Diversity and Differentiation of Duckweed Species from Israel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Survey of Duckweed Strains
2.2. Duckweed Collection
2.3. Cultivation
2.4. Morphological Identification
2.5. DNA Extraction, Fragment Amplification and Sequencing
2.6. DNA Barcoding Analysis
2.7. Fatty Acids Analysis
2.8. Nitrogen Content Analysis
3. Results
3.1. Distribution of Duckweed Species in Israel
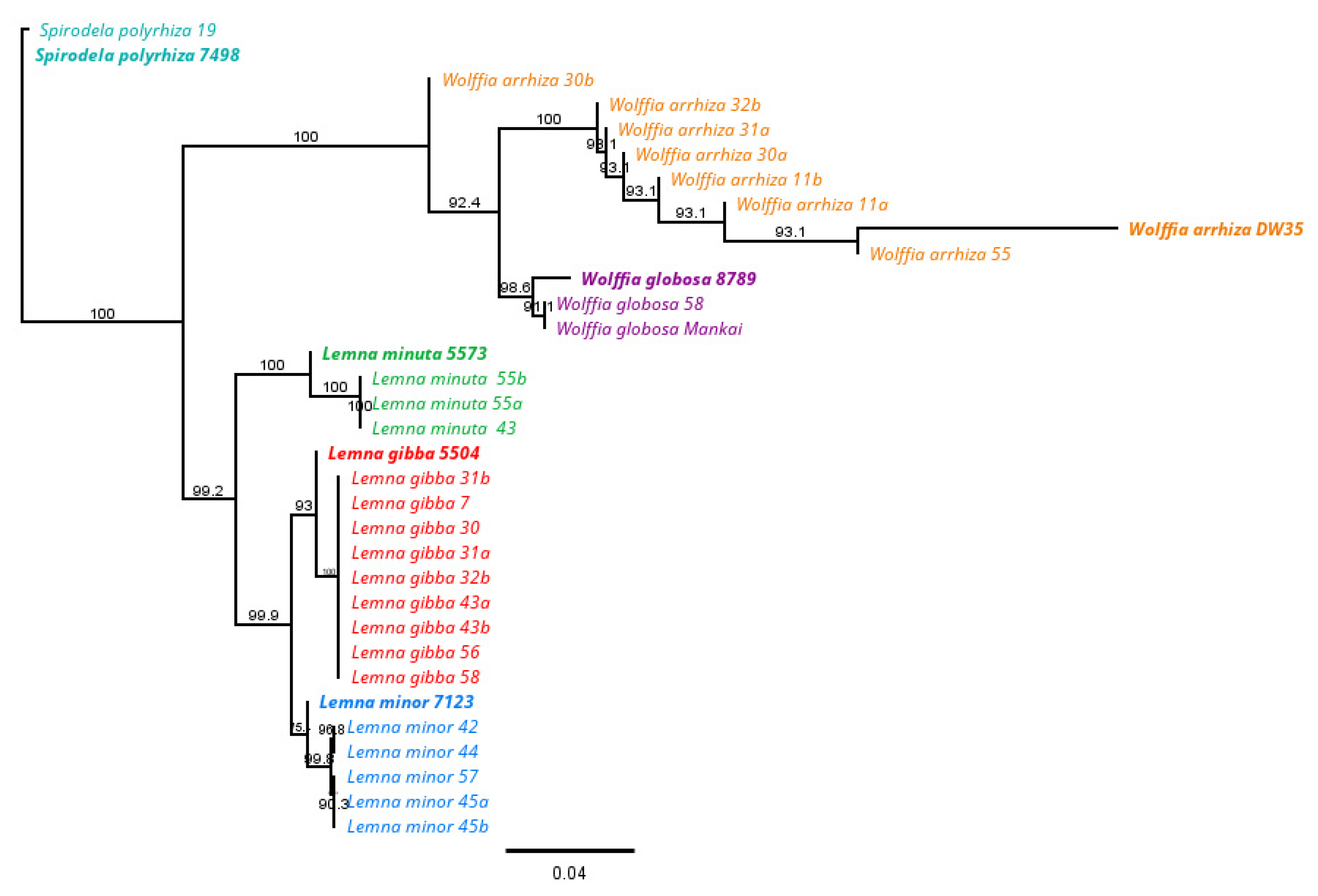
3.2. DNA Barcoding Analysis
Phylogenetic Tree
3.3. Fatty Acid Analysis
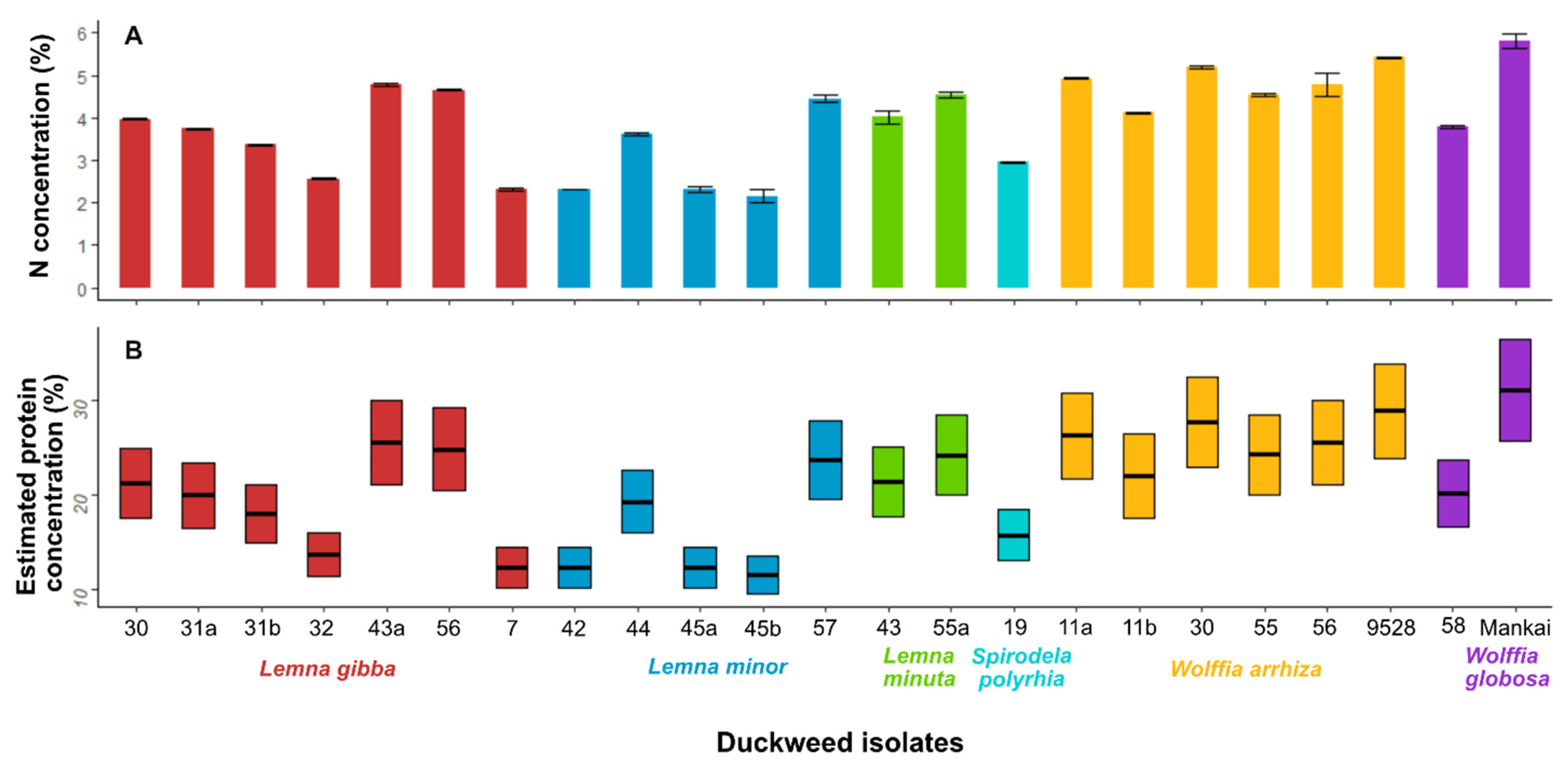
3.4. Nitrogen Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landolt, E. Biosystematic Investigations in the Family of Duckweed (Lemnaceae); Geobotanischen Inst ETH: Zurich, Switzerland, 1986. [Google Scholar]
- Bog, M.; Appenroth, K.-J.; Sree, K.S. Duckweed (Lemnaceae): Its Molecular Taxonomy. Front. Sustain. Food Syst. 2019, 3, 117. [Google Scholar] [CrossRef]
- Sree, K.S.; Sudakaran, S.; Appenroth, K.-J. How Fast Can Angiosperms Grow? Species and Clonal Diversity of Growth Rates in the Genus Wolffia (Lemnaceae). Acta Physiol. Plant 2015, 37, 204. [Google Scholar] [CrossRef]
- Appenroth, K.-J.; Sree, K.S.; Böhm, V.; Hammann, S.; Vetter, W.; Leiterer, M.; Jahreis, G. Nutritional Value of Duckweeds (Lemnaceae) as Human Food. Food Chem. 2017, 217, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Sela, I.; Yaskolka Meir, A.; Brandis, A.; Krajmalnik-Brown, R.; Zeibich, L.; Chang, D.; Dirks, B.; Tsaban, G.; Kaplan, A.; Rinott, E.; et al. Wolffia Globosa–Mankai Plant-Based Protein Contains Bioactive Vitamin B12 and Is Well Absorbed in Humans. Nutrients 2020, 12, 3067. [Google Scholar] [CrossRef] [PubMed]
- Sońta, M.; Rekiel, A.; Batorska, M. Use of Duckweed (Lemna L.) in Sustainable Livestock Production and Aquaculture—A Review. Ann. Anim. Sci. 2019, 19, 257–271. [Google Scholar] [CrossRef] [Green Version]
- Frédéric, M.; Samir, L.; Louise, M.; Abdelkrim, A. Comprehensive Modeling of Mat Density Effect on Duckweed (Lemna Minor) Growth under Controlled Eutrophication. Water Res. 2006, 40, 2901–2910. [Google Scholar] [CrossRef]
- Iqbal, J.; Javed, A.; Baig, M.A. Growth and Nutrient Removal Efficiency of Duckweed (Lemna Minor) from Synthetic and Dumpsite Leachate under Artificial and Natural Conditions. PLoS ONE 2019, 14, e0221755. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, H.; Stomp, A.-M.; Cheng, J.J. The Production of Duckweed as a Source of Biofuels. Biofuels 2012, 3, 589–601. [Google Scholar] [CrossRef]
- Ren, H.; Jiang, N.; Wang, T.; Mubashar Omar, M.; Ruan, W.; Ghafoor, A. Enhanced Biogas Production in the Duckweed Anaerobic Digestion Process. J. Energy Resour. Technol. 2018, 140, 041805. [Google Scholar] [CrossRef]
