Aqueous Extract of Artemisia annua Shows In Vitro Antimicrobial Activity and an In Vivo Chemopreventive Effect in a Small-Cell Lung Cancer Model
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Preparation of Aqueous Extract of Artemisia annua (AAE) from Leaves
2.3. Microorganisms
2.4. Determination of Antimicrobial Activity of AAE by the Well Diffusion and the Dilution Methods
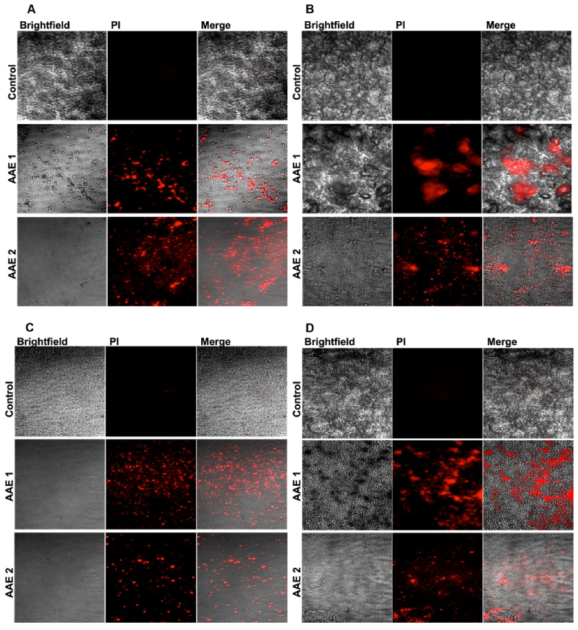
2.5. Analysis of the Microbial Death by the Confocal Microscopy
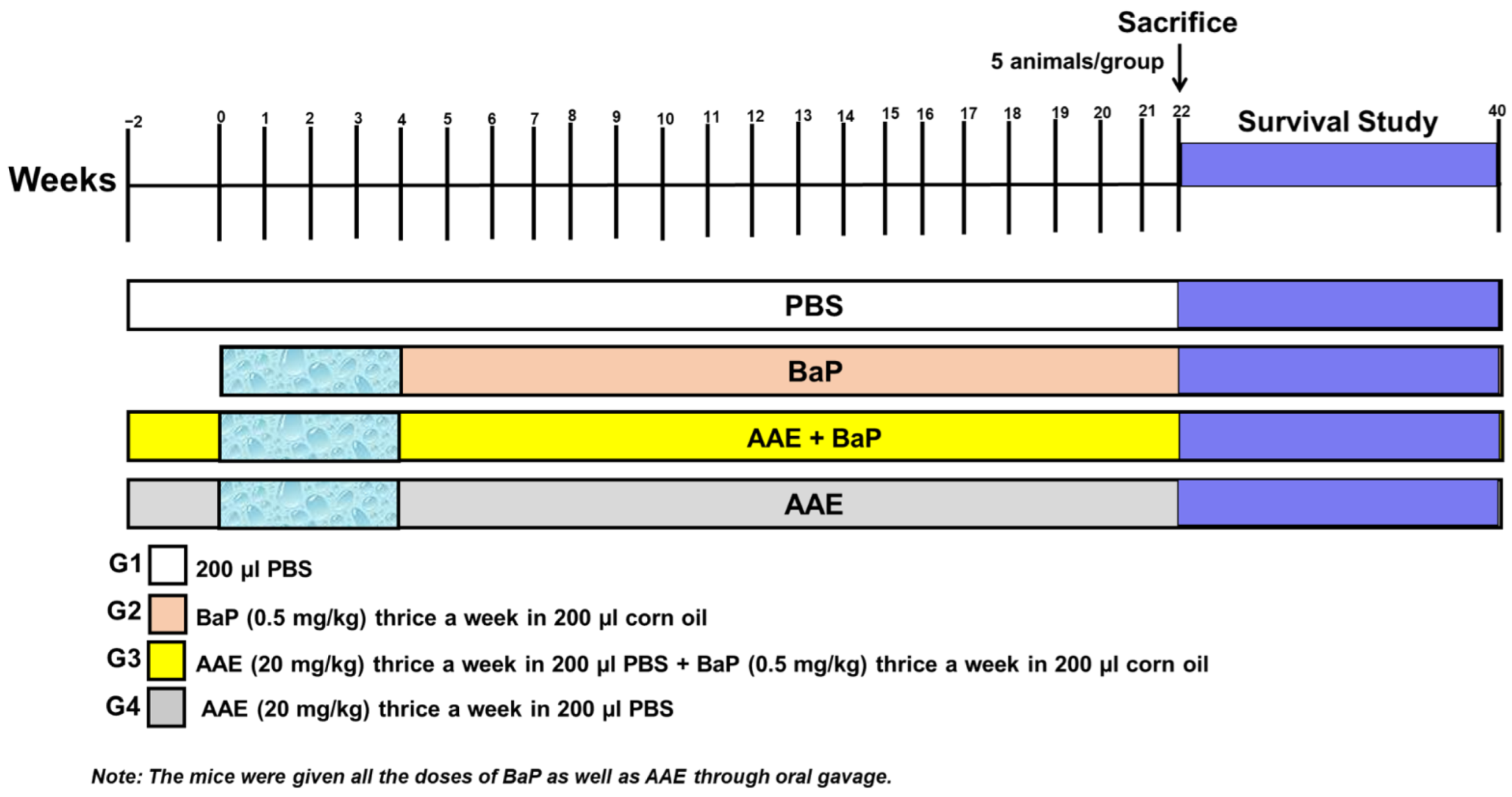
2.6. In Vivo Studies
2.6.1. Mice
2.6.2. Experimental Design
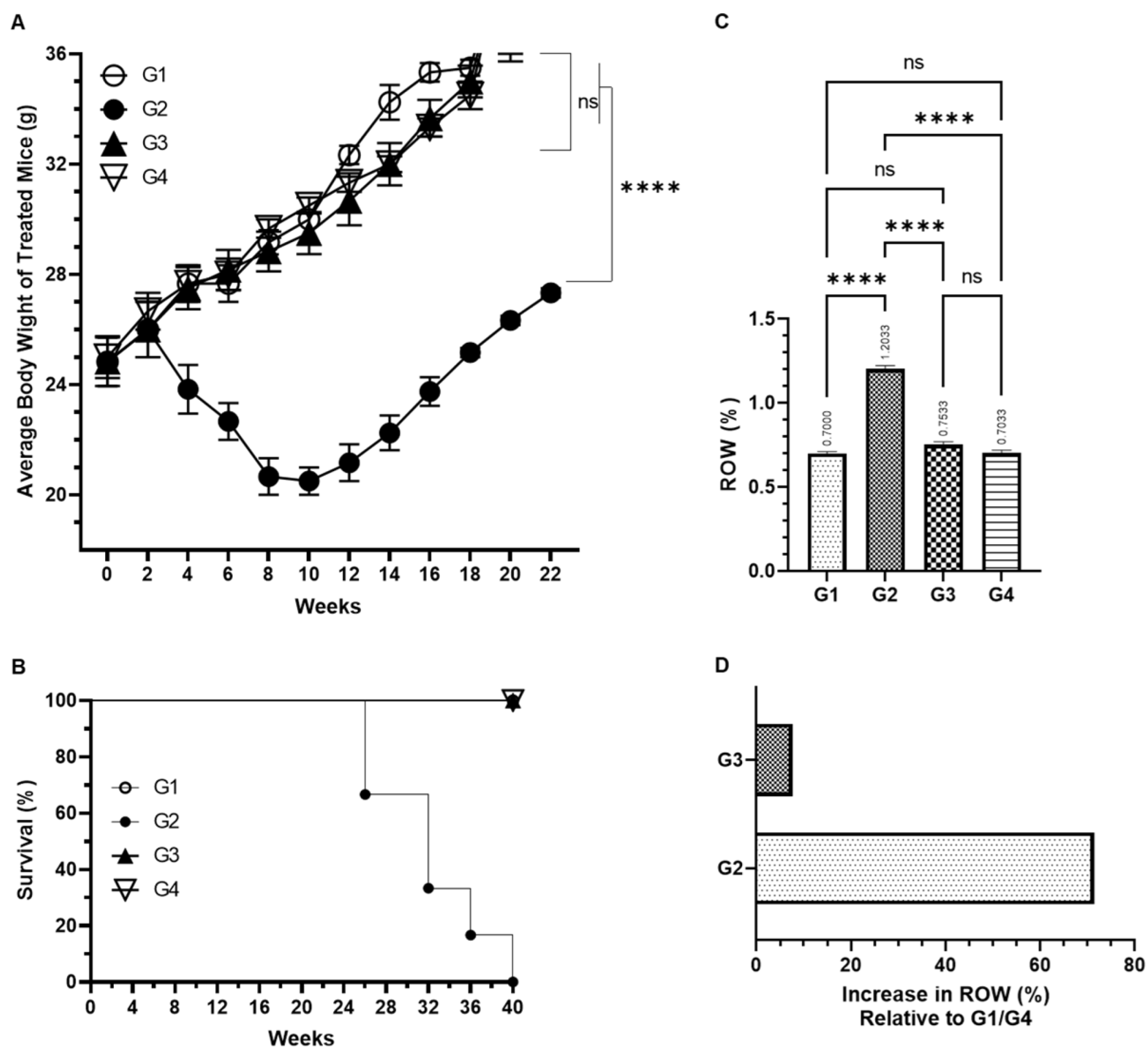
2.7. Investigation of Average Body Weight (ABW), Relative Lung Weight (RLW), and Survival Rate
2.8. Histopathological Evaluation of Lungs
2.9. Serum Biochemical Analyses
2.10. Antioxidant Enzyme Assays in Lung Tissues
2.11. Annexin V-FITC/PI Apoptosis Assay
2.12. Determination of Cellular ROS Generation by Flow Cytometry
2.13. Statistical Analysis
3. Results
3.1. AAE Yield Percentage and Its Potent Antibacterial and Antifungal Activity
3.2. Effect of AAE on BaP-Induced ABW, Survival, and RLW
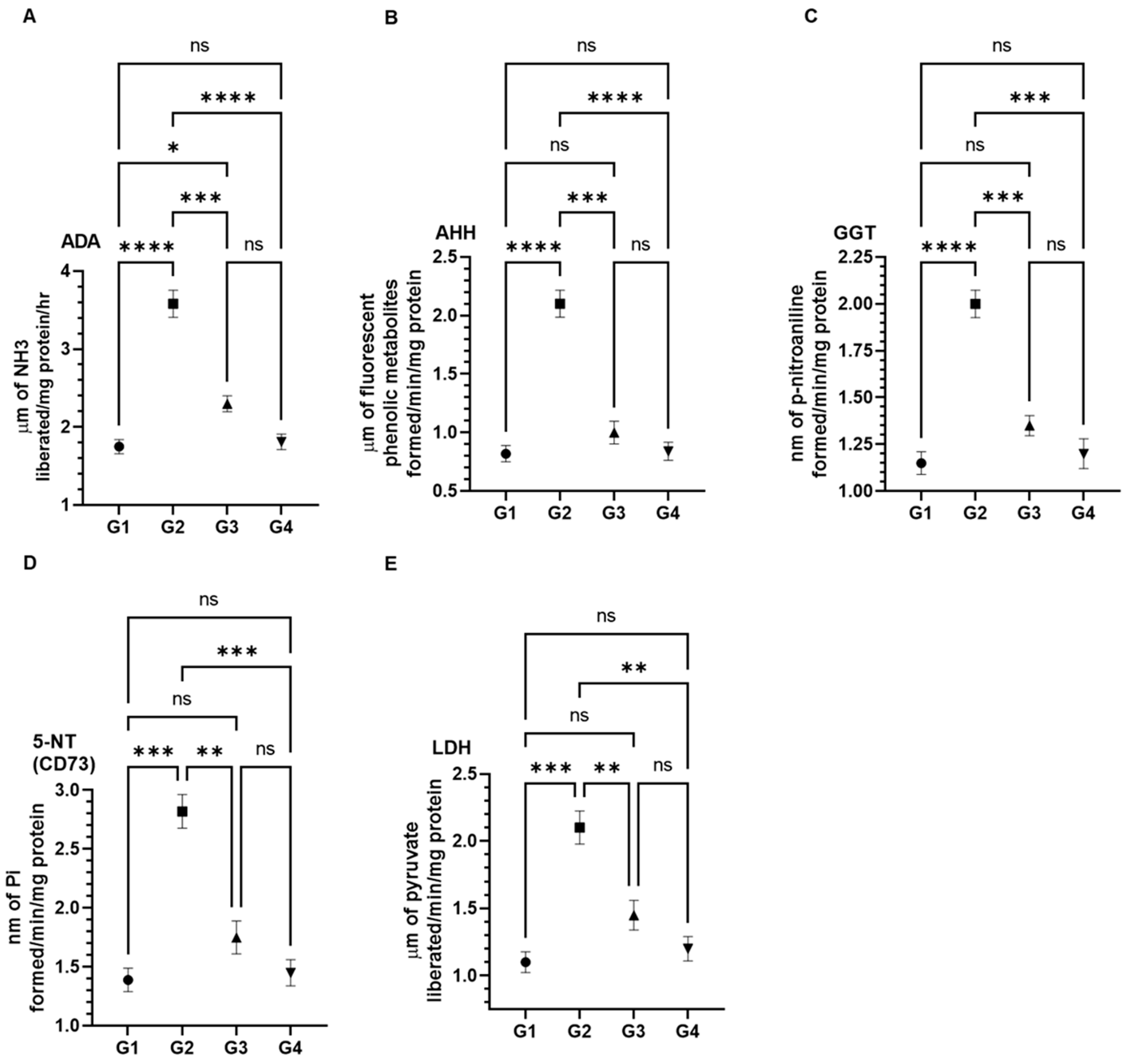
3.3. Effect of AAE on Cancer Marker Enzymes as ADA, AHH, GGT, 5-NT (CD73), and LDH in the Serum Induced by Carcinogen
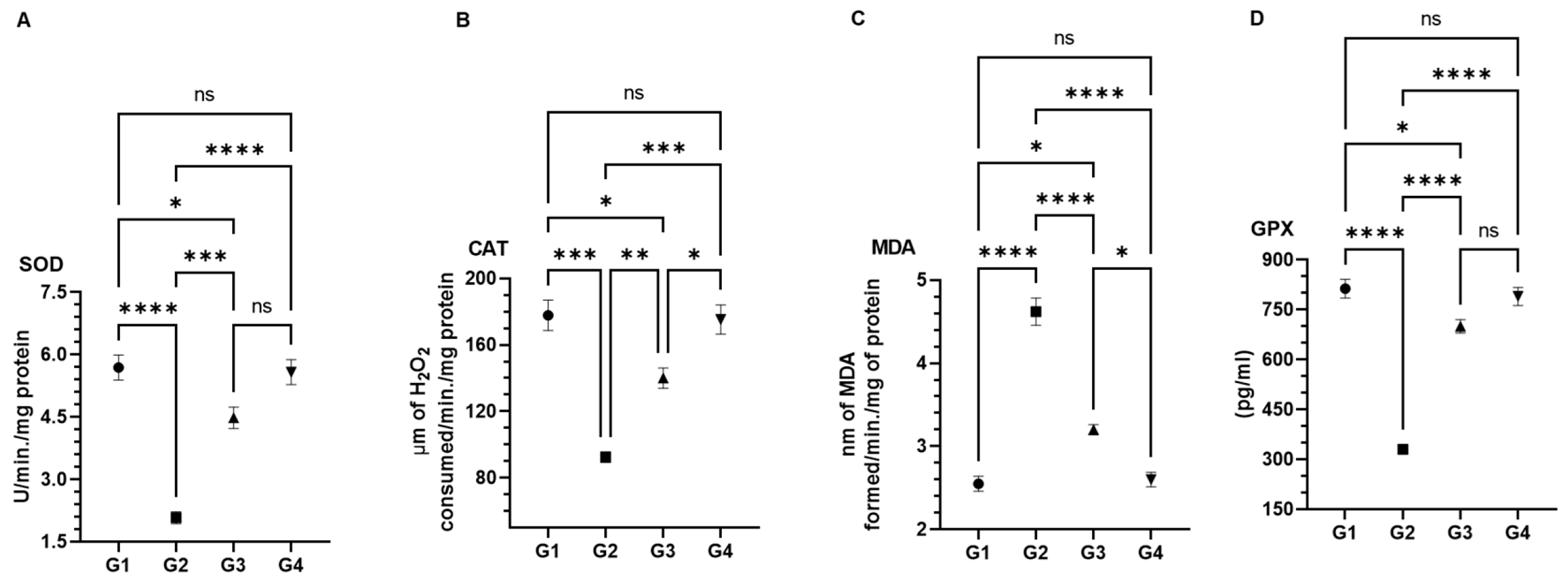
3.4. Effect of AAE on Carcinogen-Modulated Antioxidant Enzymes in Lung Tissues
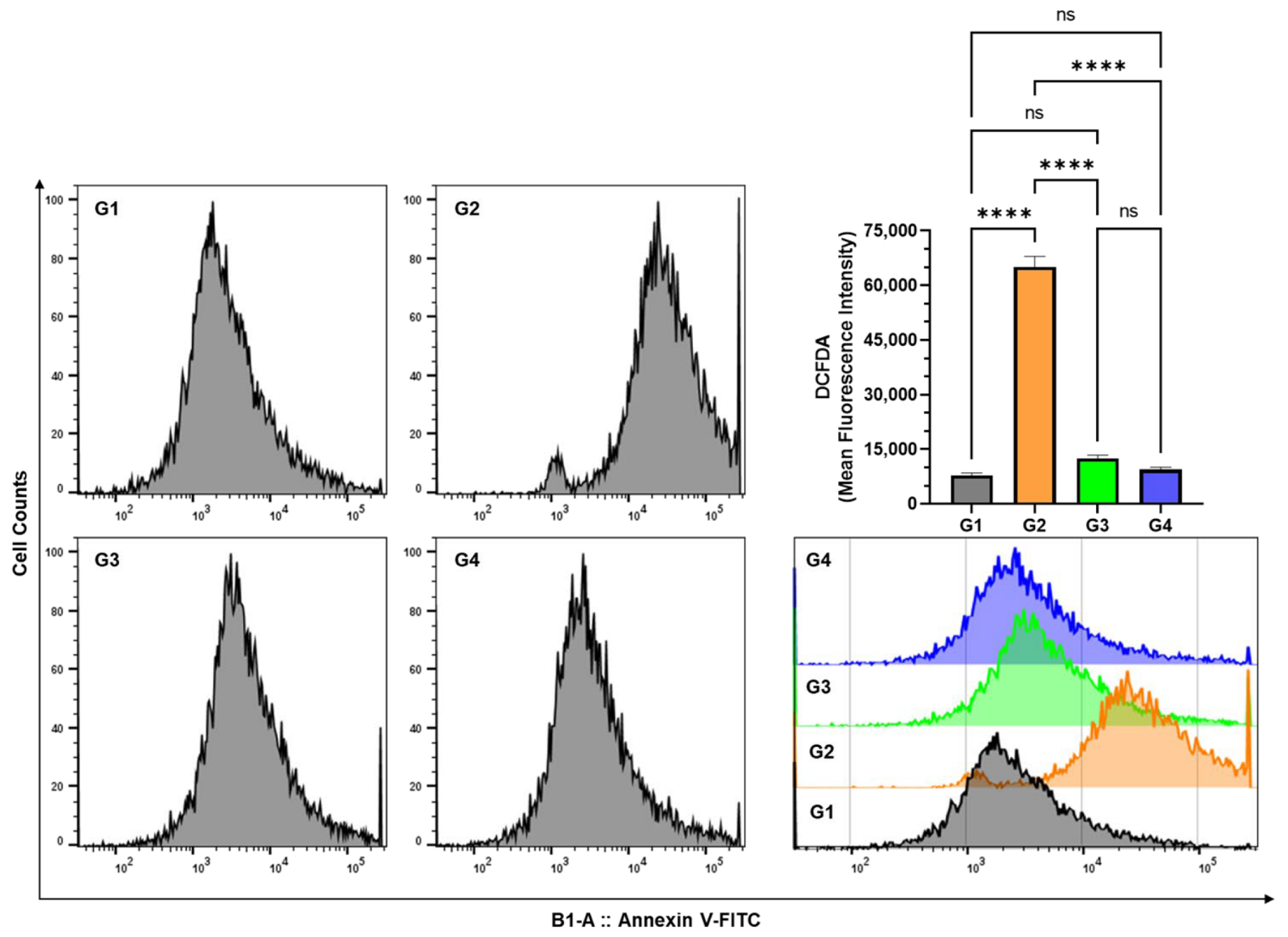
3.5. Effect of AAE on Cellular ROS in the Lung Cells by DCFDA Using Flow Cytometry
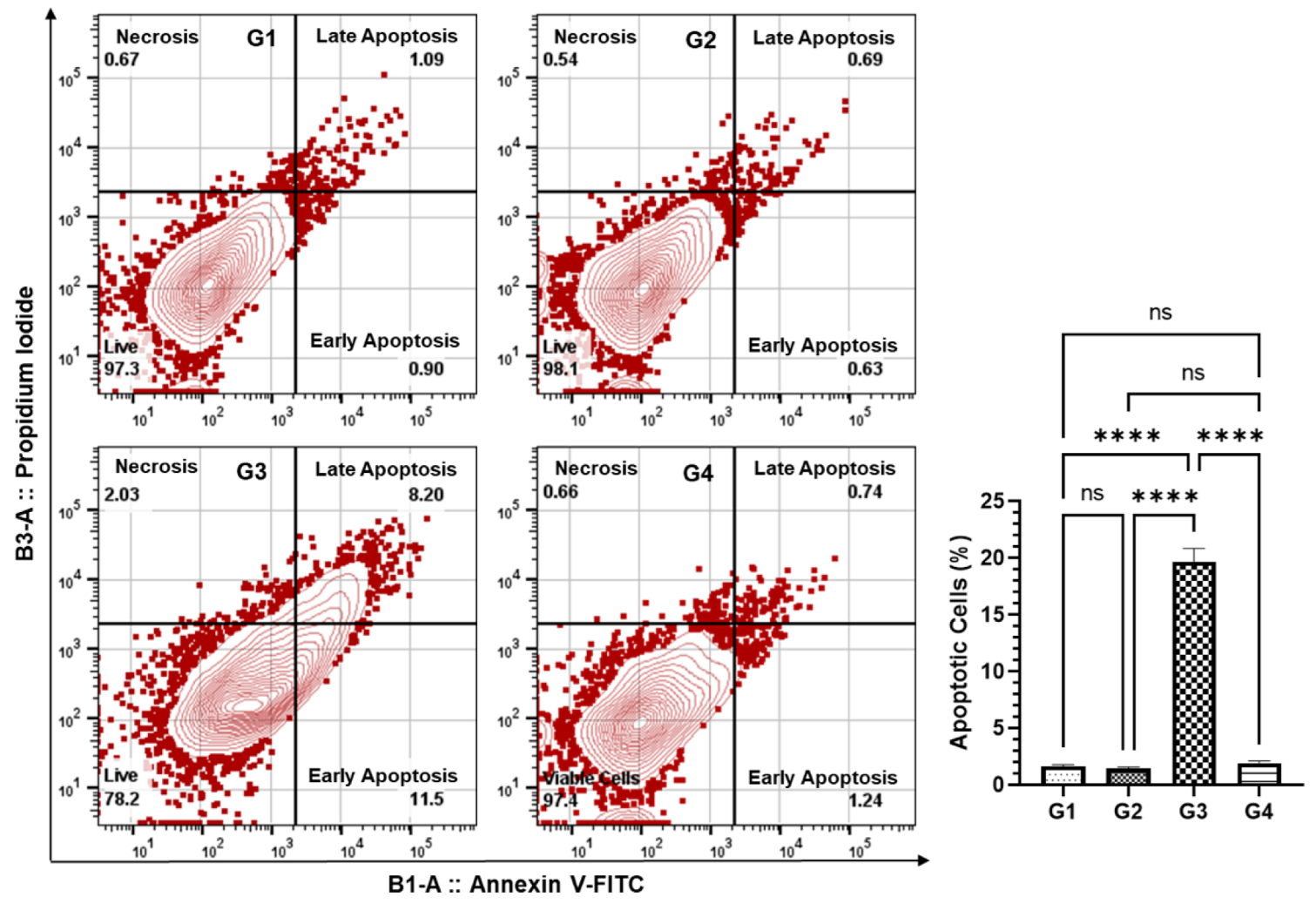
3.6. Effect of AAE on the Induction of Apoptosis in the Lung Cells by Annexin V-FITC-PI Using Flow Cytometry
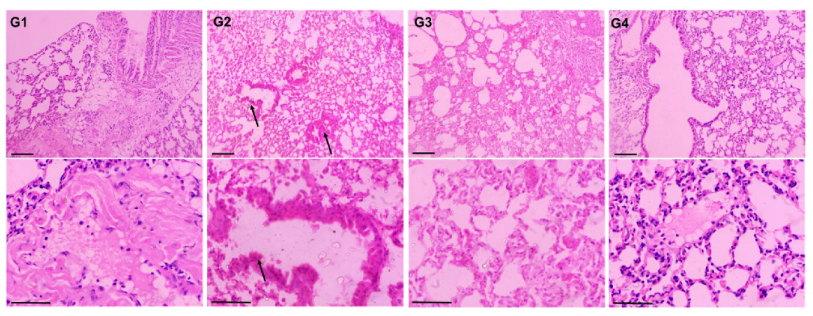
3.7. Effect of AAE on the Histopathology of BaP-Modulated Lungs
4. Discussions
5. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chopra, A.S.; Lordan, R.; Horbańczuk, O.K.; Atanasov, A.G.; Chopra, I.; Horbańczuk, J.O.; Jóźwik, A.; Huang, L.; Pirgozliev, V.; Banach, M.; et al. The current use and evolving landscape of nutraceuticals. Pharm. Res. 2022, 175, 106001. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M. International Natural Product Sciences Taskforce, Supuran CT. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Dhiman, A. Nutraceuticals: A Comparative Analysis of Regulatory Framework in Different Countries of the World. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Helal, N.A.; Eassa, H.A.; Amer, A.M.; Eltokhy, M.A.; Edafiogho, I.; Nounou, M.I. Nutraceuticals’ Novel Formulations: The Good, the Bad, the Unknown and Patents Involved. Recent Pat. Drug Deliv. Formul. 2019, 13, 105–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Qiao, Y.; Ang, E.L.; Zhao, H. Using natural products for drug discovery: The impact of the genomics era. Expert Opin. Drug Discov. 2017, 12, 475–487. [Google Scholar] [CrossRef] [Green Version]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Leena, M.M.; Silvia, M.G.; Vinitha, K.; Moses, J.A.; Anandharamakrishnan, C. Synergistic potential of nutraceuticals: Mechanisms and prospects for futuristic medicine. Food Funct. 2020, 11, 9317–9337. [Google Scholar] [CrossRef]
- Pezzani, R.; Salehi, B.; Vitalini, S.; Iriti, M.; Zuñiga, F.A.; Sharifi-Rad, J. Synergistic Effects of Plant Derivatives and Conventional Chemotherapeutic Agents: An Update on the Cancer Perspective. Medicina 2019, 55, 546. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, T.D.; Ramasamy, J.R.; Palanisamy, K. Nutraceutical potentials of synergic foods: A systematic review. J. Ethn. Foods 2019, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Ryabushkina, N.A. Synergism of Metabolite Action in Plant Responses to Stresses. Russ. J. Plant Physiol. 2005, 52, 547–552. [Google Scholar] [CrossRef]
- Enke, C.G.; Nagels, L.J. Undetected components in natural mixtures: How many? What concentrations? Do they account for chemical noise? What is needed to detect them? Anal. Chem. 2011, 83, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Devitt, N.M.; Davis, J.M.; Schure, M.R. Estimation of low-level components lost through chromatographic separations with finite detection limits. J. Chromatogr. A 2020, 1626, 461266. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [Green Version]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10 (Suppl. 1), S4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junio, H.A.; Sy-Cordero, A.A.; Ettefagh, K.A.; Burns, J.T.; Micko, K.T.; Graf, T.N.; Ritcher, S.J.; Cannon, R.E.; Oberlies, N.H.; Cech, N.B. Synergy-directed fractionation of botanical medicines: A case study with goldenseal (Hydrastis canadensis). J. Nat. Prod. 2011, 74, 1621–1629. [Google Scholar] [CrossRef] [Green Version]
