Western Spruce Budworm Effects on Forest Resilience
Abstract
1. Introduction
2. Results
2.1. Stand Conditions, WSBW Defoliation and Other Biotic Damaging Agents
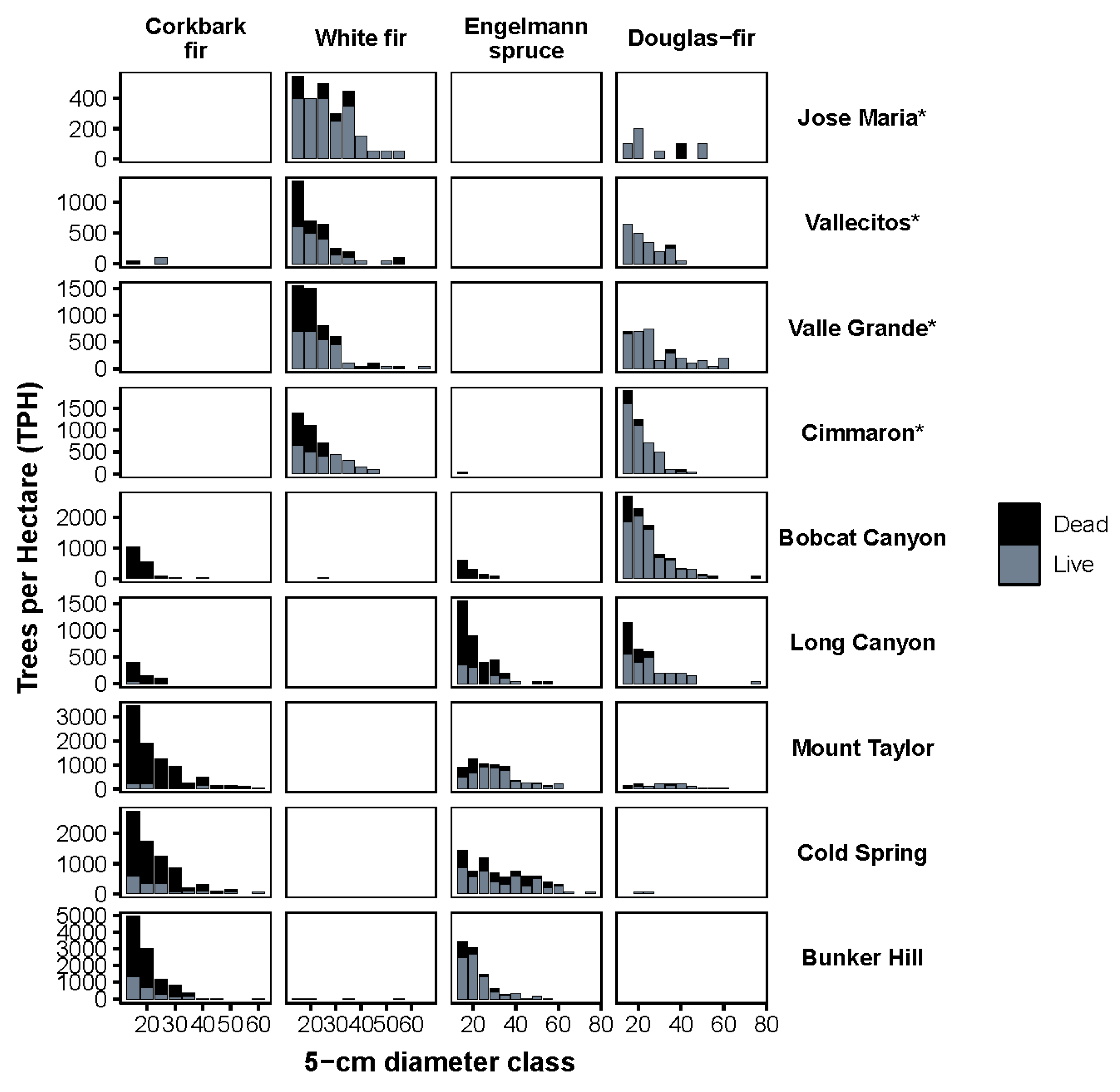
2.2. Overstory Mortality
2.3. Understory Composition, Density and Health
3. Discussion
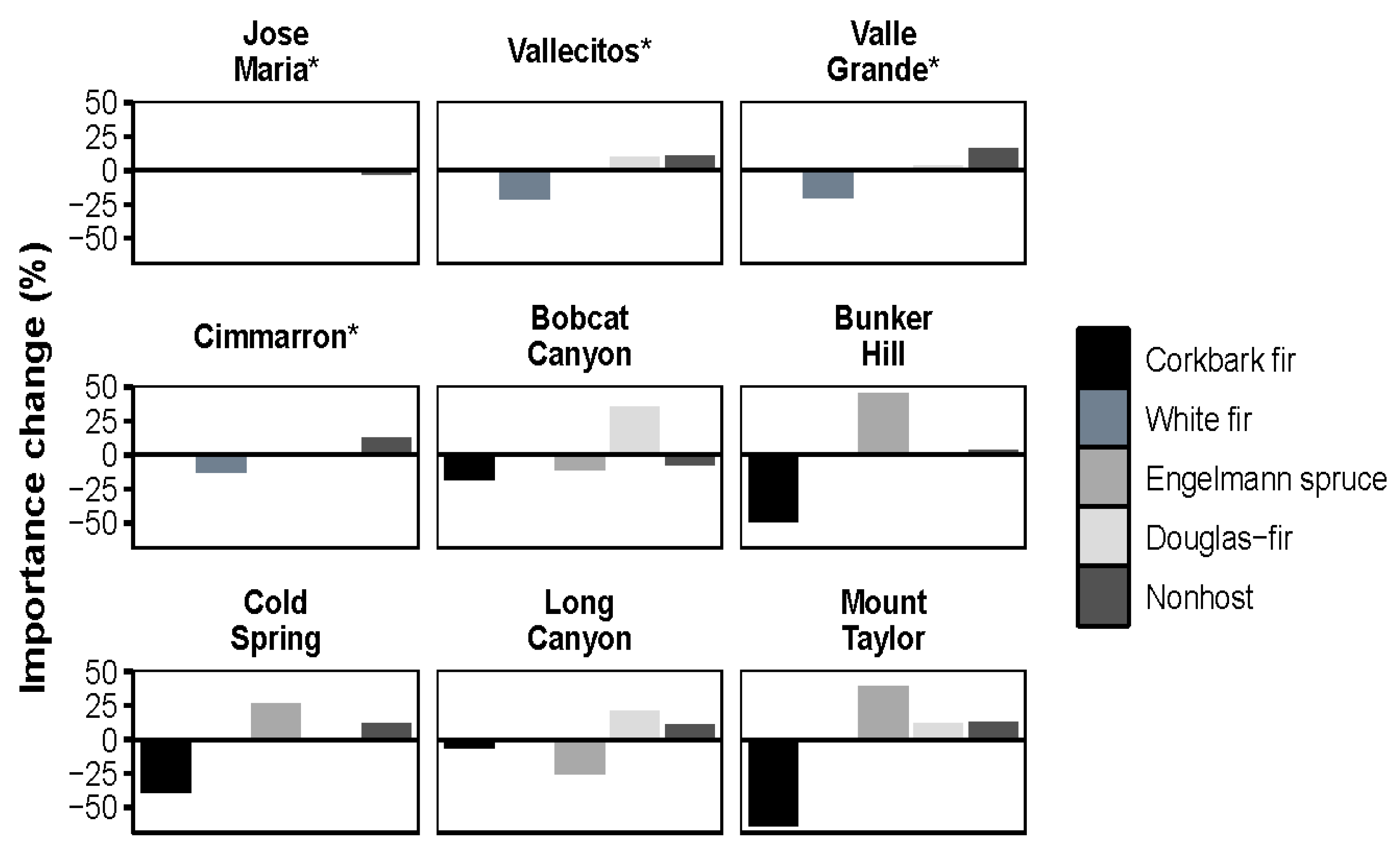
3.1. Species Composition Shifts by Forest Type
3.2. Trends by Elevation
3.3. Other Disturbances and Interactions with WSBW
3.4. Understory Condition and Trajectory
3.5. Impact of WSBW on Resistance and Resilience
4. Materials and Methods
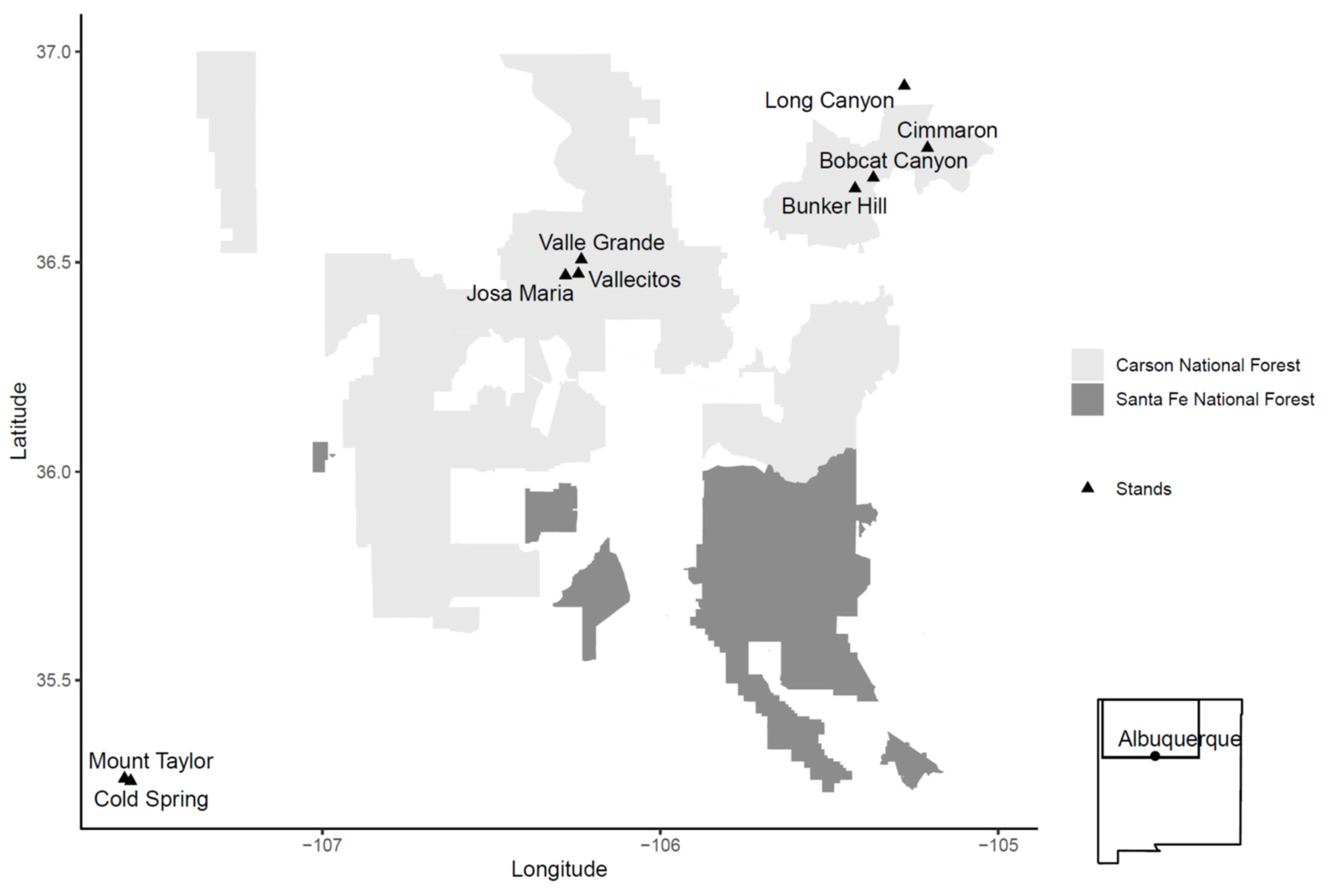
4.1. Stand Selection
4.2. Field Methods
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics; John Wiley & Sons, Inc.: New York, NY, USA, 1996. [Google Scholar]
- Schowalter, T.D. Insect Ecology: An Ecosystem Approach, 4th ed.; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Flower, C.E.; Gonzalez-Meler, M.A. Responses of Temperate Forest Productivity to Insect and Pathogen Disturbances. Annu. Rev. Plant Biol. 2015, 66, 547–569. [Google Scholar] [CrossRef]
- Schowalter, T.D. Insect Herbivore Relationship to the State of the Host Plant: Biotic Regulation of Ecosystem Nutrient Cycling through Ecological Succession. Oikos 1981, 37, 126–130. [Google Scholar] [CrossRef]
- Cooke, B.J.; Nealis, V.; Régnière, J. Insect Defoliators as Periodic Disturbances in Northern Forest Ecosystems. In Plant Disturbance Ecology: The Process and The Response; Johnson, E.A., Miyanishi, K., Eds.; Academic Press: Cambridge, MA, USA, 2007; pp. 487–525. [Google Scholar] [CrossRef]
- Furniss, R.L.; Carolin, V.M. Western Forest Insects; U.S. Department of Agriculture: Washington, DC, USA, 1977. [Google Scholar]
- Alfaro, R.I. Mortality and Top-Kill in Douglas-Fir Following Defoliation by the Western Spruce Budworm in British Columbia. J. Entomol. Soc. Br. Columbia 1986, 83, 19–26. [Google Scholar]
- Wulf, W.N.; Cates, R.G. Site and Stand Characteristics. In Western Spruce Budworm; Campbell, R., Colbert, J., Mitchell, R., Stark, R., Eds.; U.S. Department of Agriculture Forest Service Technical Bulletin 1694; U.S. Department of Agriculture: Washington, DC, USA, 1987; pp. 89–115. [Google Scholar]
- Hadley, K.S.; Veblen, T.T. Stand response to western spruce budworm and Douglas-fir bark beetle outbreaks, Colorado Front Range. Can. J. For. Res. 1993, 23, 479–491. [Google Scholar] [CrossRef]
