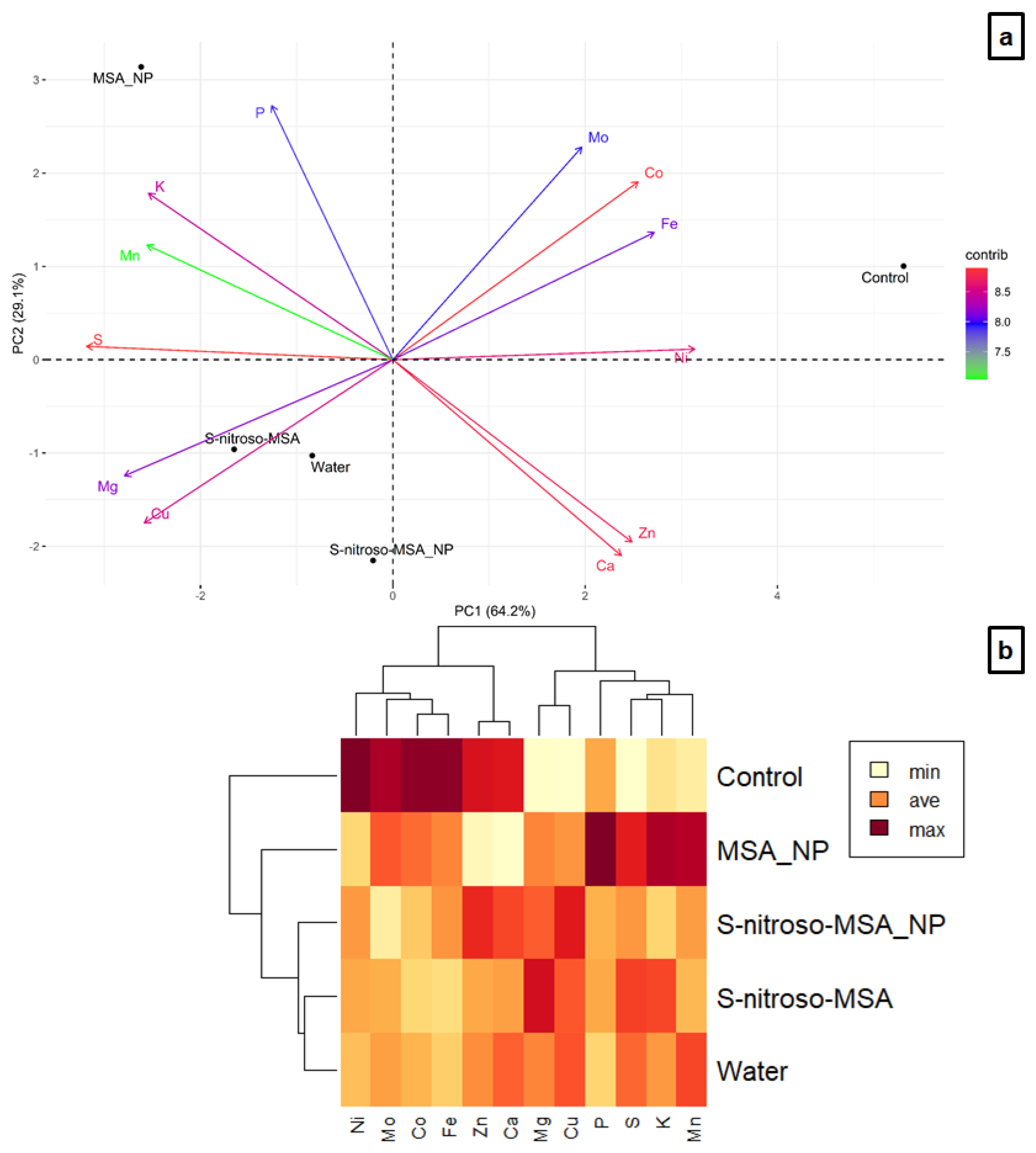
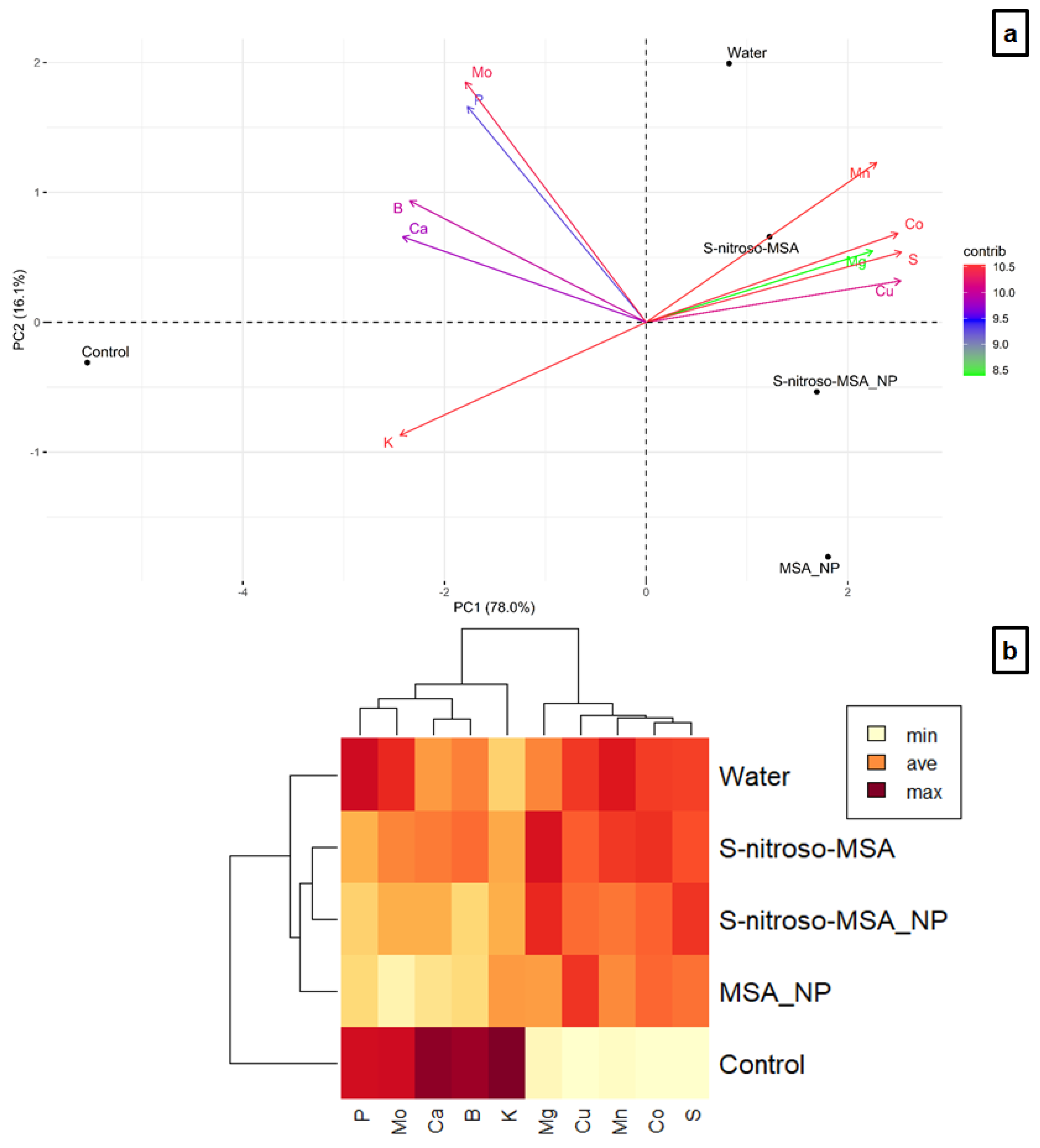
2.4. Nutrient Analysis
3. Discussion
3.1. Characterization of the Nanoparticles and the Encapsulation Efficiency of MSA
3.2. Morphophysiological Analyses
3.3. Biochemical Analyses
3.4. Nutrient Analysis
4. Materials and Methods
4.1. Synthesis of NO-Releasing CS Nanoparticles
4.2. Characterization of the Nanoparticles and the Determination of the Encapsulation Efficiency of MSA in the Nanoparticles
4.3. Soil Contamination with Cu
4.4. Biological Material, Treatments and Sample Harvesting
4.5. Morphophysiological Analyses
4.6. Biochemical Analyses
4.7. Nutrient Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NO | nitric oxide |
| S-nitroso-MSA | S-nitroso-mercaptosuccinic acid |
| RSNOs | S-nitrosothiols |
| CS | chitosan |
| S-nitroso-MSA_NP | treatment with a nanoencapsulated NO donor (S-nitroso-mercaptosuccinic acid) |
| S-nitroso-MSA | treatment with a NO donor (S-nitroso-mercaptosuccinic acid) in the free form |
| MSA_NP | treatment with nanoparticles containing the non-nitrosated mercaptosuccinic acid |
| PSII | photosystem II |
| Fv/F0 | potential activity of photosystem II |
| rETR | relative rate of the linear electron transport of photosystem II |
| A | net photosynthetic rate |
| gs | stomatal conductance |
| k | Instantaneous carboxylation efficiency |
| CD | conjugated dienes |
| SOD | superoxide dismutase |
| POD | peroxidase |
| APX | ascorbate peroxidase |
| CAT | catalase |
| RL | root length |
| RDW | root dry weight |
| SL | shoot length |
| SDW | shoot dry weight |
| LA | leaf area |
| PCA | principal component analysis |
| TEM | transmission electron microscopy |
| DLS | dynamic light scattering |
| PDI | polydispersity index |
| SNP | sodium nitroprusside |
References
- Nasrollahi, Z.; Hashemi, M.; Bameri, S.; Taghvaee, V.M. Environmental pollution, economic growth, population, industrialization, and technology in weak and strong sustainability: Using STIRPAT model. Environ. Dev. Sustain. 2020, 22, 1105–1122. [Google Scholar] [CrossRef]
- Ondrasek, G.; Badovinac, I.J.; Petravić, M.; Macan, J.; Rengel, Z. Humates and chlorides synergistically increase cd phytoaccumulation in strawberry fruits, heightening health risk from cd in human diet. Expo. Health 2022, 14, 393–410. [Google Scholar] [CrossRef]
- Abedi, T.; Gavanji, S.; Mojiri, A. Lead and zinc uptake and toxicity in maize and their management. Plants 2022, 11, 1922. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, X.; Tang, Z.; Yang, Y.; Nie, Z.; Huang, Q. Concentrations and human health implications of heavy metals in market foods from a Chinese coal-mining city. Environ. Toxicol. Pharmacol. 2017, 50, 37–44. [Google Scholar] [CrossRef]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef]
