Rumors of Psychedelics, Psychotropics and Related Derivatives in Vachellia and Senegalia in Contrast with Verified Records in Australian Acacia
Abstract
1. Introduction
2. Australian Acacia
2.1. Verified References of Psychedelics, Psychotropics and Related Derivatives in Australian Acacia
2.2. Rumours and Anecdotes of Psychedelics, Psychotropics and Related Derivatives in Australian Acacia
| Acacia Taxon | Anecdote |
|---|---|
| A. alpina F.Muell. | Dimethyltryptamine in leaf (unregulated use, expired web source) [42]. |
| A. auriculiformis A.Cunn. ex Benth. | 5-MeO-DMT speculated from TLC of stem bark extract [25]. |
| A. beauverdiana Ewart and Sharman | Claimed to be psychoactive in expired web source (www.bushfood.net, accessed on 3 November 2022), accessed using ‘Wayback Machine’ (https://web.archive.org/). |
| A. colei Maslin and L.A.J.Thomson | Media source claimed DMT in bark at 1.8% (not verifiable) [42]. |
| A. cultriformis A.Cunn. ex G.Don | Traces of tryptamine * (by TLC) and phenethylamine in leaves [21,22,27], and 5-MeO-DMT in aerial parts speculated by TLC [25]. |
| A. cuthbertsonii Luehm. | Claimed to be psychoactive in expired web source (www.bushfood.net), accessed using ‘Wayback Machine’ (https://web.archive.org/) [42]. |
| A. delibrata A.Cunn. ex Benth. | Claimed to be psychoactive in expired web source (www.bushfood.net), accessed using ‘Wayback Machine’ (https://web.archive.org/) [42]. |
| A. falcata Desf. (syn. Prosopis juliflora (Sw.) DC., var. juliflora) | Claimed to be psychoactive in expired web source (www.bushfood.net), accessed using ‘Wayback Machine’ (https://web.archive.org/) [42]. |
| A. hamiltoniana Maiden (syn. A. sieberiana) | Claims of DMT in the leaves caused by misreading of a paper that concluded none was found [43]. Claims of psychedelic effects experienced with unregulated use [42]. |
| A. implexa Benth. | Claimed to be psychoactive in the media, but no primary source found [42]. |
| A. macradenia Benth. | Claimed to be psychoactive in the media, but no primary source found [42]. |
| A. mangium Willd. | Claims to be psychoactive from unregulated use [42]. |
| A. melanoxylon R.Br. | Claims of DMT in the bark and leaves, but no primary sources confirmed this [42]. |
| A. mucronata subsp. longifolia (Benth.) Court | DMT, NMT, tryptamine * speculated from TLC migration patterns [44]. |
| A. obtusifolia A.Cunn. | Claims of NMT, tryptamine *, harman and norharman, tentative 5-MeO-DMT from a network of anonymous authors whose works appear in ‘The Entheogen Review’ (http://www.entheogenreview.com/) [42]. |
| A. penninervis Sieber ex DC. | Claimed to be psychoactive in expired web source (www.bushfood.net), accessed using ‘Wayback Machine’ (https://web.archive.org/) [42]. |
| A. pycnantha Benth. | An oral presenter claimed to have identified 0.4% DMT [42]. |
| A. retinodes Schltdl. | Speculation of DMT and NMT in aerial parts [42]. |
| A. sophorae (Labill.) R.Br. | Alkaloids in leaves, stems and unripe seed pods [20,27] possibly tryptamine alkaloids [42]. |
| A. victoriae Benth. | Speculation from TLC of DMT in aerial parts and 5-MeO-DMT in roots [25]. Unregulated users claim to have experienced psychedelic effects [42]. |
3. American Acacia, Senegalia and Vachellia
3.1. Verified References of Psychedelics, Psychotropics and Related Derivatives in American Senegalia and Vachellia
3.2. Rumours and Anecdotes of Psychedelics, Psychotropics and Related Derivatives in American Senegalia and Vachellia
4. South African Senegalia and Vachellia
5. Description and Mechanism of Psychotropics in Acacia, Senegalia and Vachellia
5.1. The Tryptamines
5.2. β-Carboline Alkaloids
5.3. Phenethylamines
5.4. Histamines
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dyer, C. New names for the African Acacia species in Vachellia and Senegalia. South. For. J. For. Sci. 2014, 76, 3. [Google Scholar] [CrossRef][Green Version]
- Murphy, D.J. A review of the classification of Acacia (Leguminosae, Mimosoideae). Muellaria 2008, 26, 10–26. [Google Scholar] [CrossRef]