- Van Sickle, G. Host Responses. In Western Spruce Budworm; Campbell, R.W., Colbert, J., Mitchell, R., Stark, R., Eds.; U.S. Department of Agriculture. Forest Service. Technical Bulletin 1694; U.S. Department of Agriculture: Washington, DC, USA, 1987; pp. 57–69. [Google Scholar]
- Vane, E.; Waring, K.; Polinko, A. The Influence of Western Spruce Budworm on Fire in Spruce-Fir Forests. Fire Ecol. 2017, 13, 16–33. [Google Scholar] [CrossRef]
- Shaw, D.C.; Beedlow, P.A.; Henry Lee, E.; Woodruff, D.R.; Meigs, G.W.; Calkins, S.J.; Reilly, M.J.; Merschel, A.G.; Cline, S.P.; Comeleo, R.L. The Complexity of Biological Disturbance Agents, Fuels Heterogeneity, and Fire in Coniferous Forests of the Western United States. For. Ecol. Manag. 2022, 525, 120572. [Google Scholar] [CrossRef]
- Cole, H.M.; Andrus, R.A.; Butkiewicz, C.; Rodman, K.C.; Santiago, O.; Tutland, N.J.; Waupochick, A.; Hart, S.J. Outbreaks of Douglas-Fir Beetle Follow Western Spruce Budworm Defoliation in the Southern Rocky Mountains, USA. Forests 2022, 13, 371. [Google Scholar] [CrossRef]
- Holling, C.S. Resilience and Stability of Ecological Systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef]
- DeRose, R.J.; Long, J.N. Resistance and Resilience: A Conceptual Framework for Silviculture. For. Sci. 2014, 60, 1205–1212. [Google Scholar] [CrossRef]
- Bryant, T.; Waring, K.; Sánchez Meador, A.; Bradford, J.B. A Framework for Quantifying Resilience to Forest Disturbance. Front. For. Glob. Change 2019, 2, 56. [Google Scholar] [CrossRef]
- Hessburg, P.F.; Miller, C.L.; Parks, S.A.; Povak, N.A.; Taylor, A.H.; Higuera, P.E.; Prichard, S.J.; North, M.P.; Collins, B.M.; Hurteau, M.D.; et al. Climate, Environment, and Disturbance History Govern Resilience of Western North American Forests. Front. Ecol. Evol. 2019, 7, 239. [Google Scholar] [CrossRef]
- Xu, B.; Hicke, J.A.; Abatzoglou, J.T. Drought and Moisture Availability and Recent Western Spruce Budworm Outbreaks in the Western United States. Forests 2019, 10, 354. [Google Scholar] [CrossRef]
- Anderson, L.; Carlson, C.E.; Wakimoto, R.H. Forest Fire Frequency and Western Spruce Budworm Outbreaks in Western Montana. For. Ecol. Manag. 1987, 22, 251–260. [Google Scholar] [CrossRef]
- Swetnam, T.W.; Lynch, A.M. A Tree-Ring Reconstruction of Western Spruce Budworm History in the Southern Rocky Mountains. For. Sci. 1989, 35, 962–986. [Google Scholar] [CrossRef]
- Swetnam, T.W.; Lynch, A.M. MultiCentury, Regional-Scale Patterns of Western Spruce Budworm Outbreaks’. Ecol. Monogr. 1993, 63, 399–424. [Google Scholar] [CrossRef]