- Dettweiler, M.; Marquez, L.; Bao, M.; Quave, C.L. Quantifying synergy in the bioassay-guided fractionation of natural product extracts. PLoS ONE 2020, 15, e0235723. [Google Scholar] [CrossRef]
- Efferth, T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 2017, 46, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Septembre-Malaterre, A.; Lalarizo Rakoto, M.; Marodon, C.; Bedoui, Y.; Nakab, J.; Simon, E.; Hoarau, L.; Savriama, S.; Starberg, D.; Guiraud, P.; et al. Artemisia annua, a Traditional Plant Brought to Light. Int. J. Mol. Sci. 2020, 21, 4986. [Google Scholar] [CrossRef]
- Brisibe, E.A.; Umoren, U.E.; Brisibe, F.; Magalhäes, P.M.; Ferreira, J.F.S.; Luthria, D.; Wu, X.; Prior, L.D. Nutritional characterisation and antioxidant capacity of different tissues of Artemisia annua L. Food Chem. 2009, 115, 1240–1246. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Juteau, F.; Masotti, V.; Bessière, J.M.; Dherbomez, M.; Viano, J. Antibacterial and antioxidant activities of Artemisia annua essential oil. Fitoterapia 2002, 73, 532–535. [Google Scholar] [CrossRef]
- Lang, S.J.; Schmiech, M.; Hafner, S.; Paetz, C.; Steinborn, C.; Huber, R.; Gaafary, M.E.; Werner, K.; Schmidt, C.Q.; Syrovets, T.; et al. Antitumor activity of an Artemisia annua herbal preparation and identification of active ingredients. Phytomedicine 2019, 62, 152962. [Google Scholar] [CrossRef]
- Appalasamy, S.; Lo, K.Y.; Ch’ng, S.J.; Nornadia, K.; Othman, A.S.; Chan, L.-K. Antimicrobial Activity of Artemisinin and Precursor Derived from In Vitro Plantlets of Artemisia annua L. Biomed. Res. Int. 2014, 2014, 215872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poiată, A.; Tuchiluş, C.; Ivănescu, B.; Ionescu, A.; Lazăr, M.I. Antibacterial activity of some Artemisia species extract. Rev. Med. Chir. Soc. Med. Nat. Iasi 2009, 113, 911–914. [Google Scholar]
- Sen, R.; Bandyopadhyay, S.; Dutta, A.; Mandal, G.; Ganguly, S.; Saha, P.; Chatterjee, M. Artemisinin triggers induction of cell-cycle arrest and apoptosis in Leishmania donovani promastigotes. J. Med. Microbiol. 2007, 56, 1213–1218. [Google Scholar] [CrossRef] [Green Version]