- Les, D.H.; Landolt, E.; Crawford, D.J. Systematics of TheLemnaceae (Duckweeds): Inferences from Micromolecular and Morphological Data. Plant Syst. Evol. 1997, 204, 161–177. [Google Scholar] [CrossRef]
- Crawford, D.J.; Landolt, E.; Les, D.H.; Kimball, R.T. Allozyme Studies in Lemnaceae: Variation and Relationships in Lemna Sections Alatae and Biformes. Taxon 2001, 50, 987–999. [Google Scholar] [CrossRef]
- Tang, J.; Li, Y.; Ma, J.; Cheng, J.J. Survey of Duckweed Diversity in Lake Chao and Total Fatty Acid, Triacylglycerol, Profiles of Representative Strains. Plant Biol. J. 2015, 17, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Bog, M.; Schneider, P.; Hellwig, F.; Sachse, S.; Kochieva, E.Z.; Martyrosian, E.; Landolt, E.; Appenroth, K.-J. Genetic Characterization and Barcoding of Taxa in the Genus Wolffia Horkel Ex Schleid. (Lemnaceae) as Revealed by Two Plastidic Markers and Amplified Fragment Length Polymorphism (AFLP). Planta 2013, 237, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Y.; Yan, Y.; Ermakova, M.; Kerstetter, R.; Messing, J. DNA Barcoding of the Lemnaceae, a Family of Aquatic Monocots. BMC Plant Biol. 2010, 10, 205. [Google Scholar] [CrossRef] [Green Version]
- Tippery, N.P.; Les, D.H. Tiny Plants with Enormous Potential: Phylogeny and Evolution of Duckweeds. In The Duckweed Genomes; Cao, X.H., Fourounjian, P., Wang, W., Eds.; Compendium of Plant Genomes; Springer International Publishing: Cham, Switzerland, 2020; pp. 19–38. ISBN 978-3-030-11044-4. [Google Scholar]
- Sree, K.S.; Adelmann, K.; Garcia, C.; Lam, E.; Appenroth, K.-J. Natural Variance in Salt Tolerance and Induction of Starch Accumulation in Duckweeds. Planta 2015, 241, 1395–1404. [Google Scholar] [CrossRef]
- Kuehdorf, K.; Jetschke, G.; Ballani, L.; Appenroth, K.-J. The Clonal Dependence of Turion Formation in the Duckweed Spirodela Polyrhiza—An Ecogeographical Approach. Physiol. Plantarum. 2014, 150, 46–54. [Google Scholar] [CrossRef]
- Sree, K.; Bog, M.; Appenroth, K. Taxonomy of Duckweeds (Lemnaceae), Potential New Crop Plants. Emir. J. Food Agric. 2016, 28, 291. [Google Scholar] [CrossRef] [Green Version]
- Les, D.H.; Crawford, D.J.; Kimball, R.T.; Moody, M.L.; Landolt, E. Biogeography of Discontinuously Distributed Hydrophytes: A Molecular Appraisal of Intercontinental Disjunctions. Int. J. Plant Sci. 2003, 164, 917–932. [Google Scholar] [CrossRef] [Green Version]
- Landolt, E. Lemnaceae. In Flowering Plants Monocotyledons; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 264–270. ISBN 978-3-642-08378-5. [Google Scholar]
- Kimball, R.T.; Crawford, D.J.; Les, D.H.; Landolt, E. Out of Africa: Molecular Phylogenetics and Biogeography of Wolffiella (Lemnaceae): WOLFFIELLA PHYLOGENETICS and BIOGEOGRAPHY. Biol. J. Linn. Soc. 2003, 79, 565–576. [Google Scholar] [CrossRef] [Green Version]