- Touchan, R.; Allen, C.D.; Swetnam, T.W. Fire History and Climatic Patterns in Ponderosa Pine and Mixed-Conifer Forests of the Jemez Mountains, Northern New Mexico; U.S. Forest Service General Technical Report RM-GTR-286; U.S. Geological Survey: Reston, VA, USA, 1996; pp. 33–46. [Google Scholar]
- Keane, R.; Ryan, K.; Veblen, T. Cascading Effects of Fire Exclusion in Rocky Mountain Ecosystems: A Literature Review; Rocky Mountain Research Station, General Technical Report RMRS-GTR-9; U.S. Department of Agriculture: Fort Collins, CO, USA, 2002; p. 24. [Google Scholar]
- Vankat, J.L. Vegetation Dynamics on the Mountains and Plateaus of the American Southwest; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Fauss, D.L.; Pierce, W.R. Stand Conditions and Spruce Budworm Damage in a Western Montana Forest. J. For. 1969, 67, 322–325. [Google Scholar] [CrossRef]
- Flower, A.; Gavin, D.G.; Heyerdahl, E.K.; Parsons, R.A.; Cohn, G.M. Drought-Triggered Western Spruce Budworm Outbreaks in the Interior Pacific Northwest: A Multi-Century Dendrochronological Record. For. Ecol. Manag. 2014, 324, 16–27. [Google Scholar] [CrossRef]
- MacLean, D.A. Impacts of Insect Outbreaks on Tree Mortality, Productivity, and Stand Development. Can. Entomol. 2016, 148 (Suppl. S1), 138–159. [Google Scholar] [CrossRef]
- Ryerson, D.E.; Swetnam, T.W.; Lynch, A.M. A Tree-Ring Reconstruction of Western Spruce Budworm Outbreaks in the San Juan Mountains, Colorado, U.S.A. Can. J. For. Res. 2003, 33, 1010–1028. [Google Scholar] [CrossRef]
- Hermann, R.K. Description of Host Species. In Western Spruce Budworm; Campbell, R., Colbert, J., Mitchell, R., Stark, R., Eds.; U.S. Department of Agriculture Forest Service Technical Bulletin 1694; U.S. Department of Agriculture: Washington, DC, USA, 1987; pp. 43–56. [Google Scholar]
- Gary, H.L.; Coltharp, G.B. Snow Accumulation and Disappearance by Aspect and Vegetation Type in the Santa Fe Basin, New Mexico; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1967. [Google Scholar]
- Redmond, M.D.; Kelsey, K.C. Topography and Overstory Mortality Interact to Control Tree Regeneration in Spruce-Fir Forests of the Southern Rocky Mountains. For. Ecol. Manag. 2018, 427, 106–113. [Google Scholar] [CrossRef]
- Reich, R.M.; Lundquist, J.E.; Hughes, K. Host-Environment Mismatches Associated with Subalpine Fir Decline in Colorado. J. For. Res. 2016, 27, 1177–1189. [Google Scholar] [CrossRef]