- Weathers, P.J.; Towler, M.; Hassanali, A.; Lutgen, P.; Engeu, P.O. Dried-leaf Artemisia annua: A practical malaria therapeutic for developing countries? World J. Pharmacol. 2014, 3, 39–55. [Google Scholar] [CrossRef]
- Mueller, M.S.; Runyambo, N.; Wagner, I.; Borrmann, S.; Dietz, K.; Heide, L. Randomized controlled trial of a traditional preparation of Artemisia annua L. (Annual Wormwood) in the treatment of malaria. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 318–321. [Google Scholar] [CrossRef]
- McIntosh, H.M.; Olliaro, P. Artemisinin derivatives for treating severe malaria. Cochrane Database Syst. Rev. 2000, 3, CD000527. [Google Scholar] [CrossRef]
- Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef]
- Su, X.-Z.; Miller, L.H. The discovery of artemisinin and the Nobel Prize in Physiology or Medicine. Sci. China Life Sci. 2015, 58, 1175–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, S.; Stebbings, S.; McNamara, D. An open-label six-month extension study to investigate the safety and efficacy of an extract of Artemisia annua for managing pain, stiffness and functional limitation associated with osteoarthritis of the hip and knee. NZ Med. J. 2016, 129, 97–102. [Google Scholar]
- Stebbings, S.; Beattie, E.; McNamara, D.; Hunt, S. A pilot randomized, placebo-controlled clinical trial to investigate the efficacy and safety of an extract of Artemisia annua administered over 12 weeks, for managing pain, stiffness, and functional limitation associated with osteoarthritis of the hip and knee. Clin. Rheumatol. 2016, 35, 1829–1836. [Google Scholar] [CrossRef]
- Rassias, D.J.; Weathers, P.J. Dried leaf Artemisia annua efficacy against non-small cell lung cancer. Phytomedicine 2019, 52, 247–253. [Google Scholar] [CrossRef]
- Singh, N.P.; Lai, H.C. Artemisinin induces apoptosis in human cancer cells. Anticancer Res. 2004, 24, 2277–2280. [Google Scholar] [PubMed]
