Exploring the Plant Growth-Promotion of Four Streptomyces Strains from Rhizosphere Soil to Enhance Cucumber Growth and Yield
Abstract
:1. Introduction
2. Results
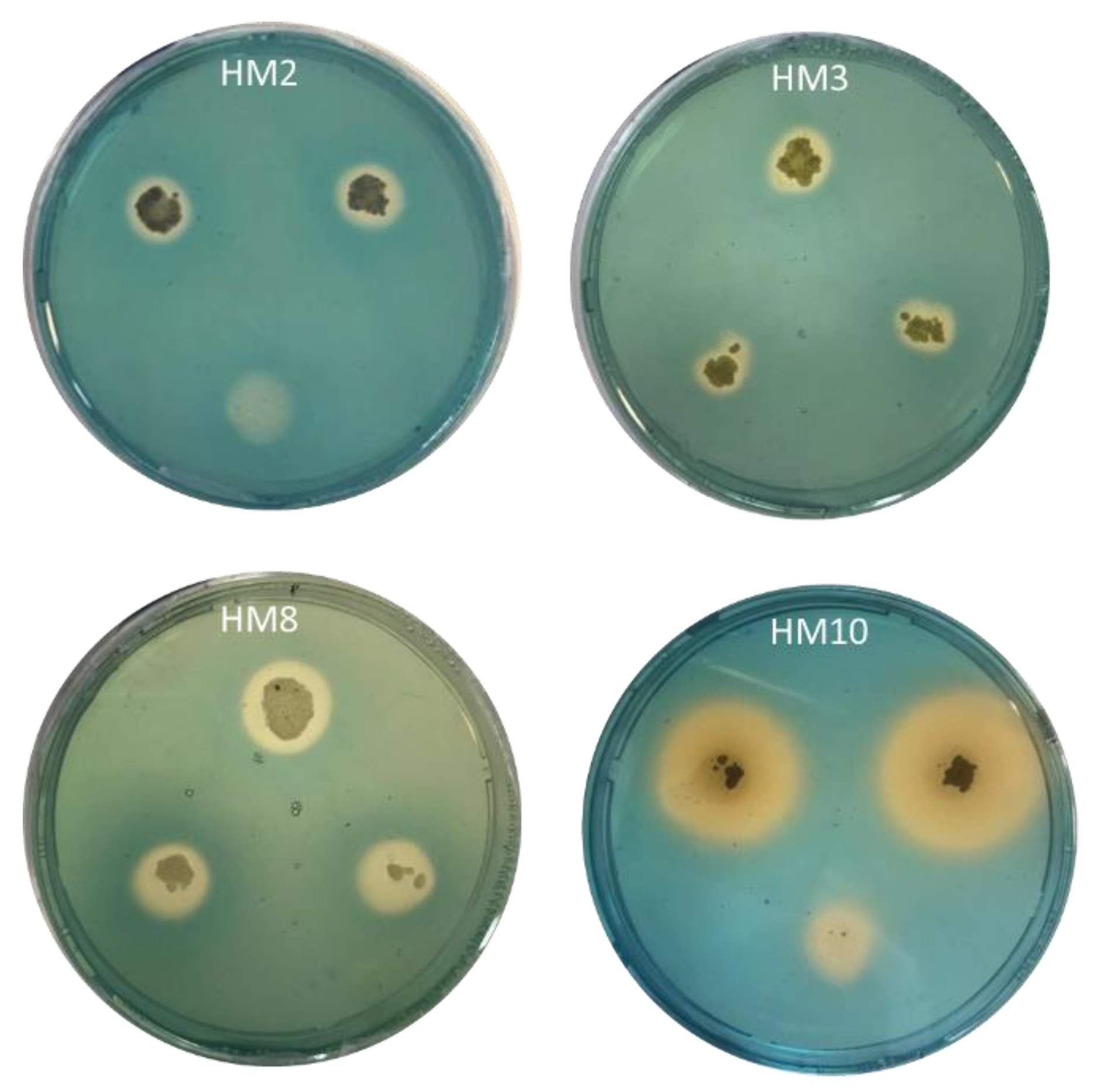
2.1. In Vitro Evaluation of Plant Growth Promotion Features
2.2. Streptomyces Strains and Their Effect on Plant Growth Promotion in Cucumber Plants
2.2.1. Growth Traits
2.2.2. Yield Traits Measurements
2.2.3. Soil and Plant Phosphorus
2.2.4. Interrelationship among Traits
3. Discussion
4. Materials and Methods
4.1. Lab Work
4.1.1. Streptomyces Strains and Cultural Conditions
4.1.2. In Vitro Evaluation of IAA, Siderophore, and Phosphate Solubilization of Streptomyces Strains
4.1.3. Extraction, Purification, and Determination of IAA by HPLC
4.2. Greenhouse Work
4.2.1. Experimental Site and Agronomic Practices
4.2.2. Streptomyces Treatments under Greenhouse
4.2.3. Measured Traits
Morphological and Growth Traits
Grain Yield and Its Related Traits
Statistical Analysis and Principal Component Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Katsenios, N.; Andreou, V.; Sparangis, P.; Djordjevic, N.; Giannoglou, M.; Chanioti, S.; Kasimatis, C.-N.; Kakabouki, I.; Leonidakis, D.; Danalatos, N.; et al. Assessment of plant growth promoting bacteria strains on growth, yield and quality of sweet corn. Sci. Rep. 2022, 12, 11598. [Google Scholar] [CrossRef] [PubMed]
- Kusale, S.P.; Attar, Y.C.; Sayyed, R.Z.; Malek, R.A.; Ilyas, N.; Suriani, N.L.; Khan, N.; El Enshasy, H. Production of plant beneficial and antioxidants metabolites by Klebsiella variicola under salinity Stress. Molecules 2021, 26, 1894. [Google Scholar] [CrossRef]
- Khumairah, F.H.; Setiawati, M.R.; Fitriatin, B.N.; Simarmata, T.; Alfaraj, S.; Ansari, M.J.; Enshasy, H.E.; Sayyed, R.Z.; Najafi, S. Halotolerant Plant Growth Promoting Rhizobacteria isolated from Saline Soil Improve Nitrogen fixation and alleviate Salt Stress. Front. Microbiol. 2022, 13, 905210. [Google Scholar] [CrossRef] [PubMed]
- Nithyapriya, S.; Lalitha, S.; Sayyed, R.Z.; Reddy, M.S.; Dailin, D.J.; El Enshasy, H.A.; Luh, N.; Herlambang, S. Production, purification, and characterization of bacillibactin siderophore of Bacillus subtilis and its application for improvement in plant growth and oil content in sesame. Sustainability 2021, 13, 5394. [Google Scholar] [CrossRef]
- Sharma, S.; Sayyed, R.; Sonawane, M.; Trivedi, M.; Thivakaran, G. Neurospora sp SR8, a novel phosphate solubiliser from rhizosphere of soil of Sorghum in Kachh, Gujarat. Indian. J. Exp. Biol. 2016, 54, 644–649. [Google Scholar]
- Baba, Z.A.; Hamid, B.; Sheikh, T.A.; Alotaibi, S.; Enshasy, H.E.; Ansari, J.A.; Zuan, A.T.K.; Sayyed, R.Z. Psychrotolerant Mesorhizobium sp. Isolated from temperate and cold desert regions solubilize Potassium and produces multiple plant growth promoting metabolites. Molecules 2021, 26, 5758. [Google Scholar] [CrossRef]
- Sagar, A.; Sayyed, R.Z.; Ramteke, P.W.; Sharma, S.; Marraiki, N.; Elgorban, A.M.; Syed, A. ACC deaminase and antioxidant enzymes producing halophilic Enterobacter sp. PR14 promotes the growth of rice and millets under salinity stress. Physiol. Mol Biol. Plants 2020, 26, 1847–1854. [Google Scholar] [CrossRef]
- Khan, N.; Al, S.; Shahi, M.A.; Mustafa, A.; Sayyed, R.Z.; Curaá, J.A. Insights into the Interactions among Roots, Rhizosphere and Rhizobacteria for Improving Plant Growth and Tolerance to Abiotic Stresses: A Review. Cells 2021, 10, 1551. [Google Scholar] [CrossRef]
- Bhat, B.A.; Tariq, L.; Nissar, S.; Islam, S.T.; Islam, S.U.; Mangral, Z.; Ilyas, N.; Sayyed, R.Z.; Muthusamy, G.; Kim, W.; et al. Plant-associated rhizobacteria in plant growth and metabolism as a tool for sustainable agriculture. J. Appl. Microbiol. 2022, 1–25. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; Enshasy, H.E. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; Enshasy, H.E.; Gafur, A.; et al. Bacterial Plant Biostimulants: A Sustainable Way towards Improving Growth, Productivity, and Health of Crops. Sustainability 2021, 21, 2856. [Google Scholar] [CrossRef]