- Green, A.J. The Importance of Waterbirds as an Overlooked Pathway of Invasion for Alien Species. Divers. Distrib. 2016, 22, 239–247. [Google Scholar] [CrossRef]
- Frankenberg, E. Will the Biogeographical Bridge Continue to Exist? Isr. J. Zool. 1999, 45, 65–74. [Google Scholar] [CrossRef]
- Frumkin, R.; Pinshow, B.; Kleinhaus, S. A Review of Bird Migration over Israel. J. Ornithol. 1995, 136, 127–147. [Google Scholar] [CrossRef]
- Kirby, J.S.; Stattersfield, A.J.; Butchart, S.H.M.; Evans, M.I.; Grimmett, R.F.A.; Jones, V.R.; O’Sullivan, J.; Tucker, G.M.; Newton, I. Key Conservation Issues for Migratory Land- and Waterbird Species on the World’s Major Flyways. Bird Conserv. Int. 2008, 18, S49–S73. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, C.; Miranda, N.A.F.; Cumming, G.S. The Role of Waterbirds in the Dispersal of Aquatic Alien and Invasive Species. Divers. Distrib. 2015, 21, 744–754. [Google Scholar] [CrossRef]
- Yan, Y.; Candreva, J.; Shi, H.; Ernst, E.; Martienssen, R.; Schwender, J.; Shanklin, J. Survey of the Total Fatty Acid and Triacylglycerol Composition and Content of 30 Duckweed Species and Cloning of a Δ6-Desaturase Responsible for the Production of γ-Linolenic and Stearidonic Acids in Lemna Gibba. BMC Plant Biol. 2013, 13, 201. [Google Scholar] [CrossRef] [Green Version]
- Jones, D. Factors for Converting Percentages of Nitrogen in Foods and Feeds into Percentages of Proteins Re; US Department of Agriculture, USDA Publications: Washington, DC, USA, 1931. [Google Scholar]
- Appenroth, K.-J.; Sree, K.S.; Bog, M.; Ecker, J.; Seeliger, C.; Böhm, V.; Lorkowski, S.; Sommer, K.; Vetter, W.; Tolzin-Banasch, K.; et al. Nutritional Value of the Duckweed Species of the Genus Wolffia (Lemnaceae) as Human Food. Front. Chem. 2018, 6, 483. [Google Scholar] [CrossRef] [Green Version]
- Borisjuk, N.; Chu, P.; Gutierrez, R.; Zhang, H.; Acosta, K.; Friesen, N.; Sree, K.S.; Garcia, C.; Appenroth, K.J.; Lam, E. Assessment, Validation and Deployment Strategy of a Two-Barcode Protocol for Facile Genotyping of Duckweed Species. Plant Biol. J. 2015, 17, 42–49. [Google Scholar] [CrossRef]
- Braglia, L.; Breviario, D.; Gianì, S.; Gavazzi, F.; De Gregori, J.; Morello, L. New Insights into Interspecific Hybridization in Lemna L. Sect. Lemna (Lemnaceae Martinov). Plants 2021, 10, 2767. [Google Scholar] [CrossRef]
- Ho, E.K.H.; Bartkowska, M.; Wright, S.I.; Agrawal, A.F. Population Genomics of the Facultatively Asexual Duckweed Spirodela Polyrhiza. New Phytol. 2019, 224, 1361–1371. [Google Scholar] [CrossRef]
- Xing, W.; Huang, W.; Liu, G. Effect of Excess Iron and Copper on Physiology of Aquatic Plant Spirodela polyrrhiza (L.) Schleid. Environ. Toxicol. 2009. [Google Scholar] [CrossRef]