- Husak, V. Copper and copper-containing pesticides: Metabolism, toxicity and oxidative stress. J. Vasyl Stefanyk Precarpathian Natl. Univ. 2015, 2, 38–50. [Google Scholar] [CrossRef]
- Amlal, F.; Drissi, S.; Makroum, K.; Dhassi, K.; Er-rezza, H.; Houssa, A.A. Influence of soil characteristics and leaching rate on copper migration: Column test. Heliyon 2020, 6, e03375. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, G.; Ferreira, P.A.A.; Melo, G.W.; Ceretta, C.A.; Toselli, M. Heavy metals in vineyards and orchard soils. Rev. Bras. Frutic. 2017, 39, e263. [Google Scholar] [CrossRef]
- Kumar, V.; Pandita, S.; Sidhu, G.P.S.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.G.; da Cunha, L.S.; Oliveira, H.C. Dose-dependent dual effect of soil copper on the initial development of Glycine max (L.) merr. cv. BRS 257 seedlings. Bull. Environ. Contam. Toxicol. 2020, 105, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.G.; Lopes-Oliveira, P.J.; Debiasi, T.V.; da Cunha, L.S.; Oliveira, H.C. Regression models to stratify the copper toxicity responses and tolerance mechanisms of Glycine max (L.) Merr. plants. Planta 2021, 253, 43. [Google Scholar] [CrossRef] [PubMed]
- Terrón-Camero, L.C.; Peláez-Vico, A.; Val, C.D.; Sandalio, L.M.; Romero-Puertas, M.C. Role of nitric oxide in plant responses to heavy metal stress: Exogenous application vs. endogenous production. J. Exp. Bot. 2019, 70, 4477–4488. [Google Scholar] [CrossRef] [PubMed]
- Emamverdian, A.; Ding, Y.; Barker, J.; Mokhberdoran, F.; Ramakrishnan, M.; Liu, G.; Li, Y. Nitric oxide ameliorates plant metal toxicity by increasing antioxidant capacity and reducing Pb and Cd translocation. Antioxidants 2021, 10, 1981. [Google Scholar] [CrossRef] [PubMed]
- do Carmo, G.C.; Iastrenski, L.F.; Debiasi, T.V.; da Silva, R.C.; Gomes, D.G.; Pelegrino, M.T.; Bianchini, E.; Stolf-Moreira, R.; Pimenta, J.A.; Seabra, A.B.; et al. Nanoencapsulation improves the protective effects of a nitric oxide donor on drought-stressed Heliocarpus popayanensis seedlings. Ecotoxicol. Environ. Saf. 2021, 225, 112713. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Silveira, N.M.; Ribeiro, R.V.; Pieretti, J.C.; Barroso, J.B.; Corpas, F.J.; Palma, J.M.; Hancock, J.T.; Petřivalský, M.; Gupta, K.J.; et al. Nitric oxide-releasing nanomaterials: From basic research to potential biotechnological applications in agriculture. New Phytol. 