Influence of Bacteria of the Genus Pseudomonas on Leguminous Plants and Their Joint Application for Bioremediation of Oil Contaminated Soils
Abstract
1. Introduction
2. Results
2.1. The Effect of Bacteria on Plants
2.2. Nitrogen Content in Soil and Plants
2.3. The Number of Microorganisms in the Rhizosphere of Plants
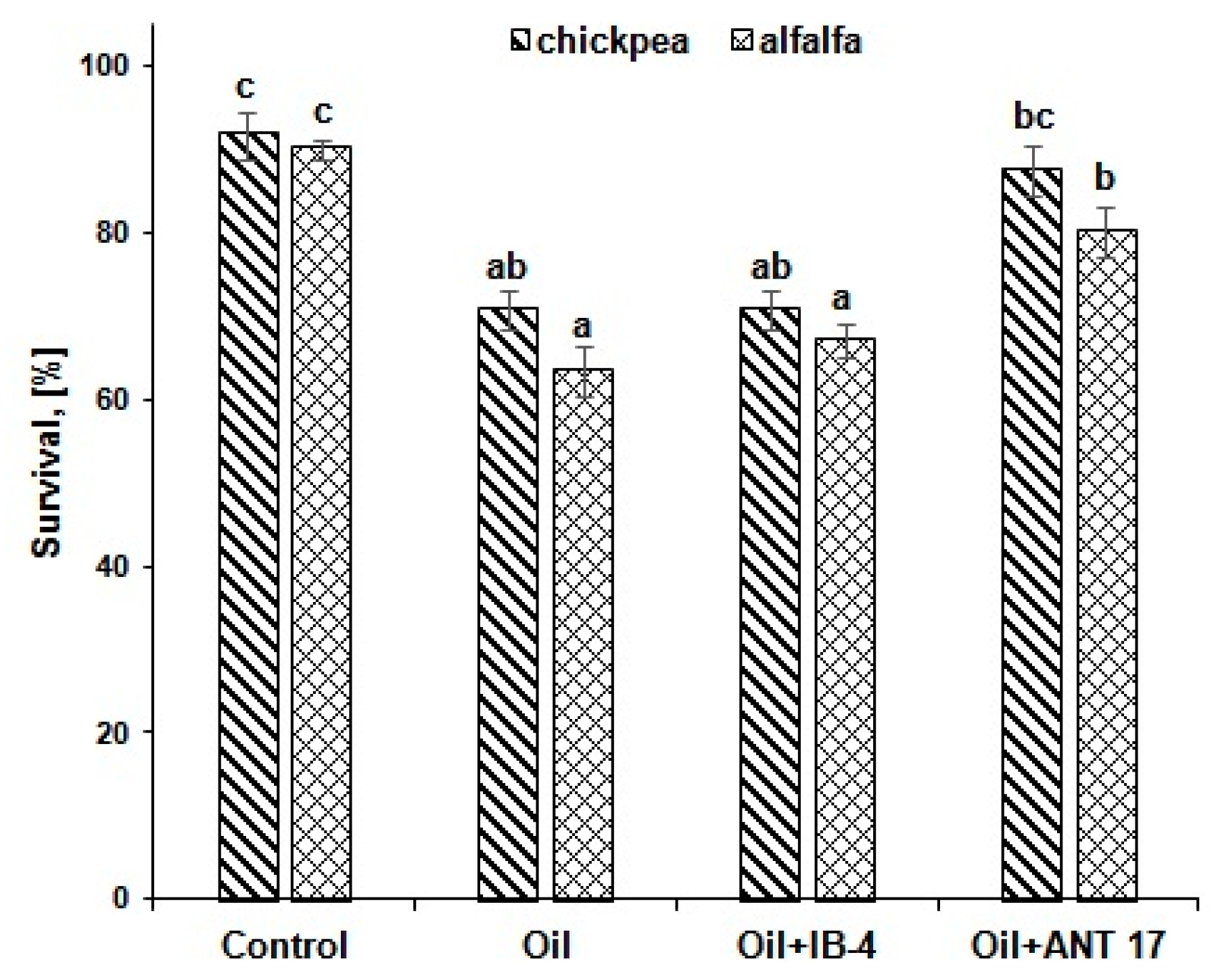
2.4. The Effect of Bacteria on Plants in Conditions of Pollution
2.5. The Number of Microorganisms in the Rhizosphere of Plants in Conditions of Pollution
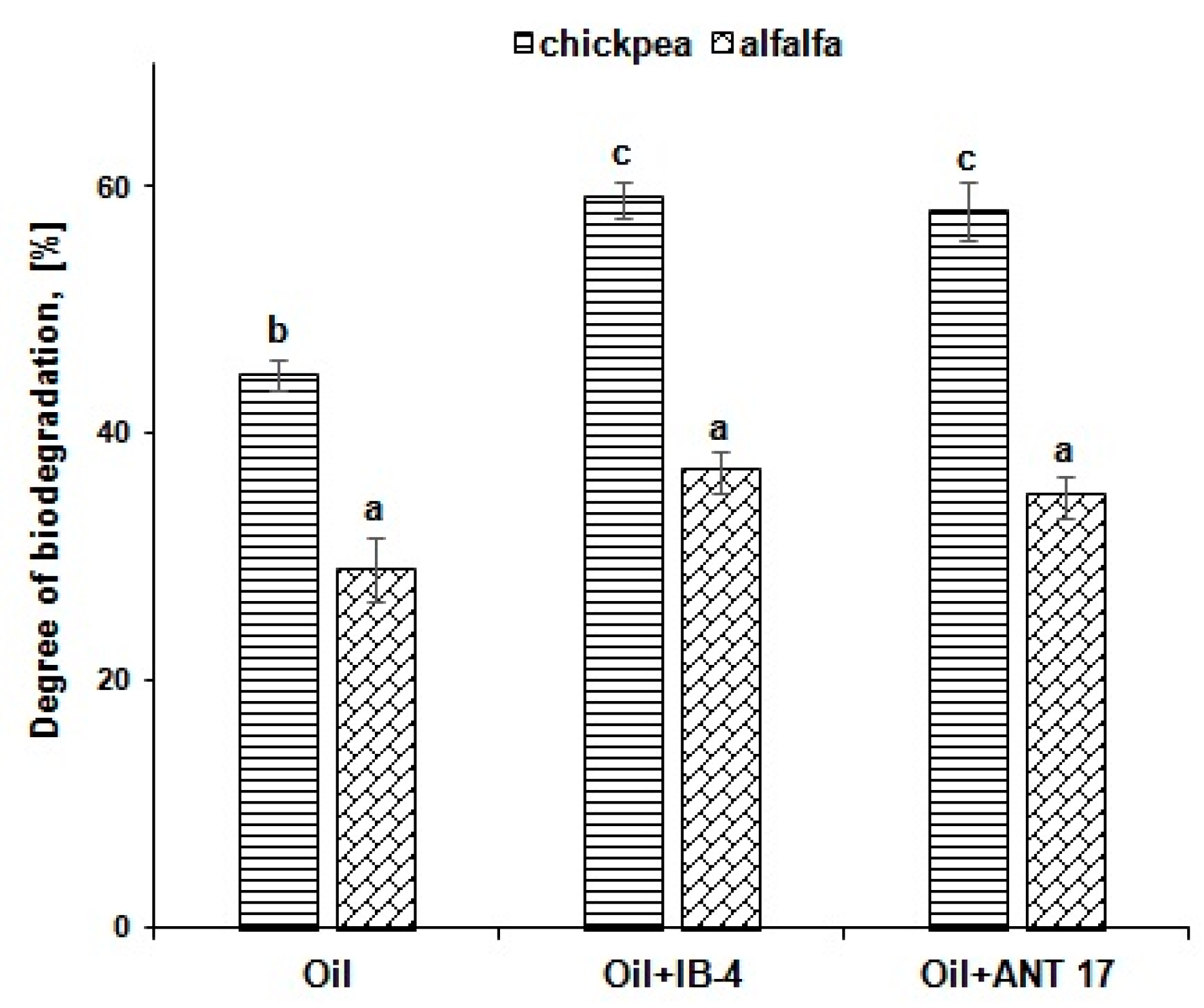
2.6. Biodegradation of Hydrocarbons
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions and Treatments
4.2. Cultivation of the Microorganisms and Analysis of Their Number
4.3. Analysis of the Content of Hydrocarbons in the Soil
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition-current knowledge and future directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed]
- Shameer, S.; Prasad, T. Plant growth promoting rhizobacteria for sustainable agricultural practices with special reference to biotic and abiotic stresses. Plant Growth Regul. 2018, 84, 603–615. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Bakaeva, M.D.; Chetverikov, S.P.; Korshunova, T.Y.; Loginov, O.N. The new bacterial strain Paenibacillus sp. IB-1: A producer of exopolysaccharide and biologically active substances with phytohormonal and antifungal activities. Appl. Biochem. Microbiol. 2017, 53, 201–208. [Google Scholar] [CrossRef]
- Majeed, A.; Abbasi, M.K.; Hameed, S.; Yasmin, S.; Hanif, M.K.; Naqqash, T.; Imran, A. Pseudomonas sp. AF-54 containing multiple plant beneficial traits acts as growth enhancer of Helianthus annuus L. under reduced fertilizer input. Microbiol. Res. 2018, 216, 56–69. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Möenne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Das, K.; Prasanna, R.; Saxena, A.K. Rhizobia: A potential biocontrol agent for soilborne fungal pathogens. Folia Microbiol. 2017, 62, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Hassen, W.; Neifar, M.; Cherif, H.; Najjari, A.; Chouchane, H.; Driouich, R.C.; Salah, A.; Naili, F.; Mosbah, A.; Souissi, Y.; et al. Pseudomonas rhizophila S211, a new plant growth-promoting rhizobacterium with potential in pesticide-bioremediation. Front. Microbiol. 2018, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Kalayu, G. Phosphate solubilizing microorganisms: Promising approach as biofertilizers. Int. J. Agron. 2019, 2019, 4917256. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Galimsyanova, N.F.; Kuzmina, L.Y.; Vysotskaya, L.B.; Sidorova, L.V.; Gabbasova, I.M.; Melentiev, A.; Kudoyarova, G. Effect of seed bacterization with plant growth-promoting bacteria on wheat productivity and phosphorus mobility in the rhizosphere. Plant Soil Environ. 2019, 65, 313–319. [Google Scholar] [CrossRef]