- Caicedo, J. Effect of Total Ammonia Nitrogen Concentration and PH on Growth Rates of Duckweed (Spirodela polyrrhiza). Water Res. 2000, 34, 3829–3835. [Google Scholar] [CrossRef]
- Ceschin, S.; Abati, S.; Leacche, I.; Zuccarello, V. Ecological Comparison between Duckweeds in Central Italy: The Invasive Lemna Minuta vs. the Native L. Minor. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2018, 152, 674–683. [Google Scholar] [CrossRef]
- Yuan, J.-X.; Pan, J.; Wang, B.-S.; Zhang, D.-M. Genetic Differentiation of Wolffia Globosa in China. J. Syst. Evol. 2011, 49, 509–517. [Google Scholar] [CrossRef]
- Lansdown, R.; Kitchener, G.; Jones, E. Wolffia Columbiana and W. Globosa (Araceae) New to Britain. Br. Ir. Bot. 2022, 4. [Google Scholar] [CrossRef]
- Walsh, É.; Cialis, E.; Dillane, E.; Jansen, M.A.K. Lemnaceae Clones Collected from a Small Geographic Region Display Diverse Traits Relevant for the Remediation of Wastewater. Environ. Technol. Innov. 2022, 28, 102599. [Google Scholar] [CrossRef]
- Fourounjian, P.; Fakhoorian, T.; Cao, X.H. Importance of Duckweeds in Basic Research and Their Industrial Applications. In The Duckweed Genomes; Cao, X.H., Fourounjian, P., Wang, W., Eds.; Compendium of Plant Genomes; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–17. ISBN 978-3-030-11044-4. [Google Scholar]
- O’Brien, A.M.; Yu, Z.H.; Luo, D.; Laurich, J.; Passeport, E.; Frederickson, M.E. Resilience to Multiple Stressors in an Aquatic Plant and Its Microbiome. Am. J. Bot. 2020, 107, 273–285. [Google Scholar] [CrossRef]

| Species | Location | No of Strains | Morphology | General Occurrence | Micrograph |
|---|---|---|---|---|---|
| Wolffia arrhiza | Golan Heights | 9 | 0.5–1.5 mm long, 0.4–1.2 mm wide; ellipsoid to spherical; upper surface is convex, opaque, bright green, with its greatest width slightly below the water surface; no veins; 30–100 stomata; no roots. | Widely distributed in temperate regions; native to Europe, South Africa; invasive in Brazil, Japan, and North America. |  |
| Wolffia globosa | HaSharon | 2 | 0. 4–0.9 mm long, 0.3–0.6 mm wide; ellipsoid; upper surface convex, translucent pale green, with its greatest width well below the water surface; no veins; 8–25 stomata; no roots. | Tropical, subtropical, and warm temperate regions; native to eastern and southeast Asia and Africa; invasive in North America. |  |
| Lemna gibba | Golan Heights, Hula Valley, HaSharon | 9 | 1–8 mm long, ~3.5 mm wide; lower surface of the fronds is usually gibbous; 4–5 veins extending from the nodes; >100 stomata; 1 root; difficult to identify due to high polymorphism. | Worldwide except Australia |  |