- Sutherland, M.G. Relationship of Site and Stand Characteristics to Outbreaks by Western Spruce Budworm in the Bitterroot National Forest. Ph.D. Thesis, Washington State University, Pullman, WA, USA, 1983. [Google Scholar]
- Vogelmann, J.E.; Tolk, B.; Zhu, Z. Monitoring Forest Changes in the Southwestern United States Using Multitemporal Landsat Data. Remote Sens. Environ. 2009, 113, 1739–1748. [Google Scholar] [CrossRef]
- Hoerling, M.P.; Dettinger, M.; Wolter, K.; Lukas, J.; Eischeid, J.; Nemani, R.; Liebmann, B.; Kunkel, K.E.; Kumar, A. Present Weather and Climate: Evolving Conditions. In Assessment of Climate Change in the Southwest United States; Kumar, A., Garfin, G., Jardine, A., Merideth, R., Black, M., LeRoy, S., Eds.; NCA Regional Input Reports; Island Press/Center for Resource Economics: Washington, DC, USA, 2013; pp. 74–100. [Google Scholar]
- Schmid, J.; Frye, R. Spruce Beetle in the Rockies; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1977; p. 38. [Google Scholar]
- Reid, M.L.; Glubish, S.S. Tree Size and Growth History Predict Breeding Densities of Douglas-Fir Beetles in Fallen Trees. Can. Entomol. 2001, 133, 697–704. [Google Scholar] [CrossRef]
- Bleiker, K.P.; Lindgren, B.S.; Maclauchlan, L.E. Characteristics of Subalpine Fir Susceptible to Attack by Western Balsam Bark Beetle (Coleoptera: Scolytidae). Can. J. For. Res. 2003, 33, 1538–1543. [Google Scholar] [CrossRef]
- Jenkins, M.J.; Hebertson, E.G.; Munson, A.S. Spruce Beetle Biology, Ecology and Management in the Rocky Mountains: An Addendum to Spruce Beetle in the Rockies. Forests 2014, 5, 21–71. [Google Scholar] [CrossRef]
- Alfaro, R.I.; Van Sickle, G.A.; Thomson, A.J.; Wegwitz, E. Tree Mortality and Radial Growth Losses Caused by the Western Spruce Budworm in a Douglas-Fir Stand in British Columbia. Can. J. For. Res. 1982, 12, 780–787. [Google Scholar] [CrossRef]
- Nealis, V.G.; Régnière, J. Insect–Host Relationships Influencing Disturbance by the Spruce Budworm in a Boreal Mixedwood Forest. Can. J. For. Res. 2004, 34, 1870–1882. [Google Scholar] [CrossRef]
- Seager, R.; Vecchi, G.A. Greenhouse Warming and the 21st Century Hydroclimate of Southwestern North America. Proc. Natl. Acad. Sci. USA 2010, 107, 21277–21282. [Google Scholar] [CrossRef]
- Seager, R.; Ting, M.; Held, I.; Kushnir, Y.; Lu, J.; Vecchi, G.; Huang, H.-P.; Harnik, N.; Leetmaa, A.; Lau, N.-C.; et al. Model Projections of an Imminent Transition to a More Arid Climate in Southwestern North America. Science 2007, 316, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Garfin, G.; Jardine, A.; Merideth, R.; Black, M.; LeRoy, S. (Eds.) Assessment of Climate Change in the Southwest United States; Island Press/Center for Resource Economics: Washington, DC, USA, 2013. [Google Scholar]