- Raheem, A.; Shaposhnikov, A.; Belimov, A.A.; Dodd, A.C.; Ali, B. Auxin production by rhizobacteria was associated with improved yield of wheat (Triticum aestivum L.) under drought stress. Arch. Agron. Soil Sci. 2018, 64, 574–587. [Google Scholar] [CrossRef]
- Großkinsky, D.K.; Tafner, R.; Moreno, M.V.; Stenglein, S.A.; García de Salamone, I.E.; Nelson, L.M.; Novák, O.; Strnad, M.; van der Graaff, E.; Roitsch, T. Cytokinin production by Pseudomonas fluorescens G20-18 determines biocontrol activity against Pseudomonas syringae in Arabidopsis. Sci. Rep. 2016, 6, 23310. [Google Scholar] [CrossRef] [PubMed]
- Trapet, P.; Avoscan, L.; Klinguer, A.; Pateyron, S.; Citerne, S.; Chervin, C.; Mazurier, S.; Lemanceau, P.; Wendehenne, D.; Besson-Bard, A. The Pseudomonas fluorescens siderophore pyoverdine weakens Arabidopsis thaliana defense in favor of growth in iron-deficient conditions. Plant Physiol. 2016, 171, 675–693. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kha, M.H.; Misra, S.; Dixit, K.V.; Khare, P.; Srivastava, S.; Chauhan, P.S. Characterisation of Pseudomonas spp. and Ochrobactrum sp. isolated from volcanic soil. Antonie Van Leeuwenhoek 2017, 110, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Asghar, H.N.; Zahir, Z.A.; Shahid, M. Impact of lead tolerant plant growth promoting rhizobacteria on growth, physiology, antioxidant activities, yield and lead content in sunflower in lead contaminated soil. Chemosphere 2018, 195, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Samaddar, S.; Anandham, R.; Kang, Y.; Kim, K.; Selvakumar, G.; Sa, T.; Chatterjee, P.; Samaddar, S.; Anandham, R.; et al. Beneficial soil bacterium Pseudomonas frederiksbergensis OS261 augments salt tolerance and promotes red pepper plant growth. Front. Plant Sci. 2017, 8, 705. [Google Scholar] [CrossRef] [PubMed]
- Saber, M.A.F.; Abdelhafez, A.A.; Hassan, E.A.; Ramadan, E.M. Characterization of fluorescent pseudomonads isolates and their efficiency on the growth promotion of tomato plant. Ann. Agric. Sci. 2015, 60, 131–140. [Google Scholar] [CrossRef]
- Kong, Z.; Deng, Z.; Glick, B.R.; Wei, G.; Chou, M. A nodule endophytic plant growth-promoting Pseudomonas and its effects on growth, nodulation and metal uptake in Medicago lupulina under copper stress. Ann. Microbiol. 2017, 67, 49–58. [Google Scholar] [CrossRef]