| Lemna minor | Galilee, Hula Valley, HaSharon | 5 | 1–10 mm long, 6–7 mm wide; upper surface shiny green, occasionally reddish; usually 3 veins, rarely 4–5; >100 stomata; 1 root. | Cooler oceanic regions; native to North America, Europe, Africa, and Western Asia. |  |
| Lemna minuta | Golan Heights, Hula Valley | 3 | 0.8–4 mm long, 0.5–2.5 mm wide; forming colonies of 2–4 fronds; circular with a slightly asymmetrical base; one vein, not very distinct; ~30 stomata; 1 root. | Temperate and subtropical regions, dry to moderately humid climate; native to America; invasive in Japan and Europe |  |
| Spirodela polyrhiza | Golan Heights | 1 | Largest duckweed: 1.5–10 mm long, 1.5–8 mm wide; usually thin fronds, rarely gibbous; maximum 16 veins; >100 stomata; 7–21 roots | Worldwide |  |
| Lab ID | Species | Region | Coordinates | Seasonality of the Water Source | atpF-atpH | Accession No | Identity (%) | psbK-psbI | Accession No | Identity (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Strain Identification | Strain Identification | |||||||||
| 32b | L. gibba | Golan Hights | 33.137779, 35.725382 | seasonal | RDSC 5504 | KX212889.1 | 100 | DW102 | OM569589.1 | 100 |
| 58 | L. gibba | HaSharon | 32.333731, 34.876507 | perennial | RDSC 5504 | KX212889.1 | 100 | DW102 | OM569589.1 | 100 |
| 56 | L. gibba | Golan Hights | 33.09779, 35.81729 | seasonal | RDSC 5504 | KX212889.1 | 100 | DW102 | OM569589.1 | 100 |
| 7 | L. gibba | Golan Hights | 32.867917, 35.770194 | seasonal | RDSC 5504 | KX212889.1 | 100 | DW102 | OM569589.1 | 100 |
| 43b | L. gibba | Hulla Vally | 33.060001, 35.615137 | seasonal | RDSC 5504 | KX212889.1 | 100 | DW102 | OM569589.1 | 100 |
| 43a | L. gibba | Hulla Vally | 33.060002, 35.615138 | seasonal | RDSC 5504 | KX212889.1 | 100 | DW102 | OM569589.1 | 100 |
| 31a | L. gibba | Golan Heights | 33.139682, 35.733806 | perennial | RDSC 5504 | KX212889.1 | 100 | DW102 | OM569589.1 | 100 |
| 31b | L. gibba | Golan Heights | 33.139682, 35.733806 | perennial | RDSC 5504 | KX212889.1 | 100 | DW102 | OM569589.1 | 100 |
| 30 | L. gibba | Golan Heights | 33.138450, 35.734019 | seasonal | RDSC 5504 | KX212889.1 | 100 | DW102 | OM569589.1 | 100 |
| 45a | L. minor | Galille | 32.912915, 35.569178 | perennial | K46 | OM569601.1 | 100 | K46 | OM569540.1 | 100 |
| 42 | L. minor | Hulla Vally | 33.015434, 35.629857 | seasonal | K46 | OM569601.1 | 100 | K46 | OM569540.1 | 100 |
| 44 | L. minor | Hulla Vally | 33.064187, 35.610817 | seasonal | K46 | OM569601.1 | 100 | K46 | OM569540.1 | 100 |
| 57 | L. minor | HaSharon | 32.363913, 34.958369 | perennial | K46 | OM569601.1 | 100 | K46 | OM569540.1 | 100 |
| 45b | L. minor | Galille | 32.912915, 35.569178 | perennial | K46 | OM569601.1 | 100 | K46 | OM569540.1 | 100 |
| 55a | L. minuta | Golan Hights | 32.801403, 35.783032 | seasonal | 5573 | MK516255.1 | 100 | 5573 | MK516236.1 | 100 |
