Volatile Oil Chemical Composition of Wild, Edible Centaurea scabiosa L. and Its Cytotoxic Activity
Abstract
1. Introduction
- (a)
- Assess the volatile oil chemical composition of C. scabiosa using the GC/MS technique;
- (b)
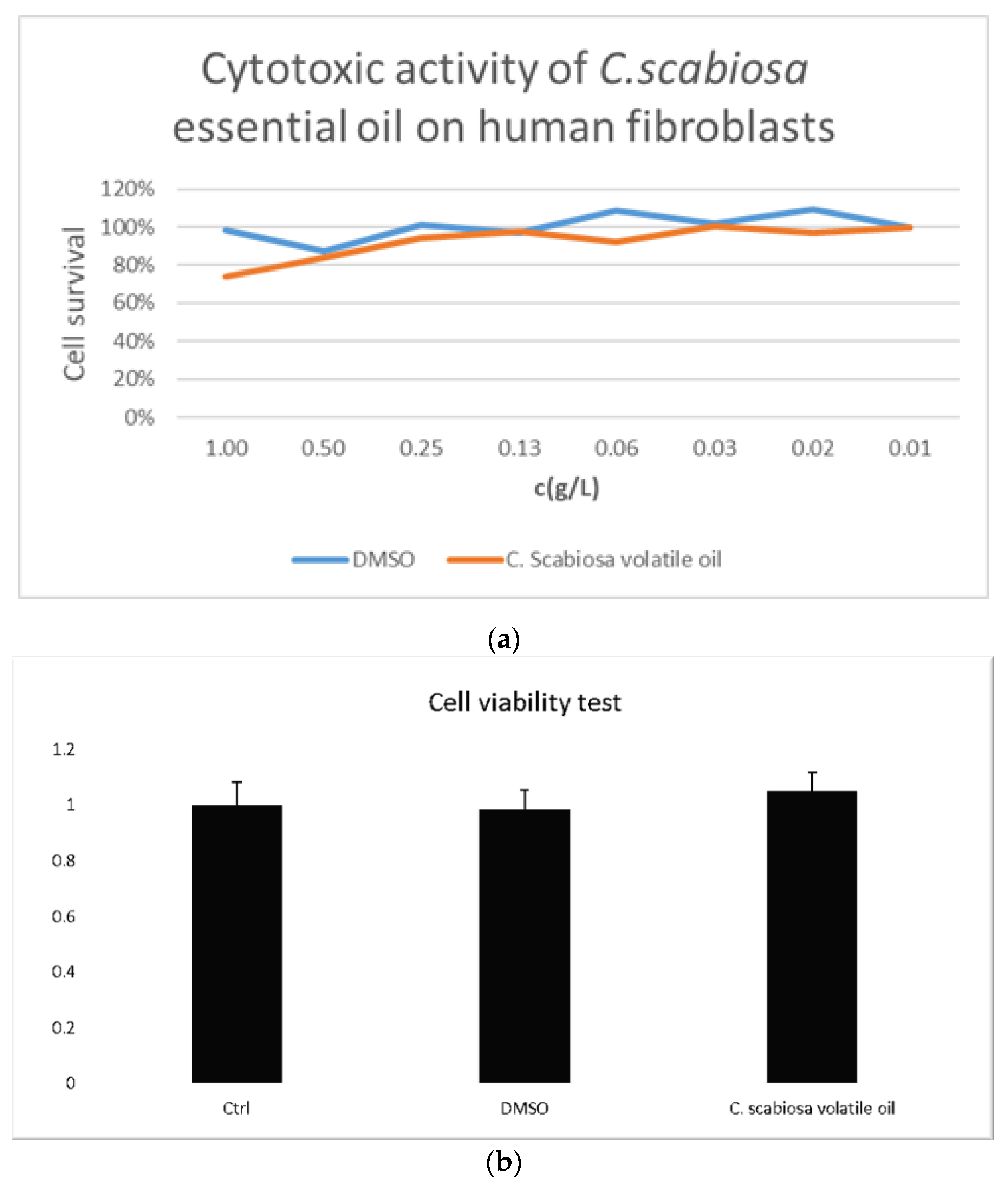
- Test C. scabiosa volatile oil cytotoxicity using the MTT assay on human fibroblasts at a concentration dose range of 0.01–1 g/L;
- (c)
- Determine genome size using flow cytometry and chromosome number using the classical karyological method.