- Maslin, B.R.; Miller, J.T.; Seigler, D.S. Overview of the generic status of Acacia (Leguminosae: Mimosoideae). Aust. Syst. Bot. 2003, 16, 1–18. [Google Scholar] [CrossRef]
- Boatwright James, S.; Van der Bank, M.; Maurin, O. Name changes in African Acacia species: Plant name changes. Veld Flora 2014, 100, 33. [Google Scholar] [CrossRef]
- van Wyk, B.; van Wyk, P. Field Guide to Trees of Southern Africa; Struik Nature: Cape Town, South Africa, 2013. [Google Scholar]
- Smit, N. Field Guide to the Acacias of South Africa; Briza Publications: Pretoria, South Africa, 2008. [Google Scholar]
- Ross, I.H. A survey of some of the pre-Linnean history of the genus Acacia. Bothalia 1980, 13, 95–110. [Google Scholar] [CrossRef][Green Version]
- Miller, P. The Gardeners Dictionary; G. Henderson: London, UK, 1835; Volume 1. [Google Scholar]
- Hitchcock, M. Wattle; Australian Government Publishing Service: Canberra, Australia, 1991. [Google Scholar]
- St John, G. Aussiewaska: A cultural history of changa and ayahuasca analogues in Australia. In The World Ayahuasca Diaspora; Routledge: London, UK, 2016; pp. 163–184. [Google Scholar]
- Elkin, A.P. Aboriginal Men of High Degree: Initiation and Sorcery in the World’s Oldest Tradition; Inner Traditions/Bear & Co.: Rochester, VT, USA, 1993. [Google Scholar]
- de Rios, M.D. This Precious Foliage. A Study of the Aboriginal Psychoactive Drug, Pituri. J. Psychoact. Drugs 1984, 16, 367–368. [Google Scholar] [CrossRef]
- Jones, G.L.; Sadgrove, N.J. Ethnopharmacology in Australia and Oceania. In Ethnopharmacology—A Reader; Heinrich, M., Jäger, A.K., Eds.; John Wiley & Sons: Chichester, UK, 2015. [Google Scholar]
- Feeney, K. The Legal Bases for Religious Peyote Use. In Psychedelic Medicine: New Evidence for Hallucinogenic Substances as Treatments; Roberts, T.B., Winkelman, M.J., Eds.; Praeger/Greenwood: Westport, CT, USA, 2007. [Google Scholar]
- Szára, S. Dimethyltryptamin: Its metabolism in man; the relation of its psychotic effect to the serotonin metabolism. Experientia 1956, 12, 441–442. [Google Scholar] [CrossRef]
- RBG-Kew. World Checklist of Vascular Plants. Available online: https://wcvp.science.kew.org/ (accessed on 6 August 2021).
- Ramos, Y.J.; Gouvêa-Silva, J.G.; de Brito Machado, D.; Felisberto, J.S.; Pereira, R.C.; Sadgrove, N.J.; de Lima Moreira, D. Chemophenetic and Chemodiversity Approaches: New Insights on Modern Study of Plant Secondary Metabolite Diversity at Different Spatiotemporal and Organizational Scales. Rev. Bras. Farmacogn. 2022. [Google Scholar] [CrossRef]
- Yaster, M.; Honorio, B.; Anderson, T.A. “Houston, We Have a Problem!”: The Role of the Anesthesiologist in the Current Opioid Epidemic. Anesth. Analg. 2017, 125, 1429–1431. [Google Scholar] [CrossRef]
- Pollan, M. How to Change Your Mind: What the New Science of Psychedelics Teaches Us about Consciousness, Dying, Addiction, Depression, and Transcendence; Penguin Press: New York, NY, USA, 2018. [Google Scholar]
- Collins, D.J.; Culvenor, C.; Lamberton, J.; Loder, J.; Price, J. Plants for Medicines: A Chemical and Pharmacological Survey of Plants in the Australian Region; CSIRO Publishing: Canberra, Australia, 1990. [Google Scholar]
- White, E.P. Legumes examined for alkaloids—Additions and corrections. N. Z. J. Sci. Technol. 1951, 33, 54–60. [Google Scholar]
- White, E.P. Evaluation of further legumes, mainly Lupinus and Acacia species for alkaloids. N. Z. J. Sci. Technol. B 1957, 38, 718–725. [Google Scholar]
- Fitzgerald, J. Alkaloids of the Australian Leguminosae. III. The occurrence of phenylethylamine derivatives in Acacia species. Aust. J. Chem. 1964, 17, 160–162. [Google Scholar] [CrossRef][Green Version]
- Repke, D.B.; Mandell, D.M.; Thomas, J.H. Alkaloids of Acacia baileyana. Lloydia 1973, 36, 211–213. [Google Scholar]
- Keeper of the Trout. Some Simple Tryptamines: A Brief Overview & Resource Compendium; MydriaticProductions, 2007; Available online: https://troutsnotes.com/pdf/SomeSimpleTryptamines_2ndEd_2007_with_addendum.pdf (accessed on 15 October 2022).