| 55b | L. minuta | Golan Hights | 32.801403, 35.783032 | seasonal | 5573 | MK516255.0 | 100 | 5573 | MK516236.2 | 100 |
| 43s | L. minuta | Hulla Vally | 33.060002, 35.615138 | seasonal | 5573 | MK516255.1 | 100 | 5573 | MK516236.3 | 100 |
| 19 | S. polyrhiza | Golan Hights | 32.969559, 35.820036 | seasonal | 7498 | MN419335.1 | 100 | RDSC 2014 | OM569580.1 | 100 |
| 58 | W. globosa | HaSharon | 32.333731, 34.876507 | perennial | DW2101-4 | KJ630544.1 | 100 | 5514 | MG812327.1 | 100 |
| 11b | W. arrhiza | Golan Heights | 32.894030, 35.775695 | seasonal | DW35 | OM569550.1 | 100 | DW35 | OM569611.1 | 99.01 |
| 30a | W. arrhiza | Golan Heights | 33.138450, 35.734019 | seasonal | DW35 | OM569550.1 | 100 | DW35 | OM569611.1 | 99.01 |
| 31b | W. arrhiza | Golan Heights | 33.139682, 35.733806 | perennial | DW35 | OM569550.1 | 100 | DW35 | OM569611.1 | 99.01 |
| 55 | W. arrhiza | Golan Heights | 32.801403, 35.783032 | seasonal | DW35 | OM569550.1 | 100 | DW32 | OM569610.1 | 99.29 |
| 32b | W. arrhiza | Golan Heights | 33.137779, 35.725382 | seasonal | DW35 | OM569550.1 | 100 | DW35 | OM569611.1 | 99.31 |
| 30b | W. arrhiza | Golan Heights | 33.138451, 35.734018 | seasonal | DW35 | OM569550.1 | 100 | |||
| 11a | W. arrhiza | Golan Heights | 32.895829, 35.776775 | seasonal | DW35 | OM569550.1 | 100 | DW35 | OM569611.1 | 99.31 |
| Fatty Acid (% of Total Fatty Acids) | ω3/ω6 18:3/18:2 | TFA (%DW) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Haplotype | 16:0 | 16:1 | 16:2 | 16:3 | 18:0 | 18:1n9 | 18:2n6 | 18:3n6 GLA | 18:3n3 ALA | 18:4n3 SDA | 20:0 | 22:0 | 24:0 | ||
| L. gibba 58 | 20.56 ± 0.43 | 3.91 ± 0.42 | 2.2 ± 0.05 | 0.52 ± 0.13 | 0.92 ± 0.04 | 1.13 ± 0.13 | 11.1 ± 0.33 | 0.61 ± 0.05 | 52.88 ± 0.65 | 2.62 ± 0.08 | 0.37 ± 0.01 | 0.44 ± 0.04 | 1.13 ± 0.03 | 4.77 | 5.25 |
| L. gibba 30 | 19.72 ± 0.46 | 4.88 ± 0.03 | 3.27 ± 0.05 | 0.75 ± 0.04 | 1.01 ± 0.16 | 1.07 ± 0.09 | 10.46 ± 0.17 | 0.89 ± 0.05 | 50.32 ± 0.36 | 3.81 ± 0 | 0.45 ± 0.01 | 0.39 ± 0.02 | 1.15 ± 0.04 | 4.81 | 4.39 |
| L. gibba 31a | 21.32 ± 0.05 | 4.28 ± 0.04 | 2.06 ± 0.01 | 0.17 ± 0.02 | 1.25 ± 0.01 | 0.78 ± 0 | 11.27 ± 0.18 | 0.72 ± 0 | 50.26 ± 0 | 4.37 ± 0.03 | 0.53 ± 0 | 0.57 ± 0.01 | 1.05 ± 0.01 | 4.46 | 3.63 |
| L. gibba 31b | 21.61 ± 0.17 | 5.46 ± 0.31 | 2.18 ± 0.02 | 0.23 ± 0.07 | 1.07 ± 0.04 | 1.11 ± 0.01 | 13.93 ± 0.05 | 0.39 ± 0 | 48.98 ± 0.01 | 1.96 ± 0.03 | 0.39 ± 0 | 0.41 ± 0.01 | 0.84 ± 0.02 | 3.52 | 3.88 |
| L. gibba 32b | 20.01 ± 0.42 | 4.65 ± 1.09 | 2.11 ± 0.03 | 0.35 ± 0.21 | 1.36 ± 0.05 | 0.94 ± 0.01 | 15.37 ± 0.09 | 0.8 ± 0.01 | 48.09 ± 0.21 | 3.21 ± 0.01 | 0.55 ± 0.02 | 0.51 ± 0.05 | 0.93 ± 0.02 | 3.13 | 4.10 |
| L. gibba 43a | 20.96 ± 0.23 | 3.69 ± 0.42 | 2.07 ± 0.06 | 0.65 ± 0.08 | 1.01 ± 0.08 | 1.47 ± 0.24 | 13.83 ± 0.02 | 0.61 ± 0.07 | 49.87 ± 0.91 | 2.06 ± 0.14 | 0.48 ± 0.02 | 0.51 ± 0.01 | 1.1 ± 0.19 | 3.60 | 4.36 |