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Volatile Oil Extraction, Gas Chromatography (GC), and Gas Chromatography—Mass Spectrometry (GC–MS) Analyses
3.3. Toxicity on Human Primary Fibroblasts
3.4. Chromosome Number and Genome Size Evaluation
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abad, M.J.; Bedoya, L.M.; Bermejo, P. Chapter 14—Essential Oils from the Asteraceae Family Active against Multidrug-Resistant Bacteria A2—Kon, Mahendra Kumar RaiKateryna Volodymyrivna. In Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components; Academic Press: San Diego, CA, USA, 2013; pp. 205–215. Available online: http://www.sciencedirect.com/science/article/pii/B9780123985392000148 (accessed on 20 November 2022).
- Cakilcioglu, U.; Turkoglu, I. An ethnobotanical survey of medicinal plants in Sivrice (Elazi{dotless}ĝ-Turkey). J. Ethnopharmacol. 2010, 132, 165–175. [Google Scholar] [CrossRef]
- Manukyan, A.; Lumlerdkij, N.; Heinrich, M. Caucasian endemic medicinal and nutraceutical plants: In-vitro antioxidant and cytotoxic activities and bioactive compounds. J. Pharm. Pharmacol. 2019, 71, 1152–1161. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85065040449&doi=10.1111%2Fjphp.13093&partnerID=40&md5=5da076fd98856d262b1d8be8248c9e3a (accessed on 21 November 2022). [CrossRef]
- Łuczaj, Ł.; Zovko Končić, M.; Miličević, T.; Dolina, K.; Pandža, M. Wild vegetable mixes sold in the markets of Dalmatia (southern Croatia). J. Ethnobiol. Ethnomed. 2013, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, Ł.; Pieroni, A. Nutritional ethnobotany in Europe: From emergency foods to healthy folk cuisines and contemporary foraging trends. In Mediterranean Wild Edible Plants: Ethnobotany and Food Composition Tables; Springer: New York, NY, USA, 2016. [Google Scholar]
- María de Cortes Sánchez-Mata, J.T. Mediterranean Wild Edible Plants; Universidad Complutense de Madrid: Madrid, Spain; IMIDRA: Alcalá de Henares, Madrid, Spain, 2016. [Google Scholar]
- Kenny, O.; Smyth, T.J.; Walsh, D.; Kelleher, C.T.; Hewage, C.M.; Brunton, N.P. Investigating the potential of under-utilised plants from the Asteraceae family as a source of natural antimicrobial and antioxidant extracts. Food Chem. 2014, 161, 79–86. Available online: http://www.sciencedirect.com/science/article/pii/S0308814614005299 (accessed on 21 November 2022). [CrossRef]
- Sharonova, N.; Nikitin, E.; Terenzhev, D.; Lyubina, A.; Amerhanova, S.; Bushmeleva, K.; Rakhmaeva, A.; Fitsev, I.; Sinyashin, K. Comparative assessment of the phytochemical composition and biological activity of extracts of flowering plants of Centaurea cyanus L., Centaurea jacea L., and Centaurea scabiosa L. Plants 2021, 10, 1279. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85108370926&doi=10.3390%2Fplants10071279&partnerID=40&md5=b76099b335e0ea81e6994facd97516ee (accessed on 20 November 2022). [CrossRef] [PubMed]
- Kaminskiy, I.P.; Yermilova, Y.V.; Kadyrova, T.V.; Lar’Kina, M.S.; D’Yakonov, A.A.; Belousov, M.V. Antiradical AC-tivity of extracts from siberian flora genus centaurea plants. Khimiya Rastit Syr’ya 2019, 4, 174–179. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.085080865421&doi=10.14258%2Fjcprm.2019045409&partnerID=40&md5=af6230da76cb5458cab5e46cd08b19d2 (accessed on 20 November 2022).