- Dils, C.; Vandendriesche, D.; White, A.; Anhold, J.; DePinte, D.; Gaylord, M.; Grady, A.; Wilhelmi, N. Forest Insect and Disease Conditions in the Southwestern Region, 2015; U.S. Department of Agriculture: Washington, DC, USA, 2016. [Google Scholar]
- Abrams, M.D.; Mostoller, S.A. Gas Exchange, Leaf Structure and Nitrogen in Contrasting Successional Tree Species Growing in Open and Understory Sites during a Drought. Tree Physiol. 1995, 15, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü.; Valladares, F. Photosynthetic Acclimation to Simultaneous and Interacting Environmental Stresses along Natural Light Gradients: Optimality and Constraints. Plant Biol. 2004, 6, 254–268. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade Tolerance, a Key Plant Feature of Complex Nature and Consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Hill, E.M.; Ex, S. Microsite Conditions in a Low-Elevation Engelmann Spruce Forest Favor Ponderosa Pine Establishment during Drought Conditions. For. Ecol. Manag. 2020, 463, 118037. [Google Scholar] [CrossRef]
- Rehfeldt, G.E.; Crookston, N.L.; Warwell, M.V.; Evans, J.S. Empirical Analyses of Plant-Climate Relationships for the Western United States. Int. J. Plant Sciences 2006, 167, 1123–1150. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Plant Associations of Arizona and New Mexico, Edition 3 Volume 1: Forests; U.S. Department of Agriculture: Washington, DC, USA, 1997. [Google Scholar]
- Menon, M.; Bagley, J.C.; Friedline, C.J.; Whipple, A.V.; Schoettle, A.W.; Leal-Sàenz, A.; Wehenkel, C.; Molina-Freaner, F.; Flores-Rentería, L.; Gonzalez-Elizondo, M.S.; et al. The Role of Hybridization during Ecological Divergence of Southwestern White Pine (Pinus strobiformis) and Limber Pine (P. flexilis). Mol. Ecol. 2018, 27, 1245–1260. [Google Scholar] [CrossRef] [PubMed]
- Soil Survey Staff Web Soil Survey. Natural Resources Conservation Service, U.S. Department of Agriculture Website. Available online: http://websoilsurvey.nrcs.usda.gov/ (accessed on 25 October 2022).
- Crookston, N.L.; Rehfeldt, G.E. Research on Forest Climate Change: Predicted Effects of Global Warming on Forests and Plant Climate Relationships in Western North America and Mexico. Available online: https://charcoal2.cnre.vt.edu/climate/ (accessed on 25 October 2022).