- König, M.; von Hagens, C.; Hoth, S.; Baumann, I.; Walter-Sack, I.; Edler, L.; Sertel, S. Investigation of ototoxicity of artesunate as add-on therapy in patients with metastatic or locally advanced breast cancer: New audiological results from a prospective, open, uncontrolled, monocentric phase I study. Cancer Chemother. Pharmacol. 2016, 77, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Wan, C.; Luo, Y.; Zhang, C.-F.; Zhang, Q.-H.; Chen, L.; Liu, Z.; Wang, D.H.; Lager, M.; Li, C.-H.; et al. Effects of dihydroartemisinin, a metabolite of artemisinin, on colon cancer chemoprevention and adaptive immune regulation. Mol. Biol. Rep. 2022, 49, 2695–2709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, F.; Wang, Y.; Xie, H.; Luo, S.; Meng, L.; Su, B.; Ye, Y.; Wu, K.; Xu, Y.; et al. Eliminating Radiation Resistance of Non-Small Cell Lung Cancer by Dihydroartemisinin Through Abrogating Immunity Escaping and Promoting Radiation Sensitivity by Inhibiting PD-L1 Expression. Front. Oncol. 2020, 10, 595466. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-J.; Zhang, J.-L.; Li, A.; Wang, Z.; Lou, X.-E. Dihydroartemisinin improves the efficiency of chemotherapeutics in lung carcinomas in vivo and inhibits murine Lewis lung carcinoma cell line growth in vitro. Cancer Chemother. Pharmacol. 2010, 66, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, A.-M.; Amrioui, F.; Fallet, V. [Risk factors and prevention of lung cancer]. Rev. Prat. 2020, 70, 852–856. [Google Scholar]
- McFarland, D.C. A Response to “Psychological symptoms and survival in patients with metastatic lung cancer: Smoking must be the first concern!”. Psycho-Oncology 2020, 29, 1504–1505. [Google Scholar] [CrossRef]
- Sasco, A.J.; Secretan, M.B.; Straif, K. Tobacco smoking and cancer: A brief review of recent epidemiological evidence. Lung Cancer 2004, 45, S3–S9. [Google Scholar] [CrossRef]
- Shen, H.; Spitz, M.R.; Qiao, Y.; Guo, Z.; Wang, L.-E.; Bosken, C.H.; Amos, C.I.; Wei, Q. Smoking, DNA repair capacity and risk of nonsmall cell lung cancer. Int. J. Cancer 2003, 107, 84–88. [Google Scholar] [CrossRef]
- Zou, X.; Fu, Y.; Wu, D.; Liu, J.; Xiao, Y.; Huang, H. [Establishment of mouse lung cancer model induced by benzo[a]pyrene dynamic inhalation exposure]. Wei Sheng Yan Jiu 2020, 49, 486–490. [Google Scholar] [CrossRef]
- Ceppi, M.; Munnia, A.; Cellai, F.; Bruzzone, M.; Peluso, M.E.M. Linking the generation of DNA adducts to lung cancer. Toxicology 2017, 390, 160–166. [Google Scholar] [CrossRef]