- Johns, S.; Lamberton, J.; Sioumis, A. Alkaloids of the Australian Leguminosae. VII. Nb-Methyltetrahydroharman from Acacia complanata A.Cunn. ex Benth. Aust. J. Chem. 1966, 19, 1539–1540. [Google Scholar] [CrossRef]
- White, E.P. Isolation of β-phenethylamine from Acacia species. N. Z. J. Sci. Techonol. B 1944, 25, 139–142. [Google Scholar]
- White, E.P. Isolation of tryptamine from some Acacia species. N. Z. J. Sci. Technol. 1944, 25B, 154–162. [Google Scholar]
- Repke, D.B. The histamine amides of Acacia longifolia. Lloydia 1975, 38, 101–105. [Google Scholar]
- Rovelli, B.; Vaughan, G. Alkaloids of Acacia. I. NbNb-Dimethyltryptamine in Acacia phlebophylla F.Muell. Aust. J. Chem. 1967, 20, 1299–1300. [Google Scholar] [CrossRef][Green Version]
- White, E.P. The occurrence of N-methyl-β-phenylethylamine in Acacia prominens A.Cunn. N. Z. J. Sci. Technol. 1954, 35B, 451–455. [Google Scholar]
- Sadgrove, N.J. The influence of indigenous food procurement techniques on populations of cyanobacteria in pre-European Australia: A potential small-scale water amelioration tool. Ecohealth 2009, 6, 390–403. [Google Scholar] [CrossRef]
- Ratsch, A.; Steadman, K.J.; Bogossian, F. The pituri story: A review of the historical literature surrounding traditional Australian Aboriginal use of nicotine in Central Australia. J. Ethnobiol. Ethnomed. 2010, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Latz, P. Bushfires and Bushtucker: Aboriginal Plant Use in Central Australia; IAD Press: Alice Springs, Australia, 2004. [Google Scholar]
- Sadgrove, N.J.; Jones, G.L. From Petri Dish to Patient: Bioavailability Estimation and Mechanism of Action for Antimicrobial and Immunomodulatory Natural Products. Front. Microbiol. 2019, 10, 2470. [Google Scholar] [CrossRef] [PubMed]
- Ott, J. Psychonautic uses of “Ayahuasca” and its analogues: Panacæa or Outré Entertainment. In The Internationalization of Ayahuasca; LIT Verlag Münster: Münster, Germany, 2011; pp. 105–122. [Google Scholar]
- Lakstygal, A.M.; Kolesnikova, T.O.; Khatsko, S.L.; Zabegalov, K.N.; Volgin, A.D.; Demin, K.A.; Shevyrin, V.A.; Wappler-Guzzetta, E.A.; Kalueff, A.V. DARK Classics in Chemical Neuroscience: Atropine, Scopolamine, and Other Anticholinergic Deliriant Hallucinogens. ACS Chem. Neurosci. 2019, 10, 2144–2159. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, C.; Gough, J.; Conlan, L.; Hegarty, M.; Palmer, B.; Krause, D. Nutritive value assessment of the tropical shrub legume Acacia angustissima: Anti-nutritional compounds and in vitro digestibility. Anim. Feed. Sci. Technol. 2005, 121, 175–190. [Google Scholar] [CrossRef]
- Skerritt, J.H.; Guihot, S.L.; McDonald, S.E.; Culvenor, R.A. Development of Immunoassays for Tyramine and Tryptamine Toxins of Phalaris aquatica L. J. Agric. Food Chem. 2000, 48, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; González, D.; Ancín-Azpilicueta, C.; Arán, V.J.; Guillén, H. β-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO). Food Chem. Toxicol. 2010, 48, 839–845. [Google Scholar] [CrossRef]
- Riba, J.; McIlhenny, E.H.; Valle, M.; Bouso, J.C.; Barker, S.A. Metabolism and disposition of N,N-dimethyltryptamine and harmala alkaloids after oral administration of ayahuasca. Drug Test. Anal. 2012, 4, 610–616. [Google Scholar] [CrossRef]
- Wikipedia. List of Acacia Species Known to Contain Psychoactive Alkaloids. Available online: https://en.wikipedia.org/wiki/List_of_Acacia_species_known_to_contain_psychoactive_alkaloids (accessed on 25 November 2022).