- Lynch, A.M. Comparison of Fixed-Area Plot Designs for Estimating Stand Characteristics and Western Spruce Budworm Damage in Southwestern U.S.A. Forests. Can. J. For. Res. 2003, 33, 1245–1255. [Google Scholar] [CrossRef]
- Beyer, H.L. Geomatics Tools: Hawth’s Tools–Free Tools for Spatial Analysis. Geomatics Tools Website. 2013. Available online: https://geomaticstools.blogspot.com/2013/02/hawths-tools-free-tools-for-spatial.html (accessed on 25 October 2022).
- ArcGIS Desktop: Release 10; Environmental Systems Research Institute (ESRI): Redlands, CA, USA, 2011.
- Raphael, M.; White, M. Use of Snags by Cavity-Nesting Birds in the Sierra Nevada. Wildl. Monogr. 1984, 3–66. [Google Scholar]
- U.S. Department of Agriculture, Forest Service. Field Guide to Insects & Diseases of Arizona and New Mexico Forests; U.S. Department of Agriculture, Forest Service, Southwestern Region: Albuquerque, NM, USA, 2013; p. 269. [Google Scholar]
- Millers, I.; Lachance, D.; Burkman, W.; Allen, D. North American Sugar Maple Decline Project: Organization and Field Methods; General Technical Report NE-154; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1991. [Google Scholar]
- Schomaker, M.E.; Zarnoch, S.J.; Bechtold, W.A.; Latelle, D.J.; Burkman, W.G.; Cox, S.M. Crown-Condition Classification: A Guide to Data Collection and Analysis; General Technical Report SRS-102; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2007. [Google Scholar]
- Hollands, J.G.; Dyre, B.P. Bias in Proportion Judgments: The Cyclical Power Model. Psychol. Rev. 2000, 107, 500–524. [Google Scholar] [CrossRef]
- Brookes, M.; Colbert, J.; Mitchell, R.G.; Stark, R.W. Managing Trees and Stands Susceptible to Western Spruce Budworm. Technical Bulletin Number 1695; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1985. [Google Scholar]
- Polinko, A.; Waring, K. Stand Inventory Data (Overstory and Understory) in Northern New Mexico Forests Affected by Western Spruce Budworm (Choristoneura freemani Razowski) Defoliation, 2012–2013 ver 1. Environmental Data Initiative. 2022. Available online: https://portal.edirepository.org/nis/mapbrowse?packageid=edi.1256.1 (accessed on 18 November 2022). [CrossRef]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Curtis, J.T.; McIntosh, R.P. An Upland Forest Continuum in the Prairie-Forest Border Region of Wisconsin. Ecology 1951, 32, 476–496. [Google Scholar] [CrossRef]
- Beers, T.W.; Dress, P.E.; Wensel, L.C. Notes and Observations: Aspect Transformation in Site Productivity Research. J. For. 1966, 64, 691–692. [Google Scholar] [CrossRef]
- Core R Team: A Language and Environment for Statistical Computing. 2013. Available online: https://www.r-project.org/ (accessed on 25 October 2022).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MJM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Akaike, H. A New Look at The Statistical Model Identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]

| Stand | Forest Type | Mean Duration Detected Defoliation (Years) | Elevation (m) | Host Species Composition (%) | Canopy Cover (%) | Aspect (⁰) | Slope (%) |
|---|---|---|---|---|---|---|---|
| Jose Maria | Mixed-conifer | 14.3 a | 2629 (1.8) a | 78.5 (0.1) ab | 51.7 (1.6) a | 91 | 14.4 (0.5) a |
| Vallecitos | Mixed-conifer | 9.7 bcde | 2792 (0.8) b | 59.3 (0.1) a | 33.4 (1.0) a | 160 | 28.0 (0.5) b |
| Valle Grande | Mixed-conifer | 8.6 b | 2817 (3.0) b | 65.5 (0.1) a | 53.6 (0.7) a | 102 | 18.8 (0.3) ab |
| Cimarron | Mixed-conifer | 9.5 bc | 2921 (2.0) c | 82.3 (0.1) ab | 37.6 (1.1) a | 100 | 17.1 (0.4) ab |