- Vallès, J.; Canela, M.Á.; Garcia, S.; Hidalgo, O.; Pellicer, J.; Sánchez-Jiménez, I.; Siljak-Yakovlev, S.; Vitales, D.; Garnatje, T. Genome size variation and evolution in the family Asteraceae. Caryologia 2013, 66, 221–235. [Google Scholar] [CrossRef]
- Siljak-Yakovlev, S.; Solic, M.E.; Catrice, O.; Brown, S.C.; Papes, D. Nuclear DNA content and chromosome number in some diploid and tetraploid Centaurea (Asteraceae: Cardueae) from the Dalmatia region. Plant Biol. 2005, 7, 397–404. Available online: http://www.ncbi.nlm.nih.gov/pubmed/16025412 (accessed on 21 January 2017). [CrossRef]
- Siljak-Yakovlev, S.; Pustahija, F.; Šolić, E.M.; Bogunić, F.; Muratović, E.; Bašić, N.; Catrice, O.; Brown, S.C. Towards a Genome Size and Chromosome Number Database of Balkan Flora: C-Values in 343 Taxa with Novel Values for 242. Adv. Sci. Lett. 2010, 3, 190–213. Available online: http://www.ingentaconnect.com/content/asp/asl/2010/00000003/00000002/art00014 (accessed on 13 July 2021). [CrossRef]
- Bennett, M.D.; Leitch, I.J. Nuclear DNA amounts in angiosperms: Targets, trends and tomorrow. Ann. Bot. 2011, 107, 467–590. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3043933/ (accessed on 20 November 2022). [CrossRef]
- Flamini, G.; Tebano, M.; Cioni, P.L.; Bagci, Y.; Dural, H.; Ertugrul, K.; Uysal, T.; Savran, A. A multivariate statistical approach to Centaurea classification using essential oil composition data of some species from Turkey. Plant Syst. Evol. 2006, 261, 217–228. [Google Scholar] [CrossRef]
- Kilic, O.; Bagci, E. Chemical Composition of Two Endemic Centaurea L. Taxa from Turkey, A Chemotaxonomic Approach. J. Essent. Oil-Bear. Plants 2016, 19, 185–193. [Google Scholar] [CrossRef]
- Zengin, G.; Aktumsek, A.; Boga, M.; Ceylan, R.; Uysal, S. Essential Oil Composition of an Uninvestigated Centaurea Species from Turkey: Centaurea patula DC. J. Essent. Oil-Bear. Plants 2016, 19, 485–491. [Google Scholar] [CrossRef]
- Polatoglu, K.; Sen, A.; Bulut, G.; Bitis, L.; Goren, N. Essential Oil Composition of Centaurea stenolepis Kerner. from Turkey. J. Essent. Oil Bear. Plants 2014, 17, 1268–1278. [Google Scholar] [CrossRef]
- Flamini, G.; Ertugrul, K.; Cioni, P.L.; Morelli, I.; Dural, H.; Bagci, Y.G. E, Flamini K.; Cioni P.L.; Morelli I.; Dural H.; Bagci Y.G.E. Volatile constituents of two endemic Centaureaspecies from Turkey: C. pseudoscabiosa subsp. pseudoscabiosa and C. hadimensis. Biochem. Syst. Ecol. 2002, 30, 953–959. [Google Scholar] [CrossRef]
- Novaković, J.; Rajčević, N.; Milanovici, S.; Marin, P.D.; Janaćković, P. Essential Oil Composition of Centaurea atropurpurea and Centaurea orientalis Inflorescences from the Central Balkans—Ecological Significance and Taxonomic Implications. Chem Biodivers. 2016, 13, 1221–1229. [Google Scholar] [CrossRef]
- Bruno, M.; Modica, A.; Catinella, G.; Canlı, C.; Arasoglu, T.; Çelik, S. Chemical composition of the essential oils of Centaurea tomentella Hand.-Mazz. and C. haussknechtii Boiss. (Asteraceae) collected wild in Turkey and their activity on microorganisms affecting historical art craft. Nat. Prod. Res. 2019, 33, 1092–1100. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85045683081&doi=10.1080%2F14786419.2018.1463531&partnerID=40&md5=595c8289b12eeb76618d4ccb0d09a8db (accessed on 20 November 2022). [CrossRef] [PubMed]
- Politeo, O.; Carev, I.; Veljaca, A. Phytochemical Composition, Antiradical and Anticholinesterase Potentials of Centaurea alba and Centaurea jacea Volatile Oils. Croat. Chem. Acta 2019, 92, 11–17. Available online: https://hrcak.srce.hr/file/316466 (accessed on 13 July 2021). [CrossRef]