- Zakaria, A.K.; Sayyed, R.Z.; Enshasy, H.E. Biosynthesis of Antibiotics by PGPR and Their Roles in Biocontrol of Plant Diseases. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management Rhizobacteria in Biotic Stress Management; Springer: Singapore, 2019; Volume II, pp. 1–36. [Google Scholar]
- Vinay, J.U.; Naik, M.K.; Rangeshwaran, R.; Chennappa, G.; Shaikh, S.S.; Sayyed, R.Z. Detection of antimicrobial traits in fluorescent pseudomonads and molecular characterization of an antibiotic pyoluteorin. 3 Biotech 2016, 6, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reshma, P.; Naik, M.K.; Aiyaz, M.; Niranjana, S.R.; Chennappa, G.; Shaikh, S.S.; Sayyed, R.Z. Induced systemic resistance by 2,4diacetylphloroglucinol positive fluorescent Pseudomonas strains against rice sheath blight. Indian J. Exp. Biol. 2018, 56, 207–212. [Google Scholar]
- Sagar, A.; Yadav, S.S.; Sayyed, R.Z.; Sharma, S.; Ramteke, P.W. Bacillus subtilis: A Multifarious Plant Growth Promoter, Biocontrol Agent, and Bioalleviator of Abiotic Stress. In Bacilli in Agrobiotechnology. Bacilli in Climate Resilient Agriculture and Bioprospecting; Islam, M.T., Rahman, M., Pandey, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; ISBN 978-3-030-85464-5. [Google Scholar]
- Patel, S.; Sayyed, R.; Saraf, R. Bacterial Determinants and Plant Defense Induction: Their Role as Bio-Control Agent. In Agriculture Plant Soil Microbes; Springer: Cham, Switzerland, 2016; pp. 187–204. [Google Scholar]
- Khan, A.; Sayyed, R.Z.; Seifi, S. Rhizobacteria: Legendary Soil Guards in Abiotic Stress Management. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management Abiotic Stress Management Sayyed; Arora, N.K., Reddy, M.S., Eds.; Springer: Singapore, 2019; Volume I, pp. 27–342. [Google Scholar]
- Lopes, M.J.D.S.; Dias-Filho, M.B.; Gurgel, E.S.C. Successful Plant Growth-Promoting Microbes: Inoculation Methods and Abiotic Factors. Front. Sustain. Food Syst. 2021, 5. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant Growth-Promoting Bacteria: Biological Tools for the Mitigation of Salinity Stress in Plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef]
- Abdelaal, K.; AlKahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The Role of Plant Growth-Promoting Bacteria in Alleviating the Adverse Effects of Drought on Plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- Schillaci, M. The Role of Plant Growth-Promoting Bacteria in the Growth of Cereals under Abiotic Stresses; Gupta, S., Ed.; IntechOpen: Rijeka, Croatia, 2019; p. 4. ISBN 978-1-78985-310-0. [Google Scholar]
- Noshin, I.; Mumtaz, K.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Khan, W.; El Enshasy, H.A.; Dailin, D.J.K.; Elsayed, E.A.; Ali, Z. Exopolysaccharides Producing Bacteria for the Amelioration of Drought Stress in Wheat. Sustainability 2020, 12, 8876. [Google Scholar] [CrossRef]
- Kusale, S.P.; Attar, Y.C.; Sayyed, R.Z.; Enshasy, H.E.; Hanapi, Z.; Ilyas, N.; Elgorban, A.M.; Bahkali, A.H.; Marraiki, N. Inoculation of Klebsiella variicola Alleviated slat stress Salinity and Improved Growth and Nutrients in Wheat and Maize. Agronomy 2021, 8, 927. [Google Scholar] [CrossRef]
- Kapadia, C.; Sayyed, R.Z.; Enshasy, H.E.E.; Vaidya, H.; Sharma, D.; Patel, V.; Malek, R.A.; Syed, A.; Elgorban, A.M.; Ahmad, K.; et al. Halotolerant microbial consortia for sustainable mitigation of salinity stress, growth promotion, and mineral uptake in tomato plant and soil nutrient enrichment. Sustainability 2021, 13, 8369. [Google Scholar] [CrossRef]
- Manasa, M.; Ravinder, P.; Gopalakrishnan, S.; Srinivas, V.; Sayyed, R.Z.; Enshasy, H.E.; Yahayu, M.; Zuan, A.T.K.; Kassem, H.S.; Hameeda, B. Co-inoculation of Bacillus spp. for growth promotion and iron fortification in sorghum. Sustainability 2021, 13, 12091. [Google Scholar] [CrossRef]