- Kim, Y.C.; Anderson, A.J. Rhizosphere pseudomonads as probiotics improving plant health. Mol. Plant Pathol. 2018, 19, 2349–2359. [Google Scholar] [CrossRef]
- Fang, K.; Bao, Z.-S.-N.; Chen, L.; Zhou, J.; Yang, Z.-P.; Dong, X.-F.; Zhang, H.-B. Growth-promoting characteristics of potential nitrogen-fixing bacteria in the root of an invasive plant Ageratina adenophora. PeerJ. 2019, 7, e7099. [Google Scholar] [CrossRef] [PubMed]
- Rafikova, G.F.; Korshunova, T.Y.; Minnebaev, L.F.; Chetverikov, S.P.; Loginov, O.N. A new bacterial strain, Pseudomonas koreensis IB-4, as a promising agent for plant pathogen biological control. Microbiology 2016, 85, 333–341. [Google Scholar] [CrossRef]
- Rafikova, G.F.; Kuzina, E.V.; Korshunova, T.Y.; Loginov, O.N. New bacterial strains of Pseudomonas laurentiana: Promising agents for agrobiotechnology. Mosc. Univ. Biol. Sci. Bull. 2020, 75, 206–211. [Google Scholar] [CrossRef]
- Stepanova, A.Y.; Gladkov, E.A.; Osipova, E.S.; Gladkova, O.V.; Tereshonok, D.V. Bioremediation of soil from petroleum contamination. Processes 2022, 10, 1224. [Google Scholar] [CrossRef]
- Lorestani, B.; Noori, R.; Kulahchi, N. Bioremediation of soil contaminated with light crude oil using Fabaceae family. J. Environ. Sci. Tech. 2016, 18, 101–108. [Google Scholar]
- Riskuwa-Shehu, M.L.; Ijah, U.J.J.; Manga, S.B.; Bilbis, L.S. Evaluation of the use of legumes for biodegradation of petroleum hydrocarbons in soil. Int. J. Environ. Sci. Technol. 2017, 14, 2205–2214. [Google Scholar] [CrossRef]
- Hall, J.; Soole, K.; Bentham, R. Hydrocarbon phytoremediation in the family Fabaceae—A review. Int. J. Phytoremed. 2011, 13, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Panchenko, L.; Muratova, A.; Turkovskaya, O. Comparison of the phytoremediation potentials of Medicago falcate L. and Medicago sativa L. in aged oil-sludge-contaminated soil. Environ. Sci. Pollut. Res. 2017, 24, 3117–3130. [Google Scholar] [CrossRef] [PubMed]
- Baghaie, A.H.; Jabari, A.G.; Sattari, R. The effect of corn and white clover intercropping on biodegradation of diesel oil in arsenic contaminated soil in the presence of Piriformospora indica. J. Hum. Environ. Health Promot. 2020, 6, 53–59. [Google Scholar] [CrossRef]
- Li, Y.; Ruperao, P.; Batley, J.; Edwards, D.; Khan, T.; Colmer, T.D.; Pang, J.; Siddique, K.H.M.; Sutton, T. Investigating drought tolerance in chickpea using genome-wide association mapping and genomic selection based on whole-genome resequencing data. Front. Plant Sci. 2018, 9, 190. [Google Scholar] [CrossRef]