- Khalil, S.K.W.; Elkheir, Y.M. Dimethyltryptamine from the leaves of certain Acacia species of Northern Sudan. Lloydia 1975, 38, 176–177. [Google Scholar]
- Voogelbreinder, S. Garden of Eden: The Shamanic Use of Psychoactive Flora and Fauna, and the Study of Consciousness; Snu Voogelbreinder: Belgrave, Australia, 2009. [Google Scholar]
- Morales-García, J.A.; de la Fuente Revenga, M.; Alonso-Gil, S.; Rodríguez-Franco, M.I.; Feilding, A.; Perez-Castillo, A.; Riba, J. The alkaloids of Banisteriopsis caapi, the plant source of the Amazonian hallucinogen Ayahuasca, stimulate adult neurogenesis in vitro. Sci. Rep. 2017, 7, 5309. [Google Scholar] [CrossRef]
- Moraes, T.F.S.; Ferraz, A.C.; da Cruz Nizer, W.S.; Tótola, A.H.; Soares, D.B.S.; Duarte, L.P.; Vieira-Filho, S.A.; Magalhães, C.L.B.; de Magalhães, J.C. A methanol extract and N,N-dimethyltryptamine from Psychotria viridis Ruiz & Pav. inhibit Zika virus infection in vitro. Arch. Virol. 2021, 166, 3275–3287. [Google Scholar] [CrossRef]
- Pachter, I.J.; Zacharias, D.E.; Ribeiro, O. Indole Alkaloids of Acer saccharinum (the Silver Maple), Dictyoloma incanescens, Piptadenia colubrina, and Mimosa hostilis. J. Org. Chem. 1959, 24, 1285–1287. [Google Scholar] [CrossRef]
- Glennon, R.A.; Dukat, M.; Grella, B.; Hong, S.-S.; Costantino, L.; Teitler, M.; Smith, C.; Egan, C.; Davis, K.; Mattson, M.V. Binding of β-carbolines and related agents at serotonin (5-HT2 and 5-HT1A), dopamine (D2) and benzodiazepine receptors. Drug Alcohol Depend. 2000, 60, 121–132. [Google Scholar] [CrossRef]
- Callaway, J.C.; McKenna, D.J.; Grob, C.S.; Brito, G.S.; Raymon, L.P.; Poland, R.E.; Andrade, E.N.; Andrade, E.O.; Mash, D.C. Pharmacokinetics of Hoasca alkaloids in healthy humans. J. Ethnopharmacol. 1999, 65, 243–256. [Google Scholar] [CrossRef]
- Husson, H.P. β-Carbolines and Carbazoles. In The Alkaloids: Chemistry and Pharmacology; Academic Press Inc.: London, UK, 1985. [Google Scholar]
- Poupat, C.; Ahond, A.; Sévenet, T. Alcaloides de Acacia simplicifolia. Phytochemistry 1976, 15, 2019–2020. [Google Scholar] [CrossRef]
- Adams, H.R.; Camp, B.J. The isolation and identification of three alkaloids from Acacia berlandieri. Toxicon 1966, 4, 85–90. [Google Scholar] [CrossRef]
- Camp, B.J.; Moore, J.A. A quantitative method for the alkaloid of Acacia berlandieri. J. Am. Pharm. Assoc. 1960, 49, 158–160. [Google Scholar] [CrossRef]
- Camp, B.J.; Norvell, M.J. The phenylethylamine alkaloids of native range plants. Econ. Bot. 1966, 20, 274–278. [Google Scholar] [CrossRef]
- Rätsch, C. The Encyclopedia of Psychoactive Plants: Ethnopharmacology and Its Applications; Simon and Schuster: New York, NY, USA, 2005. [Google Scholar]
- Ghosal, S. Occurrence of Psychodelic Substances in Some Indian Medicinal Plants. Planta Med. 1972, 21, 200–209. [Google Scholar] [CrossRef]
- Clement, B.A.; Goff, C.M.; Forbes, T.D.A. Toxic amines and alkaloids from Acacia rigidula. Phytochemistry 1998, 49, 1377–1380. [Google Scholar] [CrossRef]
- Stafford, G.I.; Pedersen, M.E.; van Staden, J.; Jäger, A.K. Review on plants with CNS-effects used in traditional South African medicine against mental diseases. J. Ethnopharmacol. 2008, 119, 513–537. [Google Scholar] [CrossRef]