| L. gibba 43b | 21.4 ± 0.31 | 4.56 ± 0.77 | 1.96 ± 0.03 | 0.43 ± 0.13 | 0.97 ± 0.01 | 1.3 ± 0.02 | 13.69 ± 0.07 | 0.47 ± 0 | 49.92 ± 0.24 | 2.02 ± 0 | 0.39 ± 0.01 | 0.41 ± 0.02 | 0.76 ± 0.03 | 3.65 | 4.12 |
| L. gibba 56 | 21.75 ± 2.27 | 5.39 ± 1.1 | 2.62 ± 0.02 | 0.23 ± 0.11 | 1.08 ± 0.31 | 1.08 ± 0.19 | 13.82 ± 1.37 | 0.52 ± 0.11 | 47.77 ± 3.37 | 2.35 ± 0.34 | 0.42 ± 0.14 | 0.45 ± 0.15 | 0.71 ± 0.2 | 3.49 | 4.09 |
| L. gibba 7 | 21.49 ± 0.04 | 4.23 ± 0.22 | 2.24 ± 0.06 | 0.4 ± 0.03 | 1.24 ± 0.03 | 0.94 ± 0.01 | 15.3 ± 0.06 | 0.44 ± 0.05 | 47.78 ± 0.51 | 2.1 ± 0.03 | 0.53 ± 0.01 | 0.5 ± 0.01 | 1.4 ± 0.13 | 3.12 | 2.83 |
| L. minor 42 | 22.73 ± 0.12 | 5.67 ± 0.37 | 1.62 ± 0.09 | 0.35 ± 0.06 | 1.8 ± 0.12 | 1.13 ± 0.02 | 16.01 ± 0.08 | 0.46 ± 0.01 | 45.8 ± 0.03 | 1.51 ± 0.14 | 0.55 ± 0 | 0.31 ± 0 | 1.01 ± 0.01 | 2.86 | 3.35 |
| L. minor 44 | 19.85 ± 0.06 | 5.5 ± 0.01 | 2.01 ± 0.01 | 0.2 ± 0.01 | 1.28 ± 0.02 | 1.45 ± 0 | 18.4 ± 0.1 | 0.5 ± 0 | 46.75 ± 0.13 | 1.75 ± 0 | 0.31 ± 0.01 | 0.29 ± 0.01 | 0.82 ± 0.04 | 2.54 | 6.34 |
| L. minor 45a | 19.97 ± 0.33 | 2.89 ± 0.05 | 2.03 ± 0.02 | 0.75 ± 0.02 | 1.4 ± 0.04 | 1.69 ± 0.01 | 18.88 ± 0.05 | 0.6 ± 0.01 | 47.37 ± 0.39 | 1.88 ± 0.08 | 0.47 ± 0.01 | 0.36 ± 0.15 | 0.72 ± 0.78 | 2.51 | 3.77 |
| L.minor 45b | 22.49 ± 0.9 | 4.1 ± 0.79 | 2.54 ± 0.43 | 0.68 ± 0.37 | 1.4 ± 0.16 | 1.57 ± 0.03 | 17.64 ± 0.15 | 0.71 ± 0.05 | 42.74 ± 0.63 | 2.68 ± 0.21 | 0.45 ± 0.01 | 0.34 ± 0.14 | 1.16 ± 0.15 | 2.42 | 3.52 |
| L. minor 57 | 18.28 ± 0.16 | 5.8 ± 0.18 | 2.77 ± 0.02 | 0.22 ± 0.04 | 0.77 ± 0.02 | 1.33 ± 0.01 | 16.1 ± 0.11 | 1.27 ± 0.01 | 48.36 ± 0.19 | 3.04 ± 0.01 | 0.17 ± 0.01 | 0.24 ± 0 | 0.71 ± 0.03 | 3.00 | 4.19 |
| L. minuta 43 | 18.48 ± 0.45 | 4.64 ± 0.03 | 2.43 ± 0.05 | 0.52 ± 0.05 | 1.24 ± 0.39 | 1.27 ± 0.02 | 14.07 ± 0.21 | 0.23 ± 0.21 | 54.43 ± 1.13 | 0.09 ± 0.02 | 0.25 ± 0.08 | 0.29 ± 0.05 | 1.13 ± 0.1 | 3.87 | 4.78 |
| L. minuta 55a | 19.92 ± 0.2 | 4.97 ± 0.52 | 1.62 ± 0 | 0.31 ± 0.13 | 0.93 ± 0.03 | 1.08 ± 0.02 | 15.87 ± 0.14 | 0 ± 0 | 53.35 ± 0.23 | 0.12 ± 0.11 | 0.15 ± 0.02 | 0.19 ± 0.03 | 0.79 ± 0.06 | 3.36 | 3.95 |
| L. minuta 55b | 20.07 ± 0.12 | 3.44 ± 0.07 | 1.6 ± 0 | 0.66 ± 0.04 | 1.03 ± 0.02 | 1.35 ± 0.07 | 15.91 ± 0.07 | 0 ± 0 | 53.51 ± 0.09 | 0.13 ± 0.18 | 0.25 ± 0 | 0.15 ± 0 | 1.17 ± 0.02 | 3.36 | 4.19 |
| S. polyrhiza 19 | 23.15 ± 0.24 | 5.43 ± 0.37 | 6.11 ± 0.06 | 0.35 ± 0.04 | 2.35 ± 0.07 | 1.17 ± 0.04 | 5.23 ± 0 | 0.04 ± 0.05 | 50.9 ± 0.48 | 0 ± 0 | 0.58 ± 0.04 | 0.62 ± 0.01 | 2.61 ± 0.05 | 9.73 | 4.27 |
| W. arrhiza 11a | 23.22 ± 1.1 | 5.64 ± 0.58 | 3.64 ± 0.23 | 0.55 ± 0.03 | 1.62 ± 0 | 2.17 ± 0.08 | 25.24 ± 0.32 | 0.28 ± 0.01 | 34.87 ± 0.64 | 0.1 ± 0.01 | 0.77 ± 0.01 | 0.64 ± 0.04 | 0.4 ± 0.03 | 1.38 | 4.38 |