- Carev, I.; Ruščić, M.; Skočibušić, M.; Maravić, A.; Siljak-Yakovlev, S.; Politeo, O. Phytochemical and Cytogenetic Characterization of Centaurea solstitialis L. (Asteraceae) from Croatia. Chem. Biodivers. 2017, 14, e1600213. Available online: http://doi.wiley.com/10.1002/cbdv.201600213 (accessed on 13 July 2021). [CrossRef] [PubMed]
- Carev, I.; Maravić, A.; Bektašević, M.; Ruščić, M.; Siljak-Yakovlev, S.; Politeo, O. Centaurea rupestris L.: Cytogenetics, essential oil chemistry and biological activity. Croat. Chem. Acta 2018, 91, 11–19. [Google Scholar]
- Riccobono, L.; Maggio, A.; Bruno, M.; Bancheva, S.; Santucci, O.; Senatore, F. Chemical composition of the essential oil of Centaurea grinensis Reuter and Centaurea apiculata Ledeb: Growing wild in Croatia and Bulgaria, respectively and PCA analysis of subgenus Lopholoma (Cass.) Dobrocz. Plant Biosyst. 2017, 151, 1035–1044. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84994845975&doi=10.1080%2F11263504.2016.1219419&partnerID=40&md5=fb094145d2beab19e69f8a813db447d9 (accessed on 20 November 2022). [CrossRef]
- Polatoğlu, K.; Şen, A.; Bulut, G.; Bitiş, L.; Gören, N. Essential Oil Composition of Centaurea kilaea Boiss. and C. cuneifolia Sm. from Turkey. Nat. Vol. Essent. Oils 2014, 1, 55–59. [Google Scholar]
- Karamenderes, C.; Demirci, B.; Baser, K.H.C. Composition of essential oils of ten Centaurea L. taxa from Turkey. J. Essent. Oil Res. 2008, 20, 342–349. [Google Scholar] [CrossRef]
- El-Lakany, S.A.; Abd-Elhamid, A.I.; Kamoun, E.A.; El-Fakharany, E.M.; Samy, W.M.; Elgindy, N.A. α-Bisabolol-loaded cross-linked zein nanofibrous 3D-scaffolds for accelerating wound healing and tissue regeneration in rats. Int. J. Nanomed. 2019, 14, 8251–8270. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85073790044&doi=10.2147%2FIJN.S224315&partnerID=40&md5=a4d631b461f2e1c782e2153bf8ba05d2 (accessed on 20 November 2022). [CrossRef]
- Geraci, A.; Amato, F.; Di Noto, G.; Bazan, G.; Schicchi, R. The wild taxa utilized as vegetables in Sicily (Italy): A traditional component of the Mediterranean diet. J. Ethnobiol. Ethnomed. 2018, 14, 14. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85042129412&doi=10.1186%2Fs13002-018-0215-x&partnerID=40&md5=090bae44fefc8fd0f6ef8488504ccf94 (accessed on 20 November 2021). [CrossRef] [PubMed]
- Şenkardeş, İ.; Bulut, G.; Doğan, A.; Tuzlaci, E. An ethnobotanical analysis on wild edible plants of the turkish asteraceae taxa. Agric. Conspec. Sci. 2019, 84, 17–28. [Google Scholar]
- Esmaeili, A.; Rustaiyan, A.; Nadimi, M.; Masoudi, S.; Tadayon, F.; Sedaghat, S.; Ebrahimpur, N.; Hajyzadeh, E. Volatile Constituents of Centaurea depressa M.B. and Carduus pycnocephalus L. Two Compositae Herbs Growing Wild in Iran. J. Essent. Oil Res. 2005, 17, 539–541. [Google Scholar] [CrossRef]
- Ertas, A.; Goren, A.C.; Boga, M.; Demirci, S.; Kolak, U. Chemical Composition of The Essential Oils of Three Centaurea Species Growing Wild in Anatolia and Their Anticholinesterase Activities. J. Essent. Oil Bear. Plants 2014, 17, 922–926. [Google Scholar] [CrossRef]
- Erel, S.B.; Demir, S.; Nalbantsoy, A.; Ballar, P.; Khan, S.; Yavasoglu, N.U.; Karaalp, C. Bioactivity screening of five Centaurea species and in vivo anti-inflammatory activity of C. athoa. Pharm. Biol. 2014, 52, 775–781. [Google Scholar] [CrossRef]
- Milosevic, T.; Argyropoulou, C.; Solujic, S.; Murat-Spahic, D.; Skaltsa, H. Chemical composition and antimicrobial activity of essential oils from Centaurea pannonica and C. jacea. Nat. Prod. Commun. 2010, 5, 1663–1668. Available online: http://europepmc.org/abstract/MED/21121269 (accessed on 13 July 2021).