- Oliver-Bever, B. Medicinal Plants in Tropical West Africa; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Alharbi, W.D.; Azmat, A. Pharmacological evidence of neuro-pharmacological activity of Acacia tortilis leaves in mice. Metab. Brain Dis. 2016, 31, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, T.M.; Gatch, M.B. Neuropharmacology of N,N-dimethyltryptamine. Brain Res. Bull. 2016, 126, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Nichols, C.; Sanders-Bush, E. A Single Dose of Lysergic Acid Diethylamide Influences Gene Expression Patterns within the Mammalian Brain. Neuropsychopharmacology 2002, 26, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.L.; Bohn, L.M. Serotonin, But Not N-Methyltryptamines, Activates the Serotonin 2A Receptor Via a β-Arrestin2/Src/Akt Signaling Complex In Vivo. J. Neurosci. 2010, 30, 13513–13524. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L.; Buell, M.R.; Masten, V.L.; Risbrough, V.B.; Geyer, M.A. Modification of the effects of 5-methoxy-N,N-dimethyltryptamine on exploratory behavior in rats by monoamine oxidase inhibitors. Psychopharmacology 2008, 201, 55–66. [Google Scholar] [CrossRef]
- Barker, S.A. N,N-Dimethyltryptamine (DMT), an Endogenous Hallucinogen: Past, Present, and Future Research to Determine Its Role and Function. Front. Neurosci. 2018, 12, 536. [Google Scholar] [CrossRef]
- Palamar, J.J.; Acosta, P. A qualitative descriptive analysis of effects of psychedelic phenethylamines and tryptamines. Hum. Psychopharmacol. Clin. Exp. 2020, 35, e2719. [Google Scholar] [CrossRef]
- Buckholtz, N.S.; Boggan, W.O. Inhibition by β-carbolines of monoamine uptake into a synaptosomal preparation: Structure-activity relationships. Life Sci. 1977, 20, 2093–2100. [Google Scholar] [CrossRef]
- Jiang, B.; Meng, L.; Zou, N.; Wang, H.; Li, S.; Huang, L.; Cheng, X.; Wang, Z.; Chen, W.; Wang, C. Mechanism-based pharmacokinetics-pharmacodynamics studies of harmine and harmaline on neurotransmitters regulatory effects in healthy rats: Challenge on monoamine oxidase and acetylcholinesterase inhibition. Phytomedicine 2019, 62, 152967. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The Expanded Biology of Serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Shih, J.C.; Chen, K.; Ridd, M.J. MONOAMINE OXIDASE: From Genes to Behavior. Annu. Rev. Neurosci. 1999, 22, 197–217. [Google Scholar] [CrossRef]
- Ferrucci, M.; Limanaqi, F.; Ryskalin, L.; Biagioni, F.; Busceti, C.L.; Fornai, F. The Effects of Amphetamine and Methamphetamine on the Release of Norepinephrine, Dopamine and Acetylcholine from the Brainstem Reticular Formation. Front. Neuroanat. 2019, 13, 48. [Google Scholar] [CrossRef]
- Harris, D.S.; Boxenbaum, H.; Everhart, E.T.; Sequeira, G.; Mendelson, J.E.; Jones, R.T. The bioavailability of intranasal and smoked methamphetamine. Clin. Pharmacol. Ther. 2003, 74, 475–486. [Google Scholar] [CrossRef]
- de la Torre, R.; Yubero-Lahoz, S.; Pardo-Lozano, R.; Farré, M. MDMA, methamphetamine, and CYP2D6 pharmacogenetics: What is clinically relevant? Front. Genet. 2012, 3, 235. [Google Scholar] [CrossRef]
- Obata, Y.; Kubota-Sakashita, M.; Kasahara, T.; Mizuno, M.; Nemoto, T.; Kato, T. Phenethylamine is a substrate of monoamine oxidase B in the paraventricular thalamic nucleus. Sci. Rep. 2022, 12, 17. [Google Scholar] [CrossRef]