2022, 234, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.C.; Gomes, B.C.R.; Pelegrino, M.T.; Seabra, A.B. Nitric oxide-releasing chitosan nanoparticles alleviate the effects of salt stress in maize plants. Nitric Oxide 2016, 61, 10–19. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rathod, S.; Manohara, K.K.; Gireesh, C.; Anantha, M.S.; Sakhare, A.S.; Parmar, B.; Yadav, B.K.; Bandumula, N.; Raihan, F.; et al. Salt stress in plants and mitigation approaches. Plants 2022, 11, 717. [Google Scholar] [CrossRef]
- Lopes-Oliveira, P.J.; Gomes, D.G.; Pelegrino, M.T.; Bianchini, E.; Pimenta, J.A.; Stolf-Moreira, R.; Seabra, A.B.; Oliveira, H.C. Effects of nitric oxide-releasing nanoparticles on neotropical tree seedlings submitted to acclimation under full sun in the nursery. Sci. Rep. 2019, 9, 17371. [Google Scholar] [CrossRef]
- Silveira, N.M.; Seabra, A.B.; Marcos, F.C.C.; Pelegrino, M.T.; Machado, E.C.; Ribeiro, R.V. Encapsulation of S-nitrosoglutathione into chitosan nanoparticles improves drought tolerance of sugarcane plants. Nitric Oxide 2019, 84, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Silveira, N.M.; Prataviera, P.J.C.; Pieretti, J.C.; Seabra, A.B.; Almeida, R.L.; Machado, E.C.; Ribeiro, R.V. Chitosan-encapsulated nitric oxide donors enhance physiological recovery of sugarcane plants after water deficit. Environ. Exp. Bot. 2021, 190, 104593. [Google Scholar] [CrossRef]
- Pande, A.; Mun, B.-G.; Methela, N.J.; Rahim, W.; Lee, D.-S.; Lee, G.-M.; Hong, J.K.; Hussain, A.; Loake, G.; Yun, B.-W. Heavy metal toxicity in plants and the potential NO-releasing novel techniques as the impending mitigation alternatives. Front. Plant. Sci. 2022, 13, 1019647. [Google Scholar] [CrossRef]
- Kumaraswamy, R.V.; Kumari, S.; Choudhary, R.C.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Engineered chitosan-based nanomaterials: Bioactivities, mechanisms and perspectives in plant protection and growth. Int. J. Biol. Macromol. 2018, 113, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Vinceković, M.; Jurić, S.; Harja, M.; Ondrasek, G. Development and characterization of a novel soil amendment based on biomass fly ash encapsulated in calcium alginate microspheres. Int. J. Mol. Sci. 2022, 23, 9984. [Google Scholar] [CrossRef] [PubMed]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef]
- de Oliveira, M.G. S-nitrosothiols as platforms for topical nitric oxide delivery. Basic Clin. Pharmacol. Toxicol. 2016, 119, 49–56. [Google Scholar] [CrossRef]
- Pelegrino, M.T.; Weller, R.B.; Chen, X.; Seabra, A.B. Chitosan nanoparticles for nitric oxide delivery in human skin. Med. Chem. Commun. 2017, 8, 713. [Google Scholar] [CrossRef]