- Rani, A.; Devi, P.; Jha, U.C.; Sharma, K.D.; Siddique, K.H.M.; Nayyar, H. Developing climate-resilient chickpea involving physiological and molecular approaches with a focus on temperature and drought stresses. Front. Plant Sci. 2020, 10, 1759. [Google Scholar] [CrossRef]
- Mishra, A.; Nautiyal, C.S. Functional diversity of the microbial community in the rhizosphere of chickpea grown in diesel fuel-spiked soil amended with Trichoderma ressei using sole-carbon-source utilization profiles. World J. Microbiol. Biotechnol. 2009, 25, 1175–1180. [Google Scholar] [CrossRef]
- Varazi, T.; Kurashvili, M.; Pruidze, M.; Khatisashvili, G.; Gagelidze, N.; Adamia, G.; Zaalishvili, G.; Gordeziani, M.; Sutton, M. A new approach and tools for perfecting phytoremediation technology. Am. J. Environ. Prot. 2015, 4, 143–147. [Google Scholar] [CrossRef]
- Bučková, M.; Puškarová, A.; Chovanová, K.; Kraková, L.; Ferianc, P.; Pangallo, D. A simple strategy for investigating the diversity and hydrocarbon degradation abilities of cultivable bacteria from contaminated soil. World J. Microbiol. Biotechnol. 2013, 29, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, Z.; Shokoohi, R.; Baharloie, J.; Alikhani, M.; Abdollahi, A.; Goodini, K. Biological removal of PAHs by bacteria from contaminated soils. Petrol. Sci. Technol. 2016, 34, 1406–1413. [Google Scholar] [CrossRef]
- Wright, M.H.; Hanna, J.G.; Pica, D.A.; Tebo, B.M. Pseudomonas laurentiana sp. nov., an Mn(III)-oxidizing bacterium isolated from the St. Lawrence Estuary. Pharm. Commun. 2018, 8, 153–157. [Google Scholar] [CrossRef]
- Benedek, T.; Szentgyörgyi, F.; Szabó, I.; Farkas, M.; Duran, R.; Kriszt, B.; Táncsics, A. Aerobic and oxygen-limited naphthalene-amended enrichments induced the dominance of Pseudomonas spp. from a groundwater bacterial biofilm. Appl. Microbiol. Biotechnol. 2020, 104, 6023–6043. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Arkhipova, T.N.; Melent’ev, A.I. Role of bacterial phytohormones in plant growth regulation and their development. In Bacterial Metabolites in Sustainable Agroecosystem; Springer: Cham, Switzerland, 2015; pp. 69–86. [Google Scholar] [CrossRef]
- Korshunova, T.Y.; Kuzina, E.V.; Rafikova, G.F.; Timergalin, M.D.; Rameev, T.V.; Chetverikova, D.V.; Feoktistova, A.V.; Nizaeva, A.A.; Chetverikov, S.P. Bacterization of forage grass seeds: Influence on spreading and growth of plants. Biomics 2021, 13, 159–165. [Google Scholar] [CrossRef]