- Shannon, H.E.; Cone, E.J.; Yousefnejad, D. Physiologic effects and plasma kinetics of beta-phenylethylamine and its N-methyl homolog in the dog. J. Pharmacol. Exp. Ther. 1982, 223, 190–196. [Google Scholar]
- Obara, I.; Telezhkin, V.; Alrashdi, I.; Chazot, P.L. Histamine, histamine receptors, and neuropathic pain relief. Br. J. Pharmacol. 2020, 177, 580–599. [Google Scholar] [CrossRef]
- de la Torre, R.; Farré, M.; Roset, P.N.; Pizarro, N.; Abanades, S.; Segura, M.; Segura, J.; Camí, J. Human Pharmacology of MDMA: Pharmacokinetics, Metabolism, and Disposition. Ther. Drug Monit. 2004, 26, 137–144. [Google Scholar] [CrossRef]
- Maršavelski, A.; Mavri, J.; Vianello, R.; Stare, J. Why Monoamine Oxidase B Preferably Metabolizes N-Methylhistamine over Histamine: Evidence from the Multiscale Simulation of the Rate-Limiting Step. Int. J. Mol. Sci. 2022, 23, 1910. [Google Scholar] [CrossRef]

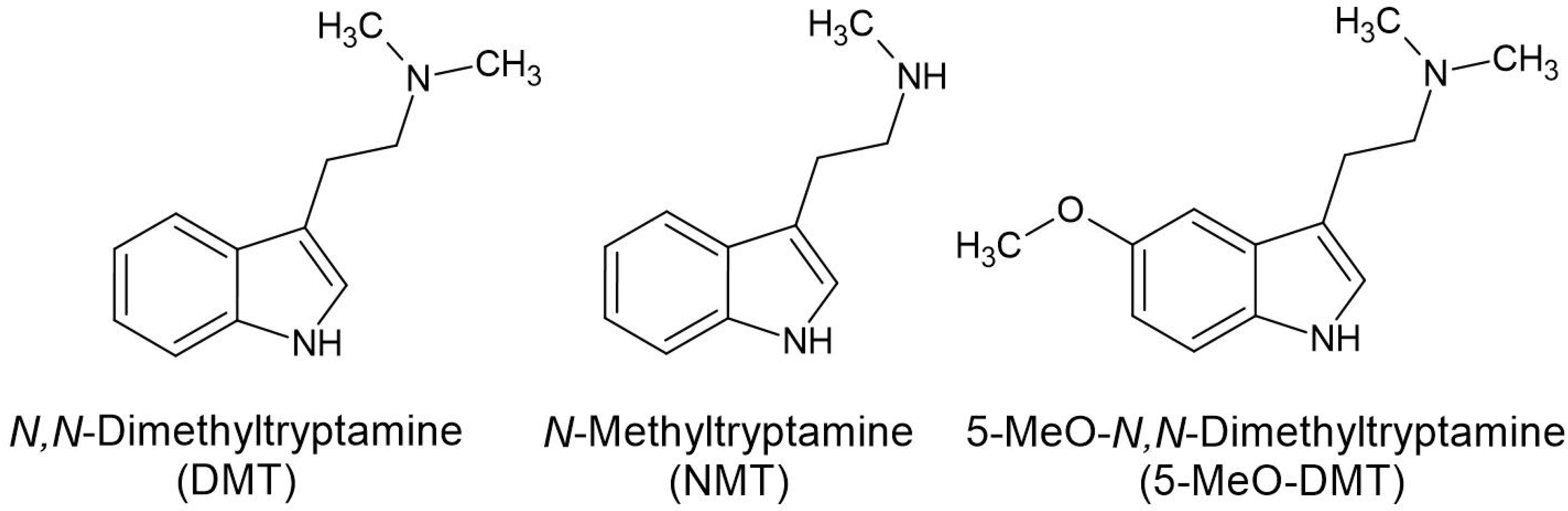

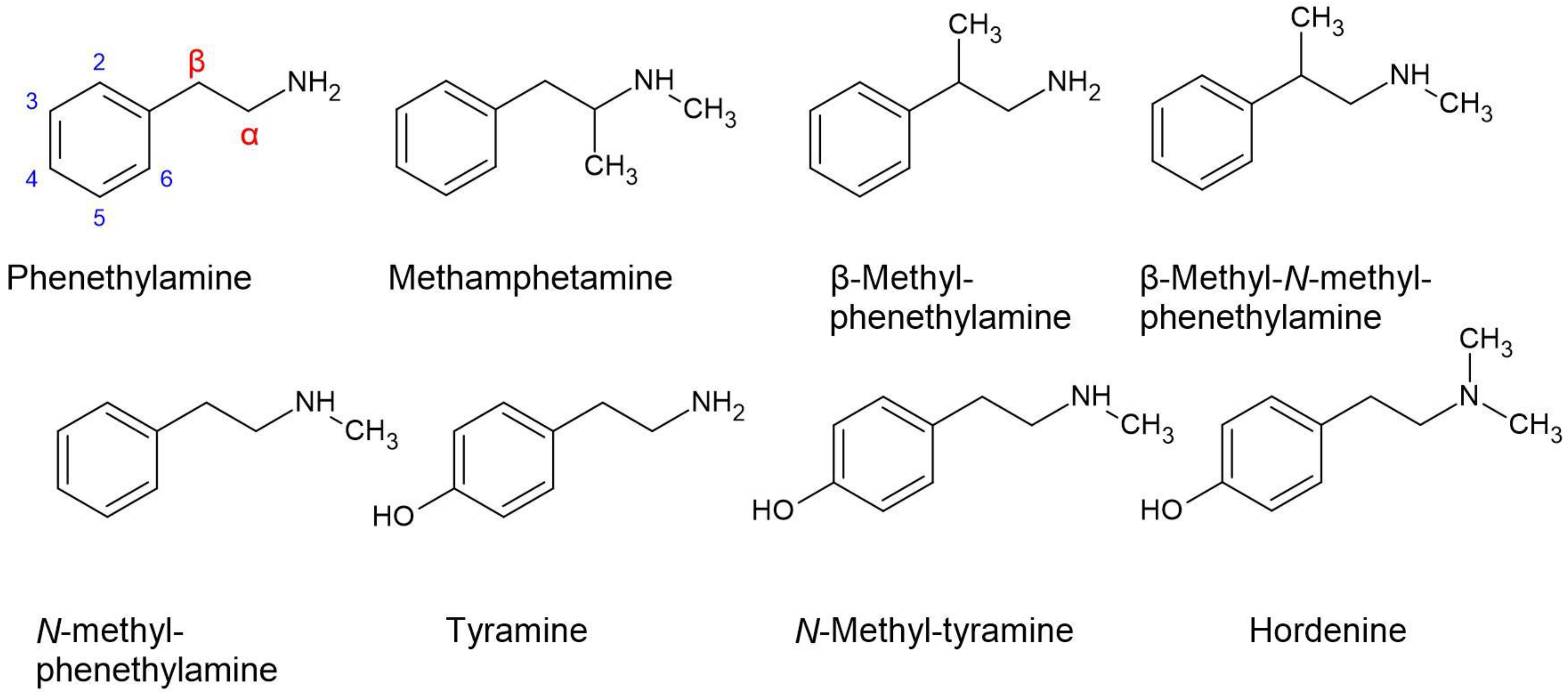

| Acacia Taxon | Description with Reference |
|---|---|
| A. acinacea Lindl. | Phenethylamine * in pods (0.08%) [21]. |
| A. acuminata Benth. | Tryptamine * in leaves and stems (0.7%) [22]. |
| A. burkittii F.Muell. ex Benth. | Tryptamine * in leaves and stems (>1%) [22]. |
| A. linearifolia J.Forbes | 2.4% of β-Methyl-phenethylamine and N-methyl-phenethylamine from leaves [23], and 3.2% alkaloids from flowers in which most was β-methyl-phenethylamine [22]. |
| A. baileyana F.Muell. | Tetrahydroharman and tryptamine * (0.02%, ratio 8:2) [24], traces of DMT speculated by TLC in seeds [25]. |
| A. buxifolia A.Cunn. | 0.6% phenethylamine from leaves and stems [21]. |
| A. cardiophylla A.Cunn. ex Benth. | Traces of tryptamine * and phenethylamine in leaves and stems of one specimen [22], but not all specimens [20]. |
| A. complanata A.Cunn. ex Benth. | N-methyl-tetrahydroharman (0.3%) with traces of tetrahydroharman in leaves and stem [26], unpublished claims of DMT in the bark. |
| A. floribunda (Vent.) Willd. | 0.2% tryptamine * and phenethylamine in flowers [27,28]. Unpublished report of DMT in leaves (0.1%) and bark (0.5%), with traces of tryptamine, harman and norharman (unregulated community anecdote) |
| A. harpophylla F.Muell. ex Benth. | Phenethylamine * and hordenine in leaves and bark (0.1–0.6%) [20,23]. |
| A. holosericea A.Cunn. ex G.Don | Hordenine (1.2%) in bark [23]. |
| A. kettlewelliae Maiden | 1–2% phenethylamine * in leaves and stems [22], and in another report, 1% β-methyl-phenethylamine from leaves [23]. |
| A. longifolia (Andrews) Willd. | Tryptamine alkaloids (0.2–1%) with traces of phenethylamine * in aerial parts [21,27,28]. Histamine amides (N-(2-imidazol-4-yl-ethyl)-trans-cinnamamide and N-(2-imidazol-4-yl-ethyl)-deca-trans-2, cis-4-dienamide) in some specimens [29]. Claims of 0.2% DMT in the unregulated community (anecdote). |
| A. maidenii F.Muell. | NMT and DMT in leaves (0.1–0.7%) and bark [20,23]. TLC method suggested 5-MeO-DMT in woody parts and NMT in root [25]. |
| A. phlebophylla F.Muell. ex H.B.Will. | DMT in leaves (0.3%) [30]. |
| A. podalyriifolia A.Cunn. ex G.Don | Tryptamine * (0.1–0.3%) and phenethylamine in aerial parts [21,22,27,28]. |