| W. arrhiza 11b | 21.63 ± 0.26 | 5.36 ± 0.53 | 3.45 ± 0.12 | 0.59 ± 0.11 | 1.48 ± 0.07 | 1.87 ± 0.03 | 25.7 ± 0.02 | 0.15 ± 0.01 | 37.32 ± 0.56 | 0.1 ± 0.01 | 0.73 ± 0.01 | 0.53 ± 0.01 | 0.37 ± 0.08 | 1.45 | 4.55 |
| W. arrhiza 30a | 20.4 ± 0.01 | 5.32 ± 0.2 | 3.46 ± 0.02 | 0.48 ± 0 | 1.17 ± 0.02 | 1.73 ± 0.01 | 22.75 ± 0.02 | 0.14 ± 0 | 42.29 ± 0.14 | 0.08 ± 0 | 0.58 ± 0 | 0.47 ± 0.01 | 0.31 ± 0.02 | 1.86 | 4.70 |
| W. arrhiza 31b | 24.28 ± 0.14 | 3.11 ± 0.22 | 3.19 ± 0.14 | 1.03 ± 0.14 | 1.35 ± 0.04 | 1.64 ± 0.04 | 23.78 ± 0.03 | 0.11 ± 0.1 | 38.74 ± 0.33 | 0.14 ± 0.02 | 0.69 ± 0.02 | 0.4 ± 0 | 0.52 ± 0.03 | 1.63 | 3.18 |
| W. arrhiza 55 | 21.85 ± 0.43 | 3.65 ± 0.25 | 3.33 ± 0.04 | 0.75 ± 0.04 | 1.37 ± 0.11 | 1.73 ± 0.04 | 25.7 ± 0.26 | 0.03 ± 0.01 | 38.97 ± 0.44 | 0.1 ± 0.01 | 0.6 ± 0.02 | 0.51 ± 0.04 | 0.34 ± 0.08 | 1.52 | 5.13 |
| W. arrhiza 9528 | 19.05 ± 0.13 | 3.72 ± 0.46 | 2.48 ± 0.03 | 0.24 ± 0.02 | 1.81 ± 0.01 | 1.79 ± 0.01 | 24.29 ± 0.12 | 0.14 ± 0.01 | 44.41 ± 0.1 | 0.07 ± 0 | 0.86 ± 0.02 | 0.66 ± 0.05 | 0.29 ± 0.02 | 1.83 | 4.44 |
| W. globosa 58 | 22.71 ± 0.05 | 4.04 ± 0.58 | 1.5 ± 0.05 | 0.35 ± 0.08 | 2.25 ± 0 | 2.41 ± 0.01 | 25.02 ± 0.01 | 0.07 ± 0.39 | 39.51 ± 0.04 | 0.02 ± 0.02 | 0.53 ± 0.01 | 0.39 ± 0.02 | 0.19 ± 0.01 | 1.58 | 4.38 |
| W. globosa Mankai | 21.73 ± 0.16 | 4.39 ± 0.64 | 2.34 ± 0.02 | 0.74 ± 0.21 | 2.14 ± 0.02 | 1.9 ± 0.01 | 19.88 ± 0.27 | 0.14 ± 0 | 44.64 ± 0.21 | 0.07 ± 0 | 0.41 ± 0.02 | 0.19 ± 0.01 | 0.33 ± 0.02 | 2.25 | 5.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedjung Yosef, A.; Ghazaryan, L.; Klamann, L.; Kaufman, K.S.; Baubin, C.; Poodiack, B.; Ran, N.; Gabay, T.; Didi-Cohen, S.; Bog, M.; et al. Diversity and Differentiation of Duckweed Species from Israel. Plants 2022, 11, 3326. https://doi.org/10.3390/plants11233326
Friedjung Yosef A, Ghazaryan L, Klamann L, Kaufman KS, Baubin C, Poodiack B, Ran N, Gabay T, Didi-Cohen S, Bog M, et al. Diversity and Differentiation of Duckweed Species from Israel. Plants. 2022; 11(23):3326. https://doi.org/10.3390/plants11233326
Chicago/Turabian StyleFriedjung Yosef, Avital, Lusine Ghazaryan, Linda Klamann, Katherine Sarah Kaufman, Capucine Baubin, Ben Poodiack, Noya Ran, Talia Gabay, Shoshana Didi-Cohen, Manuela Bog, and et al. 2022. "Diversity and Differentiation of Duckweed Species from Israel" Plants 11, no. 23: 3326. https://doi.org/10.3390/plants11233326
APA StyleFriedjung Yosef, A., Ghazaryan, L., Klamann, L., Kaufman, K. S., Baubin, C., Poodiack, B., Ran, N., Gabay, T., Didi-Cohen, S., Bog, M., Khozin-Goldberg, I., & Gillor, O. (2022). Diversity and Differentiation of Duckweed Species from Israel. Plants, 11(23), 3326. https://doi.org/10.3390/plants11233326