- Kumari, S.; Khanna, V. Effect of antagonistic rhizobacteria coinoculated with Mesorhizobium ciceri on control of fusarium wilt in chickpea (Cicer arietinum L.). Afr. J. Microbiol. Res. 2014, 8, 1255–1265. [Google Scholar] [CrossRef]
- Ummara, U.; Noreen, S.; Afzal, M.; Zafar, Z.U.; Akhter, M.S.; Iqbal, S.; Hefft, D.I.; Kazi, M.; Ahmad, P. Induced systemic tolerance mediated by plant-microbe interaction in maize (Zea mays L.) plants under hydrocarbon contamination. Chemosphere 2022, 290, 133327. [Google Scholar] [CrossRef]
- Kazmierczak, T.; Nagymihály, M.; Lamouche, F.; Barriére, Q.; Guefrachi, I.; Alunni, B.; Ouadghiri, M.; Ibijbijen, J.; Kondorosi, É.; Mergaert, P.; et al. Specific host-responsive associations between Medicago truncatula accessions and Sinorhizobium strains. Mol. Plant-Microbe Interact. 2017, 30, 399–409. [Google Scholar] [CrossRef]
- Walker, L.; Lagunas, B.; Gifford, M.L. Determinants of host range specificity in legume-rhizobia symbiosis. Front. Microbiol. 2020, 11, 585749. [Google Scholar] [CrossRef] [PubMed]
- Cornara, L.; Xiao, J.; Burlando, B. Therapeutic potential of temperate forage legumes: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Plet, J.; Wasson, A.; Ariel, F.; Le Signor, C.; Baker, D.; Mathesius, U.; Crespi, M.; Frugier, F. MtCRE1-dependent cytokinin signaling integrates bacterial and plant cues to coordinate symbiotic nodule organogenesis in Medicago truncatula. Plant J. 2011, 65, 622–633. [Google Scholar] [CrossRef] [PubMed]
- van Noorden, G.E.; Ross, J.J.; Reid, J.B.; Rolfe, B.G.; Mathesius, U. Defective long-distance auxin transport regulation in the Medicago truncatula super numeric nodules mutant. Plant Physiol. 2006, 140, 1494–1506. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Xu, X.L. Competition and mutualism between roots and rhizosphere microorganisms by nitrogen acquisition and their ecological consequences. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, P.M.; Dickinson, S.J.; Arocena, J.M. Emergence, survival and growth of selected plant species in petroleum-impacted flare pit soils. Can. J. Soil Sci. 2005, 85, 139–148. [Google Scholar] [CrossRef]
- Minoui, S.; Minai-Tehrani, D.; Shahriari, M.H. Phytoremediation of crude oil-contaminated soil by Medicago sativa (Alfalfa) and the effect of oil on its growth. In Phytoremediation for Green Energy; Springer: Dordrecht, The Netherlands, 2015; pp. 123–129. [Google Scholar] [CrossRef]