| A. pravissima F.Muell. ex Benth. | Phenethylamine * in leaves and stems (0.4%) [27]. |
| A. prominens A.Cunn. ex G.Don | Phenethylamine * and β-methyl-phenethylamine in stems and leaves (0.2–0.7%), and flowering tops (1.8%) [21,22,27,28,31]. |
| A. pruinosa A.Cunn. ex Benth. | Tryptamine * (0.02–0.1%) and traces of phenethylamine in stems, leaves, and flowers (0.4%) [27,28]. |
| A. spectabilis A.Cunn. ex Benth. | Phenethylamine * in leaves and stems (0.2–0.4%) [22]. |
| A. suaveolens (Sm.) Willd. | Phenethylamine * (1%) in leaves and stems [21,27]. |
| A. vestita Ker Gawl. | Tryptamine * in the leaves and stem (0.1–0.3%) [22]. |
| Acacia or Senegalia | Details |
|---|---|
| A. simplex (Sparrm.) Pedley (syn. A. simplicifolia) | High yield of alkaloids from leaves and stem bark (3.6%) consisting of NMT, DMT, and 2-methyl-tetrahydro-β-carboline [50,51]. |
| S. berlandieri (Benth.) Britton and Rose (sy. A. berlandieri) | Hordenine, tyramine *, N-methyltyramine, N-methylphenethylamine in leaves [52,53,54]. |
| Senegalia or Vachellia | Anecdote |
|---|---|
| S. greggii (A.Gray) Britton and Rose (syn. A. greggii) | Low yield of alkaloids from leaves (0.02%), including N-methyl-β-methyl-phenethylamine and tyramine *, speculated from migrating TLC patterns [54]. |
| S. roemeriana (Scheele) Britton and Rose (syn. A. roemeriana) | Low yield of alkaloids from leaves (0.4%), including β-methyl-phenethylamine, tyramine * and N-methyl-tyramine, speculated from migrating TLC patterns [54]. |
| V. aroma (Gillies ex Hook. and Arn.) Seigler and Ebinger (syn. A. aroma) | Speculation of tryptamine alkaloids on the internet, claiming high yield from the seeds, but no primary source cited [42]. |
| V. caven (Molina) Seigler and Ebinger (syn. A. caven) | Leaves combined with other substances and smoked for psychoactive effects [55]. Speculation of tryptamine alkaloids but no primary source cited [42]. |
| V. constricta (Benth.) Seigler and Ebinger (syn. A. constricta) | Traces of β-methyl-phenethylamine, speculated from migrating TLC patterns [54]. |
| V. cornigera (L.) Seigler and Ebinger (syn. A. cornigera) | Has been used as an aphrodisiac [55]. Speculation of tryptamines but no primary source cited [42]. |
| V. farnesiana (L.) Wight and Arn. (syn. A. farnesiana) | Both 5-MeO-DMT and a β-carboline * were speculated by migrating TLC patterns in the extract of immature seed pods [25]. Speculation of tryptamine in stem bark, peer reviewed article [56]. Speculation of β-methyl-phenethylamine from flowers, but no primary source cited [42]. |
| V. rigidula (Benth.) Seigler and Ebinger (syn. A. rigidula) | Low yield of alkaloids from leaves (0.3%), comprising N-methyl-phenethylamine and N-methyl-tyramine speculated from TLC migration patterns [54,57]. |
| V. schaffneri (S.Watson) Seigler and Ebinger (syn. A. schaffneri) | Claiming β-methyl-phenethylamine, phenethylamine *, (amphetamines? and mescaline?), but no primary source identified [42]. |
| V. schottii (Torr.) Seigler and Ebinger (syn. A. schottii) | Traces of β-methyl-phenethylamine in leaves, speculated from migrating TLC patterns [54]. |
| Senegalia or Vachellia | Country | Details of Why Evidence is Insufficient |
|---|---|---|