- Pelegrino, M.T.; de Araújo, D.; Seabra, A.B. S-nitrosoglutathione-containing chitosan nanoparticles dispersed in Pluronic F-127 hydrogel: Potential uses in topical applications. J. Drug. Deliv. Sci. Technol. 2018, 43, 211–220. [Google Scholar] [CrossRef]
- Aftab, T.; Khan, M.M.A.; Naeem, M.; Idrees, M.; da Silva, J.A.; Ram, M. Exogenous nitric oxide donor protects Artemisia annua from oxidative stress generated by boron and aluminium toxicity. Ecotoxicol. Environ. Saf. 2012, 80, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liang, X.; Dong, Y.; Xu, L.; Zhang, X.; Kong, J.; Liu, S. Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of perennial ryegrass under cadmium stress. J. Plant. Growth. Regul. 2013, 32, 721–731. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 2018, 255, 79–93. [Google Scholar] [CrossRef]
- Per, T.S.; Masood, A.; Khan, N.A. Nitric oxide improves S-assimilation and GSH production to prevent inhibitory effects of cadmium stress on photosynthesis in mustard (Brassica juncea L.). Nitric Oxide 2017, 68, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Ai, H.; Xu, X.; Chen, K.; Niu, H.; Zhu, H.; Sun, J.; Du, D.; Chen, L. Nitric oxide alleviates toxicity of hexavalent chromium on tall fescue and improves performance of photosystem II. Ecotoxicol. Environ. Saf. 2018, 164, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Mostofa, M.G.; Ahmad, M.Z.; Imtiaz, M.; Mehmood, S.; Adeel, M.; Dai, Z.; Li, Z.; Aziz, O.; Zhang, Y.; et al. Nitric oxide induces rice tolerance to excessive nickel by regulating nickel uptake, reactive oxygen species detoxification and defense-related gene expression. Chemosphere 2018, 191, 23–35. [Google Scholar] [CrossRef]
- Jafarnezhad-Moziraji, Z.; Saeidi-Sar, S.; Dehpour, A.A.; Masoudian, N. Protective effects of exogenous nitric oxide against lead toxicity in lemon balm (Melissa officinalis L.). Appl. Ecol. Environ. Res. 2017, 15, 1605–1621. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, L.; Wang, Q.; Fan, Z.; Kong, J.; Bai, X. Effects of exogenous nitric oxide on photosynthesis, antioxidative ability, and mineral element contents of perennial ryegrass under copper stress. J. Plant. Interact. 2014, 9, 402–411. [Google Scholar] [CrossRef]
- Corpas, F.J.; Chaki, M.; Begara-Morales, J.C.; Valderrama, R.; Sanchez-Calvo, B.; Barroso, J.B. Functional implications of S-nitrosothiols under nitro-oxidative stress induced by abiotic conditions. Adv. Bot. Res. 2016, 77, 79–96. [Google Scholar] [CrossRef]