- Osborne, M.R.; Brookes, P.; Beland, F.A.; Harvey, R.G. The reaction of (±)-7α, 8β-dihydroxy-9β, 10β-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene with dna. Int. J. Cancer 1976, 18, 362–368. [Google Scholar] [CrossRef]
- Khan, A.; Azam, M.; Allemailem, K.S.; Alrumaihi, F.; Almatroudi, A.; Alhumaydhi, F.A.; Ahmad, H.I.; Khan, M.U.; Khan, M.A. Coadministration of Ginger Extract and Fluconazole Shows a Synergistic Effect in the Treatment of Drug-Resistant Vulvovaginal Candidiasis. Infect. Drug Resist. 2021, 14, 1585–1599. [Google Scholar] [CrossRef]
- Khan, A.; Alsahli, M.A.; Aljasir, M.A.; Maswadeh, H.; Mobark, M.A.; Azam, F.; Allemailem, K.S.; Alrumaihi, F.; Alhumaydhi, F.A.; Almatroudi, A.A.; et al. Experimental and Theoretical Insights on Chemopreventive Effect of the Liposomal Thymoquinone Against Benzo[a]pyrene-Induced Lung Cancer in Swiss Albino Mice. J. Inflamm. Res. 2022, 15, 2263–2280. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Alhumaydhi, F.A.; Alwashmi, A.S.; Allemailem, K.S.; Alsahli, M.A.; Alrumaihi, F.A.; Almatroudi, A.; Mobark, M.A.; Mousa, A.; Khan, M.A. Diallyl Sulfide-Mediated Modulation of the Fatty Acid Synthase (FASN) Leads to Cancer Cell Death in BaP-Induced Lung Carcinogenesis in Swiss Mice. J. Inflamm. Res. 2020, 13, 1075–1087. [Google Scholar] [CrossRef]
- Alrumaihi, F.; Khan, M.A.; Babiker, A.Y.; Alsaweed, M.; Azam, F.; Allemailem, K.S.; Almatroudi, A.A.; Ahamad, S.R.; Alsugoor, M.H.; Alharbi, K.N.; et al. Lipid-Based Nanoparticle Formulation of Diallyl Trisulfide Chemosensitizes the Growth Inhibitory Activity of Doxorubicin in Colorectal Cancer Model: A Novel In Vitro, In Vivo and In Silico Analysis. Molecules 2022, 27, 2192. [Google Scholar] [CrossRef] [PubMed]
- Galal, A.M.; Ross, S.A.; Jacob, M.; ElSohly, M.A. Antifungal Activity of Artemisinin Derivatives. J. Nat. Prod. 2005, 68, 1274–1276. [Google Scholar] [CrossRef] [PubMed]
- Minda, D.; Ghiulai, R.; Banciu, C.D.; Pavel, I.Z.; Danciu, C.; Racoviceanu, R.; Soica, C.; Budu, O.D.; Muntean, D.; Diaconeasa, Z.; et al. Phytochemical Profile, Antioxidant and Wound Healing Potential of Three Artemisia Species: In Vitro and In Ovo Evaluation. Appl. Sci. 2022, 12, 1359. [Google Scholar] [CrossRef]