- Polyak, Y.M.; Bakina, L.G.; Chugunova, M.V.; Mayachkina, N.V.; Gerasimov, A.O.; Bure, V.M. Effect of remediation strategies on biological activity of oil-contaminated soil—A field study. Int. Biodeterior. Biodegrad. 2018, 126, 57–68. [Google Scholar] [CrossRef]
- Devatha, C.P.; Vishnu, V.A.; Purna Chandra Rao, J. Investigation of physical and chemical characteristics on soil due to crude oil contamination and its remediation. Appl. Water Sci. 2019, 9, 89. [Google Scholar] [CrossRef]
- Sui, X.; Wang, X.; Li, Y.; Ji, H. Remediation of petroleum-contaminated soils with microbial and microbial combined methods: Advances, mechanisms, and challenges. Sustainability 2021, 13, 9267. [Google Scholar] [CrossRef]
- Balasubramaniyam, A.; Harvey, P.J. Scanning electron microscopic investigations of root structural modifications arising from growth in crude oil-contaminated sand. Environ. Sci. Pollut. Res. 2014, 21, 12651–12661. [Google Scholar] [CrossRef]
- Muthert, L.W.F.; Izzo, L.G.; Van Zanten, M.; Aronne, G. Root tropisms: Investigations on earth and in space to unravel plant growth direction. Front. Plant Sci. 2020, 10, 1807. [Google Scholar] [CrossRef] [PubMed]
- Vysotskaya, L.B.; Arkhipova, T.N.; Kuzina, E.V.; Rafikova, G.F.; Akhtyamova, Z.A.; Ivanov, R.S.; Timergalina, L.N.; Kudoyarova, G.R. Comparison of responses of different plant species to oil pollution. Biomics 2019, 11, 86–100. [Google Scholar] [CrossRef]
- Chetverikov, S.; Vysotskaya, L.; Kuzina, E.; Arkhipova, T.; Bakaeva, M.; Rafikova, G.; Korshunova, T.; Chetverikova, D.; Hkudaygulov, G.; Kudoyarova, G. Effects of association of barley plants with hydrocarbon-degrading bacteria on the content of soluble organic compounds in clean and oil-contaminated sand. Plants 2021, 10, 975. [Google Scholar] [CrossRef] [PubMed]
- Kuzina, E.; Rafikova, G.; Vysotskaya, L.; Arkhipova, T.; Bakaeva, M.; Chetverikova, D.; Kudoyarova, G.; Korshunova, T.; Chetverikov, S. Influence of hydrocarbon-oxidizing bacteria on the growth, biochemical characteristics, and hormonal status of barley plants and the content of petroleum hydrocarbons in the soil. Plants 2021, 10, 1745. [Google Scholar] [CrossRef] [PubMed]
- Agnello, A.C.; Bagard, M.; van Hullebusch, E.D.; Esposito, G.; Huguenot, D. Comparative bioremediation of heavy metals and petroleum hydrocarbons co-contaminated soil by natural attenuation, phytoremediation, bioaugmentation and bioaugmentation-assisted phytoremediation. Sci. Total Environ. 2016, 563-564, 693–703. [Google Scholar] [CrossRef]