- Kannepalli, A.; Davranov, K.; Narimanov, A.; Enakiev, Y.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Wirth, S.; Sayyed, R.Z.; Gafur, A. Co-inoculation of rhizobacteria promotes growth, yield, and nutrient contents in soybean and improves soil enzymes and nutrients under drought conditions. Sci. Rep. 2021, 11, 22081. [Google Scholar] [CrossRef]
- Najafi, S.; Nasi, H.N.; Tuncturk, R.; Tuncturk, M.; Sayyed, R.Z.; Amirnia, R. Biofertilizer application enhances drought stress tolerance and alters the antioxidant enzymes in medicinal pumpkin (Cucurbita pepo convar. pepo var. Styriaca). Horticulturae 2021, 7, 588. [Google Scholar] [CrossRef]
- Sagar, A.; Rai, S.; Ilyas, N.; Sayyed, R.Z.; Al-Turki, A.I.; Enshasy, H.E.; Simarmata, T. Halotolerant Rhizobacteria for Salinity Stress Mitigation: Diversity, Mechanism and Molecular Approaches. Sustainability 2022, 14, 490. [Google Scholar] [CrossRef]
- Ilyas, N.; Akhtar, N.; Naseem, A.; Qureshi, R.; Majeed, A.; Sayyed, R.Z. The Potential of Bacillus subtilis and Phosphorus in Improving the Growth of Wheat under chromium Stress. J. Appl. Microbiol. 2022, 133, 3307–3321. [Google Scholar] [CrossRef]
- Zope, V.P.; El Enshasy, H.; Sayyed, R.Z. Plant Growth Promoting Rhizobacteria: An Overview in Agricultural Perspectives. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management Rhizobacteria in Biotic Stress Management; Sayyed, R.Z., Ed.; Springer: Singapore, 2019; Volume II, pp. 345–362. [Google Scholar]
- Shaikh, S.S.; Reddy, M.S.; Sayyed, R.Z. Plant Growth Promoting Rhizobacteria: An Eco-Friendly Approach for Sustainable Agro-Ecosystem Plant Soil-Microbes; Springer: Cham, Switzerland, 2016; pp. 182–201. [Google Scholar]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; Enshasy, H.E.; Dailin, D.J.; Suriani, N.L. Recent understanding of soil Acidobacteria and their ecological significance: A critical review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef]
- Amaresan, N.; Kumar, K.; Naik, J.H.; Bapatla, K.G.; Mishra, R.K. Chapter 8—Streptomyces in Plant Growth Promotion: Mechanisms and Role; Singh, B.P., Gupta, V.K., Passari, A.K.B.T.-N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 125–135. ISBN 978-0-444-63994-3. [Google Scholar]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant Growth Promoting and Biocontrol Activity of Streptomyces spp. as Endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef] [Green Version]
- Manullang, W.; Chuang, H. Streptomyces sp. mitigates abiotic stress response and promotes plant growth. J. Plant Prot. Res. 2020, 60, 263–274. [Google Scholar] [CrossRef]
- uárez-Moreno, Z.R.; Vinchira-Villarraga, D.M.; Vergara-Morales, D.I.; Castellanos, L.; Ramos, F.A.; Guarnaccia, C.; Degrassi, G.; Venturi, V.; Moreno-Sarmiento, N. Plant-Growth Promotion and Biocontrol Properties of Three Streptomyces spp. Isolates to Control Bacterial Rice Pathogens. Front. Microbiol. 2019, 10, 290. [Google Scholar] [CrossRef] [Green Version]
- de Lima Procópio, R.E.; da Silva, I.R.; Martins, M.K.; de Azevedo, J.L.; de Araújo, J.M. Antibiotics produced by Streptomyces. Braz. J. Infect. Dis. 2012, 16, 466–471. [Google Scholar] [CrossRef] [Green Version]
- Maiti, P.K.; Das, S.; Sahoo, P.; Mandal, S. Streptomyces sp SM01 isolated from Indian soil produces a novel antibiotic picolinamycin effective against multi drug resistant bacterial strains. Sci. Rep. 2020, 10, 10092. [Google Scholar] [CrossRef] [PubMed]
- Manteca, Á.; Yagüe, P. Streptomyces as a Source of Antimicrobials: Novel Approaches to Activate Cryptic Secondary Metabolite Pathways; Kırmusaoğlu, S., Ed.; IntechOpen: Rijeka, Croatia, 2019; p. 7. ISBN 978-1-78985-790-0. [Google Scholar]
- Pimentel-Elardo, S.M.; Kozytska, S.; Bugni, T.S.; Ireland, C.M.; Moll, H.; Hentschel, U. Anti-Parasitic Compounds from Streptomyces sp. Strains Isolated from Mediterranean Sponges. Mar. Drugs 2010, 8, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Myo, E.M.; Ge, B.; Ma, J.; Cui, H.; Liu, B.; Shi, L.; Jiang, M.; Zhang, K. Indole-3-acetic acid production by Streptomyces fradiae NKZ-259 and its formulation to enhance plant growth. BMC Microbiol. 2019, 19, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crane, M.; Wehner, T.C.; Naegele, R.P. Cucumber Cultivars for Container Gardening and the Value of Field Trials for Predicting Cucumber Performance in Containers. HortScience 2018, 53, 16–22. [Google Scholar] [CrossRef]