- Yazdi, H.B.; Taheri, H.N. Antimicrobial Effects of Aqueous-Alcoholic Extract of Artemisia turanica against Methicillin-Resistant Staphylococcus aureus (MRSA): An in vitro Study. J. Ilam Univ. Med. Sci. 2022, 30, 1–7. [Google Scholar]
- Abdallah, H.M.; Asfour, H.Z.; El-Halawany, A.M.; Elfaky, M.A. Saudi plants as a source of potential β-lactamase inhibitors. Pak. J. Pharm. Sci. 2018, 31, 325–332. [Google Scholar]
- Aqil, F.; Khan, M.S.A.; Owais, M.; Ahmad, I. Effect of certain bioactive plant extracts on clinical isolates of β-lactamase producing methicillin resistant Staphylococcus aureus. J. Basic Microbiol. 2005, 45, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Pain, V.M.; Randall, D.P.; Garlick, P.J. Protein synthesis in liver and skeletal muscle of mice bearing an ascites tumor. Cancer Res. 1984, 44, 1054–1057. [Google Scholar]
- Petruzzelli, M.; Wagner, E.F. Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev. 2016, 30, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Nikkhoo, B.; Sigari, N.; Ghaderi, B.; Afkhamzadeh, A.; Azadi, N.-A.; Mohsenpour, B.; Fathi, F.; Abdi, M. Diagnostic Utility of Adenosine Deaminase in Serum and Bronchoalveolar Lavage Fluid for Screening Lung Cancer in Western Iran. J. Med. Biochem. 2013, 32, 109–115. [Google Scholar] [CrossRef]
- Xie, J.; Fan, Z.; Li, Y.; Zhang, Y.; Yu, F.; Su, G.; Xie, L.Y.; Hou, Z.Q. Design of pH-sensitive methotrexate prodrug-targeted curcumin nanoparticles for efficient dual-drug delivery and combination cancer therapy. Int. J. Nanomed. 2018, 13, 1381–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, P.; Sheng, S.; Sun, X.; Liu, J.; Huang, G. Lactate dehydrogenase a in cancer: A promising target for diagnosis and therapy. IUBMB Life 2013, 65, 904–910. [Google Scholar] [CrossRef]
- Sadeck, N.E.; Ibrahim, B.M.; Alassal, M.A. Cytochrome P450-isoenzyme 1A1 in susceptibility to tobacco-related lung cancer. Asian Cardiovasc. Thorac. Ann. 2014, 22, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Nicco, C.; Laurent, A.; Chereau, C.; Weill, B.; Batteux, F. Differential modulation of normal and tumor cell proliferation by reactive oxygen species. Biomed. Pharmacother. 2005, 59, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS Signaling in Organismal Homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Oyewole, A.O.; Birch-Machin, M.A. Mitochondria-targeted antioxidants. FASEB J. 2015, 29, 4766–4771. [Google Scholar] [CrossRef]