- Lima, E.S.; Abdalla, D.S.P. Peroxidação lipídica: Mecanismos e avaliação em amostras biológicas. Braz. J. Pharm. Sci. 2001, 37, 293–303. [Google Scholar]
- Sun, C.; Lu, L.; Liu, L.; Liu, W.; Yu, Y.; Liu, X.; Hu, Y.; Jin, C.; Lin, X. Nitrate reductase-mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticum aestivum). New Phytol. 2014, 201, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y. Early generation of nitric oxide contributes to copper tolerance through reducing oxidative stress and cell death in hulless barley roots. J. Plant. Res. 2016, 129, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Sharma, R.; Bhardwaj, R.; Handa, N.; Gautam, V.; Kohli, S.K.; Bali, S.; Kaur, P.; Thukral, A.K.; Arora, S.; Ohri, P.; et al. Responses of phytochelatins and metallothioneins in alleviation of heavy metal stress in plants: An overview. In Plant Metal Interaction: Emerging Remediation Techniques; Ahmad, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 263–283. [Google Scholar]
- Pfaff, A.R.; Beltz, J.; King, E.; Ercal, N. Medicinal thiols: Current status and new perspectives. Mini. Rev. Med. Chem. 2020, 20, 513–529. [Google Scholar] [CrossRef]
- Pathak, J.; Ahmed, H.; Kumari, N.; Pandey, A.; Rajneesh; Sinha, R.P. Role of calcium and potassium in amelioration of environmental stress in plants. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; Roychoudhury, A., Tripathi, D.K., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 535–562. [Google Scholar]
- Ishfaq, M.; Wang, Y.; Yan, M.; Wang, Z.; Wu, L.; Li, C.; Li, X. Physiological essence of magnesium in plants and its widespread deficiency in the farming system of china. Front. Plant. Sci. 2022, 13, 802274. [Google Scholar] [CrossRef]
- Wang, P.G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A.J. Nitric oxide donors: Chemical activities and biological applications. Chem. Rev. 2002, 102, 1091–1134. [Google Scholar] [CrossRef]
- Kohatsu, M.Y.; Pelegrino, M.T.; Monteiro, L.R.; Freire, B.M.; Pereira, R.M.; Fincheira, P.; Rubilar, O.; Tortella, G.R.; Batista, B.L.; de Jesus, T.A.; et al. Comparison of foliar spray and soil irrigation of biogenic CuO nanoparticles (NPs) on elemental uptake and accumulation in lettuce. Environ. Sci. Pollut. Res. 2021, 28, 16350–16367. [Google Scholar] [CrossRef]
- Campos, D.V.B.; Teixeira, P.C. Microelementos. In Manual de Métodos de Análise de Solo; Teixeira, P.C., Donagema, G.K., Fontana, A., Teixeira, W.G.M., Eds.; Embrapa: Brasília, Brazil, 2017; pp. 328–333. [Google Scholar]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Ziaur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef]