| S. laeta (R.Br. ex Benth.) Seigler and Ebinger (syn. A. laeta) | Africa | Claims of DMT in the leaves caused by misreading of a paper that concluded none was found [43]. |
| S. mellifera (Benth.) Seigler and Ebinger (syn. A. mellifera) | South Africa | Claims of DMT in the leaves caused by misreading of a paper that concluded none was found [43]. |
| S. polyacantha subsp. campylacantha (Hochst. ex A.Rich.) Kyal. and Boatwr. (syn. A. campylacantha) | Africa | Old reference (1975) claims to detect traces (0.004%) of DMT in Sudanese biota, but concentration too low to be accurate with limited technology at that time [43]. |
| S. senegal (L.) Britton (syn. A. senegal) | South African | Old reference (1975) claims to detect traces (0.003%) of DMT in Sudanese biota of the same species, but concentration too low to be accurate with limited technology at that time [43]. |
| V. drepanolobium (Harms ex Y.Sjöstedt) P.J.H.Hurter (syn. A. drepanolobium) | East Africa | Claims of 1.4% DMT in bark and 0.5% in leaves (0.5–0.8%) by unregulated users [42]. |
| V. horrida (L.) Kyal. and Boatwr. (syn. A. horrida) | East Africa | Claimed to be psychoactive, but no primary source found [42]. |
| V. karroo (Hayne) Banfi and Galasso (syn. A. karroo) | South African | Roots used in Zimbabwean ethnobotany for psychoactive effects [58]. |
| V. nilotica (L.) P.J.H.Hurter and Mabb. (syn. A. nilotica) | South African | Claims of DMT in the leaves caused by misreading of a paper that concluded none was found [43]. Speculation from TLC results of DMT [25]. |
| V. nilotica subsp. adstringens (Schumach.) Kyal. and Boatwr. (syn. A. nilotica subsp. adstringens) | South African | Claims of DMT and harmane * in book [59], but not primary source found. |
| V. oerfota (Forssk.) Kyal. and Boatwr. (syn. A. oerfota) | Africa | Traces of DMT in leaves, speculated from TLC migration patterns [25]. |
| V. seyal (Delile) P.J.H.Hurter (syn. A. seyal) | East Africa | Claims of DMT in the leaves caused by misreading of a paper that concluded none was found [43]. |
| V. tortilis (Forssk.) Galasso and Banfi (syn. A. tortilis) | South Africa | Claims of DMT in the leaves caused by misreading of a paper that concluded none was found [43]. Another study used TLC, but with an Rf value of 0.9–0.95, but this finding is grossly inaccurate [60]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadgrove, N.J. Rumors of Psychedelics, Psychotropics and Related Derivatives in Vachellia and Senegalia in Contrast with Verified Records in Australian Acacia. Plants 2022, 11, 3356. https://doi.org/10.3390/plants11233356
Sadgrove NJ. Rumors of Psychedelics, Psychotropics and Related Derivatives in Vachellia and Senegalia in Contrast with Verified Records in Australian Acacia. Plants. 2022; 11(23):3356. https://doi.org/10.3390/plants11233356
Chicago/Turabian StyleSadgrove, Nicholas J. 2022. "Rumors of Psychedelics, Psychotropics and Related Derivatives in Vachellia and Senegalia in Contrast with Verified Records in Australian Acacia" Plants 11, no. 23: 3356. https://doi.org/10.3390/plants11233356
APA StyleSadgrove, N. J. (2022). Rumors of Psychedelics, Psychotropics and Related Derivatives in Vachellia and Senegalia in Contrast with Verified Records in Australian Acacia. Plants, 11(23), 3356. https://doi.org/10.3390/plants11233356