- Waseem, K.; Kamran, Q.; Jilani, M. Effect of different nitrogen levels on growth and yield of cucumber (Cucumis sativus L.). J. Agric. Res. 2008, 46, 259–266. [Google Scholar]
- Agriculture, M. Statistical Yearbook; UN iLibrary: Riyadh, Saudi Arabia, 2020. [Google Scholar]
- Hunt, R.; Causton, D.R.; Shipley, B.; Askew, A.P. A modern tool for classical plant growth analysis. Ann. Bot. 2002, 90, 485–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esitken, A.; Eken, C. Effects of mycorrhiza isolates on symbiotic germination of terrestrial orchids (Orchis palustris Jacq. and Serapias vomeracea subsp. vomeracea (Burm.f.) Briq.) in Turkey. Symbiosis 2005, 38, 59–68. [Google Scholar]
- Zhao, Y.; Wang, P.; Li, J.; Chen, Y.; Ying, X.; Liu, S. The effects of two organic manures on soil properties and crop yields on a temperate calcareous soil under a wheat–maize cropping system. Eur. J. Agron. 2009, 31, 36–42. [Google Scholar] [CrossRef]
- El-Shaikh, K.A.; Mohammed, M.S. Enhancing fresh and seed yield of okra and reducing chemical phosphorus fertilizer via using VA-mycorrhizal inoculants. World J. Agric. Sci. 2009, 5, 810–818. [Google Scholar]
- Dursun, A.; Ekinci, M.; Dönmez, M.F. Effects of foliar application of plant growth promoting bacterium on chemical contents, yield and growth of tomato (Lycopersicon esculentum L.) and cucumber (Cucumis sativus L.). Pakistan J. Bot. 2010, 42, 3349–3356. [Google Scholar]
- Isfahani, F.M.; Besharati, H. Effect of biofertilizers on yield and yield components of cucumber. J. Biol. Earth Sci. 2012, 2, 83–92. [Google Scholar]
- Isfahani, F.M.; Isfahani, S.M.; Besharati, H.; Tarighaleslami, M. Yield and concentration of some macro and micro nutrients of cucumber as influenced by bio-fertilizers. Ann. Biol. Res. 2013, 4, 61–67. [Google Scholar]
- de Sousa, J.A.; Olivares, F.L. Plant growth promotion by streptomycetes: Ecophysiology, mechanisms and applications. Chem. Biol. Technol. Agric. 2016, 3, 24. [Google Scholar] [CrossRef] [Green Version]
- Dimpika, D.C.; Jia, Z.; McLean, M.J.; Britt, D.W.; Zhan, J.; Anderson, A.J. Production of Indole-3-Acetic Acid via the Indole-3-Acetamide Pathway in the Plant-Beneficial Bacterium Pseudomonas chlororaphis O6 Is Inhibited by ZnO Nanoparticles but Enhanced by CuO Nanoparticles. Appl. Environ. Microbiol. 2012, 78, 1404–1410. [Google Scholar] [CrossRef] [Green Version]
- Khoi, N. Isolation and Characterization of Indole Acetic Acid Producing Halophilic Bacteria from Salt Affected Soil of Rice–Shrimp Farming System in the Mekong Delta, Vietnam. Agric. For. Fish. 2017, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Lebrazi, S.; Fadil, M.; Chraibi, M.; Fikri-Benbrahim, K. Screening and optimization of indole-3-acetic acid production by Rhizobium sp. strain using response surface methodology. J. Genet. Eng. Biotechnol. 2020, 18, 21. [Google Scholar] [CrossRef]
- Kaur, T.; Manhas, R.K. Evaluation of ACC deaminase and indole acetic acid production by Streptomyces hydrogenans DH16 and its effect on plant growth promotion. Biocatal. Agric. Biotechnol. 2022, 42, 102321. [Google Scholar] [CrossRef]
- Anwar, S.; Ali, B.; Sajid, I. Screening of Rhizospheric Actinomycetes for Various In-vitro and In-vivo Plant Growth Promoting (PGP) Traits and for Agroactive Compounds. Front. Microbiol. 2016, 7, 1334. [Google Scholar] [CrossRef]
- Rehan, M.; Barakat, H.; Almami, I.S.; Qureshi, K.A.; Alsohim, A.S. Production and Potential Genetic Pathways of Three Different Siderophore Types in Streptomyces tricolor Strain HM10. Fermentation 2022, 8, 346. [Google Scholar] [CrossRef]
- Chouyia, F.E.; Romano, I.; Fechtali, T.; Fagnano, M.; Fiorentino, N.; Visconti, D.; Idbella, M.; Ventorino, V.; Pepe, O. P-Solubilizing Streptomyces roseocinereus MS1B15 With Multiple Plant Growth-Promoting Traits Enhance Barley Development and Regulate Rhizosphere Microbial Population. Front. Plant Sci. 2020, 11, 1137. [Google Scholar] [CrossRef]