- Lograda, M.; Chalard, P.; Figueredo, G.; Khalfoune, K.; Silin, H.; Ramdani, T. Phytochemistry, antibacterial activity and chromosome number of Centaurea solstitialis L. Grown in Algeria. Glob. J. Res. Med. Plants Indig. Med. 2013, 2, 675–684. [Google Scholar]
- Esmaeili, A.; Akbari, M.T.; Moazami, N.; Masoudi, S.; Amiri, H.R.A. Composition of the Essential Oils of Xanthium strumarium L. and Cetaurea solstitialis L. from Iran. J. Essent. Oil Res. 2006, 18, 427. [Google Scholar] [CrossRef]
- Senatore, F.; Formisano, C.; Raio, A.; Bellone, G.; Bruno, M. Volatile components from flower-heads of Centaurea nicaeensis All., C-parlatoris Helder and C-solstitialis L. ssp schouwii (DC.) Dostal growing wild in southern Italy and their biological activity. Nat. Prod. Res. 2008, 22, 825–832. [Google Scholar] [CrossRef]
- Ayromlou, A.; Masoudi, S.; Mirzaie, A. Chemical composition, antioxidant, antibacterial, and anticancer activities of scorzonera calyculata boiss. And centaurea irritans wagenitz. Extracts, endemic to iran. J. Rep. Pharm. Sci. 2020, 9, 118–127. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85090256725&doi=10.4103%2Fjrptps.JRPTPS_97_19&partnerID=40&md5=17095ff168771f2f0e45d7ebf1c534dd (accessed on 13 July 2021).
- Çelikezen, F.Ç.; Hayta, Ş.; Özdemir, Ö.; Türkez, H. Cytotoxic and antioxidant properties of essential oil of Centaurea behen L. in vitro. Cytotechnology 2019, 71, 345–350. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85059565066&doi=10.1007%2Fs10616-018-0290-9&partnerID=40&md5=6e54b48789ceff32c4fe85c0a4e6d769 (accessed on 13 July 2021). [CrossRef]
- Mirzaie, A.; Karizi, S.Z. Study of chemical composition and characteristics of Centurea cyanus extract on colon cancer cell line and analysis of apoptosis gene expression. Tehran. Univ. Med. J. 2016, 74, 626–634. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85012131945&partnerID=40&md5=c49852b97531dbb08c1d2fd272f3e07f (accessed on 13 July 2021).
- Toğar, B.; Türkez, H.; Geyikoğlu, F.; Hacimüftüoğlu, A.; Tatar, A. Antiproliferative, genotoxic and oxidant activities of cyclosativene in rat neuron and neuroblastoma cell lines. Arch. Biol. Sci. 2014, 66, 1171–1177. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84908219865&doi=10.2298%2FABS1403171T&partnerID=40&md5=75bae9d720affb1f80f9498db2cb2342 (accessed on 13 July 2021). [CrossRef]
- Bancheva, S.; Greilhuber, J. Genome size in Bulgarian Centaurea S.L. (Asteraceae). Plant Syst. Evol. 2006, 257, 95–117. [Google Scholar] [CrossRef]
- Munzbergova, Z. The effect of genome size on detailed species traits within closely related species of the same habitat. Bot. J. Linn. Soc. 2009, 160, 290–298. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2005. [Google Scholar]
- Lepers-Andrzejewski, S.; Siljak-Yakovlev, S.; Brown, S.C.; Wong, M.; Dron, M. Diversity and dynamics of plant genome size: An example of polysomaty from a cytogenetic study of Tahitian vanilla (Vanilla× tahitensis, Orchidaceae). Am. J. Bot. 2011, 98, 986–997. [Google Scholar] [CrossRef]