| Bobcat Canyon | Spruce-fir | 12.2 ad | 3017 (4.8) d | 86.3 (0.0) ab | 51.5 (3.9) bc | 17 | 17.4 (1.5) ab |
| Long Canyon | Spruce-fir | 7.1 e | 3053 (3.8) e | 55.5 (0.1) a | 34.6 (3.7) b | 16 | 15.6 (2.6) ab |
| Mount Taylor | Spruce-fir | 11.6 acd | 3115 (13.1) ef | 80.1 (0.1) ab | 57.6 (3.4) c | 18 | 19.8 (2.3) ab |
| Cold Spring | Spruce-fir | 9.7 c | 3173 (11.1) f | 83.7 (0.1) ab | 51.4 (3.2) bc | 20 | 16.3 (1.7) a |
| Bunker Hill | Spruce-fir | 8.7 bce | 3288 (9.9) g | 92.9 (0.0) b | 51.2 (3.5) bc | 38 | 20.1 (1.8) ab |
| Corkbark Fir | White Fir | Engelmann Spruce | Douglas-Fir | |||||
|---|---|---|---|---|---|---|---|---|
| Stand | Sketch | 6-Class | Sketch | 6-Class | Sketch | 6-Class | Sketch | 6-Class |
| Jose Maria | – | – | 19.3 (1.9) | 26–50% | – | – | 11.7 (9.6) | 26–50% |
| Vallecitos | 15.5 (3.3) | – | 37.1 (3.2) | – | – | – | 23.6 (2.9) | – |
| Valle Grande | – | – | 15.6 (1.7) | 26–50% | – | – | 7.4 (1) | 1–25% |
| Cimarron | – | – | 9.5 (2.2) | 1–25% | – | – | 7.7 (1.1) | 1–25% |
| Bobcat Canyon | – | – | – | – | 28.7 (–) | 26–50% | 22.1 (0.8) | 26–50% |
| Long Canyon | 16.7 (–) | 26–50% | – | – | 6.8 (1.5) | 26–50% | 12.1 (1) | 26–50% |
| Mount Taylor | 35.2 (5.2) | 51–75% | – | – | 35.2 (2.2) | 26–50% | 14.9 (2.6) | 26–50% |
| Cold Spring | 13.2 (1.2) | 26–50% | – | – | 11.8 (0.7) | 26–50% | 14 (6.5) | 26–50% |
| Bunker Hill | 12.2 (1) | 26–50% | 8.3 (2.1) | 26–50% | 10.9 (0.6) | 26–50% | – | – |
| a. Mixed-Conifer | Jose Maria | Vallecitos | Valle Grande | Cimarron | ||||||
| TPH | BA | TPH | BA | TPH | BA | TPH | BA | |||
| White fir | ||||||||||
| Live | 143.3 (32.7) | 9.2 (2.3) | 92.5 (22.4) | 4.1 (1.0) | 130.0 (18.6) | 6.4 (1.4) | 127.5 (32.3) | 7.1 (1.9) | ||
| Dead | 30.0 (11.8) | 1.4 (0.8) | 75.0 (17.2) | 3.6 (1.1) | 115.0 (23.0) | 4.8 (1.4) | 82.5 (36.1) | 2.3 (1.2) | ||
| Douglas-fir | ||||||||||
| Live | 30.0 (16.0) | 2.1 (1.4) | 100.0 (26.9) | 4.3 (1.2) | 162.5 (32.4) | 12.1 (2.1) | 205.0 (44.1) | 8.0 (1.7) | ||
| Dead | 6.7 (4.5) | 0.8 (0.5) | 2.5 (2.5) | 0.2 (0.2) | 5.0 (3.4) | 0.3 (0.2) | 25.0 (11.2) | 0.8 (0.5) | ||
| Ponderosa pine | ||||||||||
| Live | 50.0 (23.4) | 5.0 (2.4) | 105.0 (22.3) | 10.1 (2.6) | 27.5 (9.2) | 2.4 (0.9) | 15.0 (8.2) | 0.8 (0.4) | ||
| Dead | 6.7 (4.5) | 1.0 (0.8) | 7.5 (4.1) | 0.5 (0.3) | 2.5 (2.5) | 0.2 (0.2) | 0 (0) | 0 (0) | ||
| Quaking aspen | ||||||||||
| Live | 16.7 (13.5) | 1.0 (0.9) | 27.5 (17.6) | 0.9 (0.5) | 122.5 (25.0) | 6.7 (1.6) | 20.0 (12.2) | 1.6 (1.0) | ||
| Dead | 0 (0) | 0 (0) | 25.0 (12.3) | 0.6 (0.3) | 40.0 (16.1) | 1.4 (0.6) | 10.0 (5.8) | 0.6 (0.4) | ||
| Other species * | ||||||||||
| Live | 3.3 (3.3) | 0.1 (0.1) | 5.0 (5.0) | 0.3 (0.3) | 0 (0) | 0 (0) | 60.0 (26.1) | 2.0 (0.9) | ||
| Dead | 0 (0) | 0 (0) | 2.5 (2.5) | 0 (0) | 0 (0) | 0 (0) | 2.5 (2.5) | 0 (0) | ||
| b. Spruce-fir | Bobcat Canyon | Long Canyon | Mount Taylor | Cold Spring | Bunker Hill | |||||
| TPH | BA | TPH | BA | TPH | BA | TPH | BA | TPH | BA | |
| Corkbark fir | ||||||||||
| Live | 0 (0) | 0 (0) | 2.5 (2.5) | 0 (0) | 35.0 (10.3) | 2.3 (0.8) | 91.7 (28.9) | 5.1 (1.4) | 137.5 (41.2) | 4.8 (1.7) |
| Dead | 90.0 (38.0) | 2.5 (0.9) | 30.0 (13.3) | 0.8 (0.4) | 402.5 (48.7) | 19.1 (2.7) | 319.4 (51.8) | 14.0 (2.2) | 395.0 (68.4) | 13.5 (2.4) |
| Engelmann spruce | ||||||||||
| Live | 2.5 (2.5) | 0.2 (0.2) | 47.5 (12.8) | 2.0 (0.5) | 235.0 (41.5) | 20.3 (3.2) | 263.9 (62.1) | 27.4 (4.3) | 395.0 (50.4) | 17.4 (2.3) |
| Dead | 55.0 (14.5) | 1.7 (0.5) | 135.0 (26.7) | 5.5 (1.2) | 82.5 (29.3) | 4.5 (1.8) | 147.2 (33.7) | 13.2 (4.0) | 87.5 (13.5) | 2.8 (0.6) |
| Douglas-fir | ||||||||||