- Cui, K.; Xu, T.; Chen, J.; Yang, H.; Liu, X.; Zhuo, R.; Peng, Y.; Tang, W.; Wang, R.; Chen, L.; et al. Siderophores, a potential phosphate solubilizer from the endophyte Streptomyces sp. CoT10, improved phosphorus mobilization for host plant growth and rhizosphere modulation. J. Clean. Prod. 2022, 367, 133110. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, S. Identification and Characterization of the Phosphate-Solubilizing Bacterium Pantoea sp. S32 in Reclamation Soil in Shanxi, China. Front. Microbiol. 2019, 10, 2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eifediyi, E.K.; Remison, S.U. The effects of inorganic fertilizer on the yield of two varieties of cucumber (Cucumis sativus L.). Rep. Opin. 2010, 2, 1–5. [Google Scholar]
- Li, Y.; Mattson, N.S. Effect of Organic Fertilizer Source and Rate on Growth and Nutrient Leachate Profile of Greenhouse-grown Cucumber. HortTechnology Hortte 2019, 29, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Kareem, M.; Almubarak, N. Effect of organic and bio fertilizers on the vegetative traits of the sugarcane plant (Saccharum officinarum L.). Plant Arch. 2020, 20, 5653–5660. [Google Scholar]
- AL-Taey, D.K.A.; AL-Shmary, R.F. The Impact of Bio-Organic and N, P, K Fertilizers on the Growth and Yield of Potato; Yildiz, M., Ozgen, Y., Eds.; IntechOpen: Rijeka, Croatia, 2021; p. 3. ISBN 978-1-83969-167-6. [Google Scholar]
- Ye, S.; Peng, B.; Liu, T. Effects of organic fertilizers on growth characteristics and fruit quality in Pear-jujube in the Loess Plateau. Sci. Rep. 2022, 12, 13372. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Bairwa, H.L.; Kumar, U.; Javed, T.; Asad, M.; Lal, K.; Mahawer, L.N.; Sharma, S.K.; Singh, P.; Hassan, M.M.; et al. Influence of organic manures on soil nutrient content, microbial population, yield and quality parameters of pomegranate (Punica granatum L.) cv. Bhagwa. PLoS ONE 2022, 17, e0266675. [Google Scholar] [CrossRef]
- Zainuddin, N.; Keni, M.F.; Ibrahim, S.A.S.; Masri, M.M.M. Effect of integrated biofertilizers with chemical fertilizers on the oil palm growth and soil microbial diversity. Biocatal. Agric. Biotechnol. 2022, 39, 102237. [Google Scholar] [CrossRef]
- Moradzadeh, S.; Moghaddam, S.S.; Rahimi, A.; Pourakbar, L.; Sayyed, R.Z. Combined bio-chemical fertilizers, ameliorate agro-biochemical attributes of black cumin (Nigella sativa L.). Sci. Rep. 2021, 11, 11399. [Google Scholar] [CrossRef]
- Bastami, A.; Amirnia, R.; Sayyed, R.Z.; Enshasy, H.E. The effect of mycorrhizal fungi and organic fertilizers on quantitative and qualitative traits of two important Satureja species. Agronomy 2021, 11, 1285. [Google Scholar] [CrossRef]
- Rahimi, A.; Amirnia, R.; Moghaddam, S.S.; Enshasy, H.A.E.; Hanapi, S.Z.; Sayyed, R.Z. Effect of different biological and organic fertilizer sources on the quantitative and qualitative traits of Cephalaria syriaca. Horticulturae 2021, 7, 397. [Google Scholar] [CrossRef]
- Faridvand, S.; Amirnia, R.; Tajbakhsh, M.; El Enshasy, H.A.; Sayyed, R.Z. The effect of foliar application of magnetic water and nano, organic, and chemical fertilizers on phytochemical and yield characteristics of different landraces of fennel (Foeniculum vulgare Mill). Horticulturae 2021, 7, 475. [Google Scholar] [CrossRef]
- Sagar, A.; Sayyed, R.Z.; Ramteke, P.W.; Ramakrishna, W.; Poczai, P.; Obaid, S.A.; Ansari, M.J. Synergistic effect of Azotobacter nigricans and NPK fertilizer on agronomic and yield traits of Maize (Zea mays L.). Front. Plant Sci. 2022, 13, 952212. [Google Scholar] [CrossRef]
- El Karamany, M.F.; Sadak, M.S.; Bakry, B.A. Improving quality and quantity of mungbean plant via foliar application of plant growth regulators in sandy soil conditions. Bull. Natl. Res. Cent. 2019, 43, 61. [Google Scholar] [CrossRef]