- Bourge, M.; Brown, S.C.; Siljak-Yakovlev, S. Flow cytometry as tool in plant sciences, with emphasis on genome size and ploidy level assessment. Genet. Appl. 2018, 2, 1. [Google Scholar] [CrossRef]
| Compound Name | KI | Identification | ||
|---|---|---|---|---|
| Terpene Compounds | ||||
| Non-oxygenated sesquiterpenes | 1.23 | |||
| 1 | Longifolene | 0.43 | 1409 | KI, MS |
| 2 | Aromadendrene | 0.08 | 1421 | KI, MS |
| 3 | γ-elemene | tr | 1435 | KI, MS |
| 4 | cis-ß-farnesene | tr | 1443 | KI, MS |
| 5 | α-hummulene | 0.18 | 1452 | KI, MS |
| 6 | trans-ß-farnesene | tr | 1478 | KI, MS |
| 7 | Germacrene D | 0.54 | 1482 | KI, MS |
| Oxygenated sesquiterpenes | 41.09 | |||
| 8 | Spathulenol | 0.75 | 1568 | KI, MS |
| 9 | Caryophyllene oxide | 10.90 | 1583 | KI, MS |
| 10 | Aromadendrene oxide | 2.20 | 1626 | KI, MS |
| 11 | Isospathulenol | 3.52 | 1639 | KI, MS |
| 12 | Alloaromadendrene epoxide | 10.57 | 1655 | KI, MS |
| 13 | α-bisabolol | 4.99 | 1692 | KI, MS |
| 14 | α-cyperone | 8.16 | 1752 | KI, MS |
| Oxygenated diterpene | 1.41 | |||
| 15 | Phytol | 1.41 | 2119 | KI, MS |
| Non-terpene compounds | ||||
| Hydrocarbons | 31.10 | |||
| 16 | Tricosane | 0.39 | 2300 | KI, MS |
| 17 | Tetracosane | 0.21 | 2400 | KI, MS |
| 18 | Pentacosane | 4.64 | 2500 | KI, MS |
| 19 | Hexacosane | 0.94 | 2600 | KI, MS |
| 20 | Heptacosane | 19.62 | 2700 | KI, MS |
| 21 | Octacosane | 0.52 | 2800 | KI, MS |
| 22 | Nonacosane | 4.78 | 2900 | KI, MS |
| Aldehydes | 3.67 | |||
| 23 | Benzene acetaldehyde | 0.35 | 1051 | KI, MS |
| 24 | Longifolene aldehyde | 3.32 | 1609 | MS |
| Acids | 7.00 | |||
| 25 | Hexadecanoic acid | 4.03 | 1977 | KI, MS |
| 26 | α-linolenic acid | 2.86 | 2165 | KI, MS |
| 27 | Octadecanoic acid | 0.11 | 2197 | KI, MS |
| Esters | 4.03 | |||
| 28 | Benzoic acid methyl ester | 3.64 | 1091 | KI, MS |
| 29 | 3,5-heptadienal-2-ethylidiene-6-methyl | 0.39 | 1345 | KI, MS |
| 30 | Other compounds | 0.68 | ||
| 31 | 4-vinylguaiacol | 0.46 | 1330 | KI, MS |
| 32 | Eugenol | 0.22 | 1363 | KI, MS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carev, I.; Golemac, A.; Siljak-Yakovlev, S.; Pellay, F.X.; Politeo, O. Volatile Oil Chemical Composition of Wild, Edible Centaurea scabiosa L. and Its Cytotoxic Activity. Plants 2022, 11, 3267. https://doi.org/10.3390/plants11233267
Carev I, Golemac A, Siljak-Yakovlev S, Pellay FX, Politeo O. Volatile Oil Chemical Composition of Wild, Edible Centaurea scabiosa L. and Its Cytotoxic Activity. Plants. 2022; 11(23):3267. https://doi.org/10.3390/plants11233267
Chicago/Turabian StyleCarev, Ivana, Anja Golemac, Sonja Siljak-Yakovlev, Francois Xavier Pellay, and Olivera Politeo. 2022. "Volatile Oil Chemical Composition of Wild, Edible Centaurea scabiosa L. and Its Cytotoxic Activity" Plants 11, no. 23: 3267. https://doi.org/10.3390/plants11233267
APA StyleCarev, I., Golemac, A., Siljak-Yakovlev, S., Pellay, F. X., & Politeo, O. (2022). Volatile Oil Chemical Composition of Wild, Edible Centaurea scabiosa L. and Its Cytotoxic Activity. Plants, 11(23), 3267. https://doi.org/10.3390/plants11233267