- Bodduluru, L.N.; Kasala, E.R.; Madhana, R.M.; Barua, C.C.; Hussain, M.I.; Haloi, P.; Borah, P. Naringenin ameliorates inflammation and cell proliferation in benzo(a)pyrene induced pulmonary carcinogenesis by modulating CYP1A1, NFκB and PCNA expression. Int. Immunopharmacol. 2016, 30, 102–110. [Google Scholar] [CrossRef]
- Kasala, E.R.; Bodduluru, L.N.; Barua, C.C.; Gogoi, R. Antioxidant and antitumor efficacy of Luteolin, a dietary flavone on benzo(a)pyrene-induced experimental lung carcinogenesis. Biomed. Pharmacother. 2016, 82, 568–577. [Google Scholar] [CrossRef]
- Hudlikar, R.R.; Pai, V.; Kumar, R.; Thorat, R.A.; Kannan, S.; Ingle, A.D.; Maru, G.B.; Mahimkar, M.B. Dose-Related Modulatory Effects of Polymeric Black Tea Polyphenols (PBPs) on Initiation and Promotion Events in B(a)P and NNK-Induced Lung Carcinogenesis. Nutr. Cancer 2019, 71, 508–523. [Google Scholar] [CrossRef]

| Microorganism | Zone of Inhibition MEA (6 mg) | Zone of Inhibition MEA (3 mg) |
|---|---|---|
| Candida albicans | 24 mm | 20 mm |
| Enterococcus faecalis | 25 mm | 21 mm |
| MRSA | 21 mm | 18 mm |
| Klebsiella pneumoniae | 14 mm | 12 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allemailem, K.S. Aqueous Extract of Artemisia annua Shows In Vitro Antimicrobial Activity and an In Vivo Chemopreventive Effect in a Small-Cell Lung Cancer Model. Plants 2022, 11, 3341. https://doi.org/10.3390/plants11233341
Allemailem KS. Aqueous Extract of Artemisia annua Shows In Vitro Antimicrobial Activity and an In Vivo Chemopreventive Effect in a Small-Cell Lung Cancer Model. Plants. 2022; 11(23):3341. https://doi.org/10.3390/plants11233341
Chicago/Turabian StyleAllemailem, Khaled S. 2022. "Aqueous Extract of Artemisia annua Shows In Vitro Antimicrobial Activity and an In Vivo Chemopreventive Effect in a Small-Cell Lung Cancer Model" Plants 11, no. 23: 3341. https://doi.org/10.3390/plants11233341
APA StyleAllemailem, K. S. (2022). Aqueous Extract of Artemisia annua Shows In Vitro Antimicrobial Activity and an In Vivo Chemopreventive Effect in a Small-Cell Lung Cancer Model. Plants, 11(23), 3341. https://doi.org/10.3390/plants11233341