- Rezk, E.; Abd, N.; Robin, P.; Akkal-Corfini, N.; El-Rahman, L. Effects of Different Organic and Inorganic Fertilizers on Cucumber Yield and Some Soil Properties. World J. Agric. Sci. 2009, 5, 408–414. [Google Scholar]
- Ashraf, M.Y.; Azhar, N.; Hussain, M. Indole acetic acid (IAA) induced changes in growth, relative water contents and gas exchange attributes of barley (Hordeum vulgare L.) grown under water stress conditions. Plant Growth Regul. 2006, 50, 85–90. [Google Scholar] [CrossRef]
- Abdrabbo, M.; Desoky, H. Enhancing Organic Production of Cucumber by using Plant Growth Promoting Rhizobacteria and Compost Tea under Sandy Soil Condition. Res. J. Agric. Biol. Sci. 2014, 10, 162–169. [Google Scholar]
- Azarmi, R.; Torabi Giglou, M.; Hajieghrari, B. The effect of sheep-manure vermicompost on quantitative and qualitative properties of cucumber (Cucumis sativus L.) grown in the greenhouse. Afr. J. Biotechnol. 2009, 8. [Google Scholar] [CrossRef]
- Abo-Yousef, M. Application for Hybrid Rice Technology at Egypt. Ann. Agric. Sci. Moshtohor 2018, 56, 3–16. [Google Scholar] [CrossRef]
- Nair, A.; Ngouajio, M. Integrating Rowcovers and Soil Amendments for Organic Cucumber Production: Implications on Crop Growth, Yield, and Microclimate. HortScience 2010, 45, 566–574. [Google Scholar] [CrossRef] [Green Version]
- Zapata-Sifuentes, G.; Hernandez-Montiel, L.G.; Saenz-Mata, J.; Fortis-Hernandez, M.; Blanco-Contreras, E.; Chiquito-Contreras, R.G.; Preciado-Rangel, P. Plant Growth-Promoting Rhizobacteria Improve Growth and Fruit Quality of Cucumber under Greenhouse Conditions. Plants 2022, 11, 1612. [Google Scholar] [CrossRef]
- Tahir, S.M.; Kabir, A.K.M.R.; Ibrahim, H.H.; Sufiyanu, S. Studies on the performance of organic and inorganic fertilizer on the growth and yield of cucumber (Cucumis sativus L.). Sci. World J. 2019, 14, 156–163. [Google Scholar]
- Valdenegro, M.; Bernales, M.; Knox, M.; Vinet, R.; Caballero, E.; Ayala-Raso, A.; Kučerová, D.; Kumar, R.; Viktorová, J.; Ruml, T.; et al. Characterization of Fruit Development, Antioxidant Capacity, and Potential Vasoprotective Action of Peumo (Cryptocarya alba), a Native Fruit of Chile. Antioxidants 2021, 10, 1997. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Radhakrishnan, R.; You, Y.-H.; Khan, A.L.; Park, J.-M.; Lee, S.-M.; Lee, I.-J. Cucumber performance is improved by inoculation with plant growth-promoting microorganisms. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2015, 65, 36–44. [Google Scholar] [CrossRef]
- Jog, R.; Nareshkumar, G.; Rajkumar, S. Plant growth promoting potential and soil enzyme production of the most abundant Streptomyces spp. from wheat rhizosphere. J. Appl. Microbiol. 2012, 113, 1154–1164. [Google Scholar] [CrossRef]
- Sadeghi, A.; Karimi, E.; Dahaji, P.A.; Javid, M.G.; Dalvand, Y.; Askari, H. Plant growth promoting activity of an auxin and siderophore producing isolate of Streptomyces under saline soil conditions. World J. Microbiol. Biotechnol. 2012, 28, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Jog, R.; Pandya, M.; Nareshkumar, G.; Rajkumar, S. Mechanism of phosphate solubilization and antifungal activity of Streptomyces spp. isolated from wheat roots and rhizosphere and their application in improving plant growth. Microbiology 2014, 160, 778–788. [Google Scholar] [CrossRef] [Green Version]
- Sreevidya, M.; Gopalakrishnan, S.; Kudapa, H.; Varshney, R.K. Exploring plant growth-promotion actinomycetes from vermicompost and rhizosphere soil for yield enhancement in chickpea. Braz. J. Microbiol. 2016, 47, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Awan, S.A.; Ikram, R.; Rizwan, M.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Ali, S.; Ilyas, N. 24-Epibrassinolide regulated antioxidants and osmolyte defense and endogenous hormones in two wheat varieties under drought stress. Physiol. Plant. 2020, 172, 696–706. [Google Scholar] [CrossRef]
- Sheikh, T.; Baba, Z.; Hamid, B.; Iqbal, S.; Yatoo, A.; Fatima, S.; Nabi, A.; Kanth, R.; Dar, K.; Hussain, N.; et al. Extracellular polymeric substances in psychrophilic cyanobacteria: A potential bioflocculant and carbon sink to mitigate cold stress. Biocatal. Agric. Biotechnol. 2022, 42, 102375. [Google Scholar] [CrossRef]