- Juliatti, F.C.; Azevedo, L.A.S.; Juliatti, F.C. Strategies of chemical protection for controlling soybean rust. In Soybean: The Basis of Yield, Biomass and Productivity; Kasai, M., Ed.; IntechOpen: London, UK, 2017; pp. 35–62. [Google Scholar]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2021, 24, 1337–1344. [Google Scholar] [CrossRef]
- Boveris, A.; Cadenas, E.; Chance, B. Low-level chemiluminescence of the lipoxygenase reaction. Photobiochem. Photobiophys. 1980, 1, 175–182. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superóxido dismutases. I. occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Peixoto, H.P.P.; Cambraia, J.; San’t ana, R.; Mosquim, P.R.; Moreira, A.M. Aluminium effects on lipid peroxidation and the activities of enzymes of oxidative metabolism in sorghum. Rev. Bras. Fisiol. Veg. 1999, 11, 137–143. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Aebi, H. Methods in enzymology. In Catalase In Vitro; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: Gulf Breeze, FL, USA, 1984; pp. 114–121. [Google Scholar]
- Anderson, M.D.; Prasad, T.K.; Stewart, C.R. Changes in isozyme profiles of catalase, peroxidase and gluthione reductase during acclimation to chilling in mesocotylus of maize seedlings. Plant Physiol. 1995, 109, 1247–1257. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rengel, Z.; Clode, P.L.; Kilburn, M.R.; Guagliardo, P.; Romić, D. Zinc and cadmium mapping by NanoSIMS within the root apex after short-term exposure to metal contamination. Ecotoxicol. Environ. Saf. 2019, 171, 571–578. [Google Scholar] [CrossRef]
- Ondrasek, G.; Clode, P.L.; Kilburn, M.R.; Guagliardo, P.; Romić, D.; Rengel, Z. Zinc and cadmium mapping in the apical shoot and hypocotyl tissues of radish by high-resolution secondary ion mass spectrometry (nanosims) after short-term exposure to metal contamination. Int. J. Environ. Res. Public. Health 2019, 16, 373. [Google Scholar] [CrossRef]
- Paniz, F.P.; Pedron, T.; Freire, B.M.; Torres, D.P.; Silva, F.F.; Batista, B.L. Effective procedures for the determination of As, Cd, Cu, Fe, Hg, Mg, Mn, Ni, Pb, Se, Th, Zn, U and rare earth elements in plants and foodstuffs. Anal. Methods 2018, 10, 4094–4103. [Google Scholar] [CrossRef]
- Savić, R.; Ondrasek, G.; Zemunac, R.; Kovacic, M.B.; Kranjcec, F.; Jokanovic, V.N.; Bezdan, A. Longitudinal distribution of macronutrients in the sediments of Jegricka watercourse in Vojvodina, Serbia. Sci. Total Environ. 2020, 754, 142138. [Google Scholar] [CrossRef]
- R, Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: http://www.R-project.org/ (accessed on 15 August 2022).