| Live | 380.0 (39.6) | 21.1 (2.4) | 112.5 (30.5) | 7.5 (2.4) | 52.5 (21.6) | 5.7 (1.9) | 5.6 (3.8) | 0.2 (0.2) | 0 (0) | 0 (0) |
| Dead | 80.0 (18.3 | 4.5 (1.4 | 47.5 (15.6) | 1.2 (0.4) | 12.5 (6.2) | 0.5 (0.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Quaking aspen | ||||||||||
| Live | 87.5 (31.2) | 2.5 (1.0) | 177.5 (41.6) | 8.3 (1.9) | 127.5 (35.6) | 6.5 (1.9) | 75.0 (24.3) | 5.5 (1.8) | 40.0 (24.0) | 0.8 (0.5) |
| Dead | 100.0 (27.9) | 2.1 (0.6) | 152.5 (31.7) | 4.3 (0.9) | 72.5 (21.3) | 2.2 (0.7) | 19.4 (10.0) | 0.5 (0.3) | 17.5 (10.4) | 0.4 (0.3) |
| Other species * | ||||||||||
| Live | 20.0 (11.1) | 1.1 (0.8) | 20.0 (11.1) | 0.6 (0.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 20.0 (14.2) | 1.3 (0.9) |
| Dead | 7.5 (5.5) | 0.6 (0.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7.5 (5.5) | 0.4 (0.3) |
| a. Mixed-Conifer | Jose Maria | Vallecitos | Valle Grande | Cimarron | |
| White fir | |||||
| Sapling | 330.0 (79.2) | 100.0 (38.9) | 30.0 (17.1) | 120.0 (49.8) | |
| Seedling | 1040.0 (150.4) | 210.0 (71.5) | 50.0 ( 21.9) | 250.0 (70.2) | |
| Douglas-fir | |||||
| Sapling | 110.0 (49.0) | 90.0 (37.9) | 0.0 (0.0) | 60.0 (34.3) | |
| Seedling | 180.0 ( 47.9) | 40.0 (19.7) | 50.0 (21.9) | 100.0 (30.2) | |
| Ponderosa pine | |||||
| Sapling | 10.0 (10.0) | 10.0 (10.0) | 0.0 (0.0) | 10.0 (10.0) | |
| Seedling | 20.0 (14.1) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | |
| Quaking aspen | |||||
| Sapling | 20.0 (14.1) | 10.0 (10.0) | 0.0 (0.0) | 10.0 (10.0) | |
| Seedling | 480.0 (103.9) | 40.0 (31.5) | 880.0 (151.9) | 60.0 (31.2) | |
| Other species * | |||||
| Sapling | 50.0 (21.9) | 260.0 (86.0) | 0.0 (0.0) | 0.0 (0.0) | |
| Seedling | 10.0 (10.0) | 210.0 (64.0) | 0.0 (0.0) | 200.0 (72.5) | |
| b. Spruce-fir | Bobcat Canyon | Long Canyon | Mount Taylor | Cold Spring | Bunker Hill |
| Corkbark fir | |||||
| Sapling | 0.0 (0.0) | 20.0 (20.0) | 80.0 (38.8) | 320.0 (129.2) | 921.6 (227.2) |
| Seedling | 1100.0 (227.2) | 580.0 (212.1) | 2520.0 (604.2) | 380.0 (207.8) | 11660.0 (2304.3) |
| Engelmann spruce | |||||
| Sapling | 20.0 (20.0) | 160.0 (77.5) | 180.0 (68.2) | 40.0 (28.0) | 254.9 (107.9) |
| Seedling | 680.0 (214.6) | 700.0 (254.3) | 460.0 (171.9) | 40.0 (28.0) | 1560.0 (390.9) |
| Douglas-fir | |||||
| Sapling | 40.0 (28.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Seedling | 1060.0 (204.9) | 420.0 (151.4) | 220.0 (131.8) | 0.0 (0.0) | 20.0 (20.0) |
| Quaking aspen | |||||
| Sapling | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Seedling | 1220.0 (305.4) | 2000.0 (344.0) | 5140.0 (1386.5) | 1280.0 (330.8) | 1200.0 (387.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polinko, A.D.; Rapp, M.A.; Sánchez Meador, A.J.; Graves, A.D.; Ryerson, D.E.; Waring, K.M. Western Spruce Budworm Effects on Forest Resilience. Plants 2022, 11, 3266. https://doi.org/10.3390/plants11233266
Polinko AD, Rapp MA, Sánchez Meador AJ, Graves AD, Ryerson DE, Waring KM. Western Spruce Budworm Effects on Forest Resilience. Plants. 2022; 11(23):3266. https://doi.org/10.3390/plants11233266
Chicago/Turabian StylePolinko, Adam D., Marguerite A. Rapp, Andrew J. Sánchez Meador, Andrew D. Graves, Daniel E. Ryerson, and Kristen M. Waring. 2022. "Western Spruce Budworm Effects on Forest Resilience" Plants 11, no. 23: 3266. https://doi.org/10.3390/plants11233266
APA StylePolinko, A. D., Rapp, M. A., Sánchez Meador, A. J., Graves, A. D., Ryerson, D. E., & Waring, K. M. (2022). Western Spruce Budworm Effects on Forest Resilience. Plants, 11(23), 3266. https://doi.org/10.3390/plants11233266