- Kapadia, C.; Patel, N.; Rana, A.; Vaidya, H.; Alfarraj, A.; Ansari, M.J.; Gafur, A.; Poczai, P.; Sayyed, R.Z. Evaluation of Plant Growth Promoting and Salinity Ameliorating Potential of Halophillic Bacteria Isolated from Saline Soil. Front. Plant Sci. 2022, 13, 946217. [Google Scholar] [CrossRef] [PubMed]
- Gowtham, H.G.; Singh, S.B.; Shilpa, N.; Aiyaz, M.; Nataraj, K.; Udayashankar, A.C.; Amruthesh, K.N.; Murali, M.; Poczai, P.; Gafur, A.; et al. Insight into Recent Progress and Perspectives in Improvement of Antioxidant Machinery Upon PGPR Augmentation in Plants Under Drought Stress: A Review. Antioxidants 2022, 11, 1763. [Google Scholar] [CrossRef] [PubMed]
- Fazeli-Nasab, B.; Sayyed, R.Z. Plant Growth Promoting Rhizobacteria and Salinity Stress: A Journey into the Soil. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management Vol 1 Abiotic Stress Management Sayyed; Arora, N.K., Reddy, M.S., Eds.; Springer: Singapore, 2019; pp. 21–34. [Google Scholar]
- Sherpa, M.T.; Bag, N.; Das, S.; Haokip, P.; Sharma, L. Isolation and characterization of plant growth promoting rhizobacteria isolated from organically grown high yielding pole type native pea (Pisum sativum L.) variety Dentami of Sikkim, India. Curr. Res. Microb. Sci. 2021, 2, 100068. [Google Scholar] [CrossRef] [PubMed]
- Rehan, M.; ALSohim, A.S.; Abidou, H.; Rasheed, Z.; Abdulmonem, W.A.L. Isolation, Identification, Biocontrol Activity, and Plant Growth Promoting Capability of a Superior Streptomyces tricolor Strain HM10. Pol. J. Microbiol. 2021, 70, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Analysis of Indole-3-acetic Acid (IAA) Production in Klebsiellaby LC-MS/MS and the Salkowski Method. Bio.-Protocol. 2019, 9, e3230. [Google Scholar] [CrossRef]
- Louden, B.C.; Haarmann, D.; Lynne, A.M. Use of Blue Agar CAS Assay for Siderophore Detection. J. Microbiol. Biol. Educ. 2011, 12, 51–53. [Google Scholar] [CrossRef] [Green Version]
- Arora, N.K.; Verma, M. Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech 2017, 7, 381. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.R.; Shaikh, S.S.; Sayyed, R.Z. Modified chrome azurol S method for detection and estimation of siderophores having affinity for metal ions other than iron. Environ. Sustain. 2018, 1, 81–87. [Google Scholar] [CrossRef]
- Payne, S.M. Iron acquisition in microbial pathogenesis. Trends Microbiol. 1993, 1, 66–69. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Maynard, D.N.; Hochmuth, G.J. Knott’s Handbook for Vegetable Growers; Wiley: Hoboken, NJ, USA, 2013; ISBN 9781118686102. [Google Scholar]
- Page, A.L. Methods of soil analysis. In Part 2—Chemical and Microbiological Properties; The American Society of Agronomy, Inc., Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar]
- Rathje. Jackson, M.L.: Soil chemical analysis. Verlag: Prentice Hall, Inc., Englewood Cliffs, NJ. 1958, 498 S. DM 39.40. Z. Für Pflanz. Düngung Bodenkd. 1959, 85, 251–252. [Google Scholar] [CrossRef]
- Cho, Y.Y.; Oh, S.; Oh, M.M.; Son, J.E. Estimation of individual leaf area, fresh weight, and dry weight of hydroponically grown cucumbers (Cucumis sativus L.) using leaf length, width, and SPAD value. Sci. Hortic. 2007, 111, 330–334. [Google Scholar] [CrossRef]
- Ding, X.; Yu, L.; Jiang, Y.; Yang, S.; He, L.; Zhou, Q.; Yu, J.; Huang, D. Changes in Leaf Length, Width, Area, and Photosynthesis of Fruit Cucumber in a Greenhouse Production System. HortScience Horts 2020, 55, 995–999. [Google Scholar] [CrossRef]
- Evans, G.C. The Quantitative Analysis of Plant Growth; University of California Press: Berkeley, CA, USA, 1972; Volume 1, ISBN 0520094328. [Google Scholar]
- Cottenie, A. Soil and Plant Testing as a Basis of Fertilizer Recommendations. F.A.O. Soils Bulletin. 1980. no. 38/2. p. 118. Available online: https://www.cabdirect.org/cabdirect/abstract/19806734748 (accessed on 25 October 2022).