| Natural Cu Content | Cu Supplementation (164 mg kg−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Control 1 | Water | S-nitroso-MSA_NP | S-nitroso-MSA | MSA_NP | |||||
| RL (cm) | 29.21 ± 0.83 2 | b 3 | 28.84 ± 0.71 | b | 33.14 ± 0.97 | a | 31.82 ± 0.75 | ab | 28.63 ± 0.67 | b |
| RDW (g) | 0.2073 ± 0.01 | c | 0.2101 ± 0.01 | bc | 0.2461 ± 0.01 | a | 0.2314 ± 0.01 | ab | 0.2440 ± 0.01 | a |
| SL (cm) | 17.51 ± 0.28 | b | 16.97 ± 0.31 | b | 19.02 ± 0.23 | a | 17.26 ± 0.42 | b | 17.36 ± 0.31 | b |
| SDW (g) | 0.4351 ± 0.01 | b | 0.4312 ± 0.01 | b | 0.4798 ± 0.01 | a | 0.4506 ± 0.01 | ab | 0.4502 ± 0.01 | ab |
| LA (cm2) | 143.27 ± 3.02 | ab | 137.13 ± 2.04 | ab | 146.72 ± 2.91 | a | 143.56 ± 3.78 | ab | 134.32 ± 3.32 | b |
| Natural Cu content | Cu supplementation (244 mg kg−1) | |||||||||
| Variables | Control | Water | S-nitroso-MSA_NP | S-nitroso-MSA | MSA_NP | |||||
| RL (cm) | 28.72 ± 0.86 | a | 15.32 ± 0.88 | c | 20.63 ± 0.67 | b | 20.59 ± 1.03 | b | 20.36 ± 0.45 | b |
| RDW (g) | 0.1578 ± 0.01 | a | 0.1134 ± 0.01 | c | 0.1488 ± 0.01 | ab | 0.1418 ± 0.01 | ab | 0.1358 ± 0.01 | b |
| SL (cm) | 17.33 ± 0.37 | a | 14.47 ± 0.41 | c | 16.62 ± 0.18 | ab | 15.64 ± 0.35 | bc | 15.01 ± 0.18 | c |
| SDW (g) | 0.3658 ± 0.01 | a | 0.3286 ± 0.01 | b | 0.3624 ± 0.01 | a | 0.3448 ± 0.01 | ab | 0.3317 ± 0.01 | b |
| LA (cm2) | 116.74 ± 4.90 | a | 95.92 ± 7.51 | b | 110.56 ± 2.41 | ab | 102.13 ± 3.80 | ab | 94.54 ± 4.06 | b |
| Experimental Group | Soil 1 | Formulation |
|---|---|---|
| Control | Natural Cu content | Distilled water |
| Water | Cu supplementation | Distilled water |
| S-nitroso-MSA_NP | Cu supplementation | Chitosan/sodium tripolyphosphate nanoparticles containing S-nitroso-MSA (1 mM) |
| S-nitroso-MSA | Cu supplementation | S-nitroso-MSA in the free form (1 mM) |
| MSA_NP | Cu supplementation | Nanoparticles containing the non-nitrosated MSA (1 mM) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, D.G.; Debiasi, T.V.; Pelegrino, M.T.; Pereira, R.M.; Ondrasek, G.; Batista, B.L.; Seabra, A.B.; Oliveira, H.C. Soil Treatment with Nitric Oxide-Releasing Chitosan Nanoparticles Protects the Root System and Promotes the Growth of Soybean Plants under Copper Stress. Plants 2022, 11, 3245. https://doi.org/10.3390/plants11233245
Gomes DG, Debiasi TV, Pelegrino MT, Pereira RM, Ondrasek G, Batista BL, Seabra AB, Oliveira HC. Soil Treatment with Nitric Oxide-Releasing Chitosan Nanoparticles Protects the Root System and Promotes the Growth of Soybean Plants under Copper Stress. Plants. 2022; 11(23):3245. https://doi.org/10.3390/plants11233245
Chicago/Turabian StyleGomes, Diego G., Tatiane V. Debiasi, Milena T. Pelegrino, Rodrigo M. Pereira, Gabrijel Ondrasek, Bruno L. Batista, Amedea B. Seabra, and Halley C. Oliveira. 2022. "Soil Treatment with Nitric Oxide-Releasing Chitosan Nanoparticles Protects the Root System and Promotes the Growth of Soybean Plants under Copper Stress" Plants 11, no. 23: 3245. https://doi.org/10.3390/plants11233245
APA StyleGomes, D. G., Debiasi, T. V., Pelegrino, M. T., Pereira, R. M., Ondrasek, G., Batista, B. L., Seabra, A. B., & Oliveira, H. C. (2022). Soil Treatment with Nitric Oxide-Releasing Chitosan Nanoparticles Protects the Root System and Promotes the Growth of Soybean Plants under Copper Stress. Plants, 11(23), 3245. https://doi.org/10.3390/plants11233245