- Khiddir, S.M. A Statistical Approach in the Use of Parametric Systems Applied to the FAO Framework for Land Evaluation; Rijksuniversiteit Gent. Faculteit van de Wetenschappen: Ghent, Belgium, 1986. [Google Scholar]
- Webster, R. Quantitative and Numerical Methods in Soil Classification and Survey; Transport and Road Research Laboratory (TRRL): Oxford, UK; Clarendon Press: London, UK, 1977. [Google Scholar]
- Addinsoft XLSTAT. Statistical and Data Analysis Solution; Addinsoft XLSTAT: New York, NY, USA, 2019. [Google Scholar]






| Treatments | Plant Height (cm) | No. of Leaves Plant−1 | Root Length (cm) | Leaf DM (%) | Stem DM (%) | Root DM (%) |
|---|---|---|---|---|---|---|
| T1 | 133.00 c | 17.33 c | 21.33 bc | 13.55 b | 5.57 a | 5.84 ab |
| T2 | 124.00 c | 16.67 c | 17.00 c | 16.11 a | 6.42 a | 6.21 ab |
| T3 | 187.33 ab | 25.00 ab | 19.33 c | 13.08 b | 5.60 a | 5.32 b |
| T4 | 198.33 a | 26.67 a | 44.67 a | 14.60 ab | 5.79 a | 6.67 ab |
| T5 | 195.33 a | 26.33 a | 32.67 b | 14.01 ab | 6.02 a | 7.21 ab |
| T6 | 196.33 a | 25.00 ab | 27.67 bc | 14.74 ab | 5.85 a | 7.34 ab |
| T7 | 177.33 b | 22.67 b | 25.33 bc | 14.48 ab | 5.63 a | 11.10 a |
| Treatments | Leaf Area (LA) (cm2) | Net Assimilation Rate (NAR) (g·cm2·day−1) | Relative Growth Rate (RGR) (g·g−1·day−1) |
|---|---|---|---|
| T1 | 188.167 c | 0.008 a | 0.117 a |
| T2 | 210.067 c | 0.006 a | 0.120 a |
| T3 | 307.333 ab | 0.007 a | 0.117 a |
| T4 | 303.200 ab | 0.007 a | 0.120 a |
| T5 | 323.000 a | 0.007 a | 0.137 a |
| T6 | 323.200 a | 0.006 a | 0.127 a |
| T7 | 267.933 b | 0.006 a | 0.117 a |
| Treatments | Fruit Length (cm) | Fruit Diameter (cm) | No. of Fruit per Plant | Fruit Fresh Weight (g) | Fruit Firmness (Lbs. Inch−2) |
|---|---|---|---|---|---|
| T1 | 11.83 ab | 2.10 b | 10.00 cd | 735.68 b | 4.92 a |
| T2 | 11.50 b | 2.10 b | 8.33 d | 512.66 c | 4.83 a |
| T3 | 13.83 ab | 2.80 a | 13.33 a | 810.83 ab | 5.00 a |
| T4 | 14.33 a | 2.83 a | 13.83 a | 920.00 a | 4.90 a |
| T5 | 13.16 ab | 2.75 a | 13.16 ab | 884.50 ab | 5.00 a |
| T6 | 13.16 ab | 2.43 ab | 12.66 ab | 782.50 ab | 4.83 a |
| T7 | 13.33 ab | 2.58 a | 11.00 bc | 786.00 ab | 4.83 a |
| Treatments | Soil P (mg/kg) | Plant P (%) | ||
|---|---|---|---|---|
| Before Planting | 21 DAT | At Harvest | ||
| T1 | 2.06 c | 12.52 f | 17.92 d | 0.43 c |
| T2 | 1.16 e | 18.34 c | 13.90 g | 0.52 c |
| T3 | 5.20 a | 18.50 c | 15.02 f | 0.52 c |
| T4 | 2.48 b | 30.76 a | 26.36 a | 0.91 a |
| T5 | 1.76 cd | 27.76 b | 22.40 b | 0.75 b |
| T6 | 1.44 de | 17.42 d | 18.50 c | 0.55 c |
| T7 | 1.51 de | 13.75 e | 16.08 e | 0.53 c |
| Properties | Value | |
|---|---|---|
| Soil | Water | |
| Physical properties | ||
| Sand (%) | 94.1 | - |
| Silt (%) | 3.6 | - |
| Clay (%) | 2.3 | - |
| Texture | Sand | - |
| Chemical properties | ||
| 1 pH | 7.84 | 7.17 |
| 2 EC (dS·m−1) | 0.508 | 1.55 |
| 3 Nutrient (ppm) | ||
| Total N | 168 | - |
| Available P | 0.450 | - |
| Available K | 45.0 | 40.0 |
| 4 Dissolved Ions (meq·L−1) | ||
| (1) Dissolved anions (meq·L−1) | ||
| Cl− | 1.0 | 12.0 |
| HCO3−1 + CO3−2 | 3.0 | 3.0 |
| (2) Dissolved cations (meq·L−1) | ||
| Na+ | 1.3 | 11.9 |
| Ca++ | 3.0 | 4.0 |
| Mg++ | 1.0 | 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, A.F.; Abdelmageed, A.H.A.; Al-Turki, A.; Abdelhameid, N.M.; Sayyed, R.Z.; Rehan, M. Exploring the Plant Growth-Promotion of Four Streptomyces Strains from Rhizosphere Soil to Enhance Cucumber Growth and Yield. Plants 2022, 11, 3316. https://doi.org/10.3390/plants11233316
Omar AF, Abdelmageed AHA, Al-Turki A, Abdelhameid NM, Sayyed RZ, Rehan M. Exploring the Plant Growth-Promotion of Four Streptomyces Strains from Rhizosphere Soil to Enhance Cucumber Growth and Yield. Plants. 2022; 11(23):3316. https://doi.org/10.3390/plants11233316
Chicago/Turabian StyleOmar, Ayman F., Adil H. A. Abdelmageed, Ahmad Al-Turki, Noha M. Abdelhameid, R. Z. Sayyed, and Medhat Rehan. 2022. "Exploring the Plant Growth-Promotion of Four Streptomyces Strains from Rhizosphere Soil to Enhance Cucumber Growth and Yield" Plants 11, no. 23: 3316. https://doi.org/10.3390/plants11233316