- Sotnikova, Y.M.; Grigoriadi, A.S.; Fedyaev, V.V.; Novoselova, E.I.; Yamaleeva, A.A.; Gabidullina, G.F.; Farkhutdinov, R.G. Application of microbiological preparations and lucern plants for phytoremediating activities on oil contaminated soils. J. Agric. Environ. 2022, 5. [Google Scholar] [CrossRef]
- Chernysh, Y.; Ablieieva, I.; Makarekno, N.; Plyatsuk, L.; Trunova, I.; Burla, O. Investigation of the directions of using a hybrid composition bioproduct for detoxification of a soil ecosystem contaminated with heavy metals and oil products. Biodivers. Environ. 2021, 13, 80–94. [Google Scholar]
- Abdal-Satter, O.K.; Alobaidi, K.H.; Ibrahim, K.M. Enhancing the ability of three different plant seeds to germinate under elevated concentrations of oil sludge using three different bacterial isolates. Plant Arch. 2020, 20, 3977–3980. [Google Scholar]
- Gilan, R.S.; Parvizi, Y.; Pazira, E.; Rejali, F. Bioremediation of petroleum-contaminated soil in arid region using different arid -tolerant tree, shrub, and grass plant species with bacteria. Int. J. Environ. Sci. Technol. 2022, 19, 11879–11890. [Google Scholar] [CrossRef]
- Jambon, I.; Thijs, S.; Weyens, N.; Vangronsveld, J. Harnessing plant-bacteria-fungi interactions to improve plant growth and degradation of organic pollutants. J. Plant Interact. 2018, 13, 119–130. [Google Scholar] [CrossRef]
- Shtark, O.Y.; Borisov, A.Y.; Zhukov, V.A.; Nemankin, T.A.; Tikhonovich, I.A. Multicomponent symbiosis of legumes with beneficial soil microorganisms: Genetic and evolutionary bases of application in sustainable crop production. Russ. J. Genet. Appl. Res. 2012, 2, 177–189. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Pan, D.; Ge, X.; Jin, X.; Chen, S.; Wu, F. p-Coumaric can alter the composition of cucumber rhizosphere microbial communities and induce negative plant-microbial interactions. Biol. Fertil. Soils 2018, 54, 363–372. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Terenzi, V. Rhizosphere microbial communities and heavy metals. Microorganisms 2021, 9, 1462. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Plaza, P.; Navas, M.J.; Wybraniec, S.; Michałowski, T.; Asuero, A.G. An overview of the Kjeldahl method of nitrogen determination. Part II. Sample preparation, working scale, instrumental finish, and quality control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar] [PubMed]
- Raymond, R.L. Microbial oxidation of n-paraffinic hydrocarbons. Dev. Ind. Microbiol. 1961, 2, 23–32. [Google Scholar]
- Zvyagintsev, D.G. (Ed.) Methods in Soil Microbiology and Biochemistry; MGU Publishing: Moscow, Russia, 1991; p. 304. [Google Scholar]

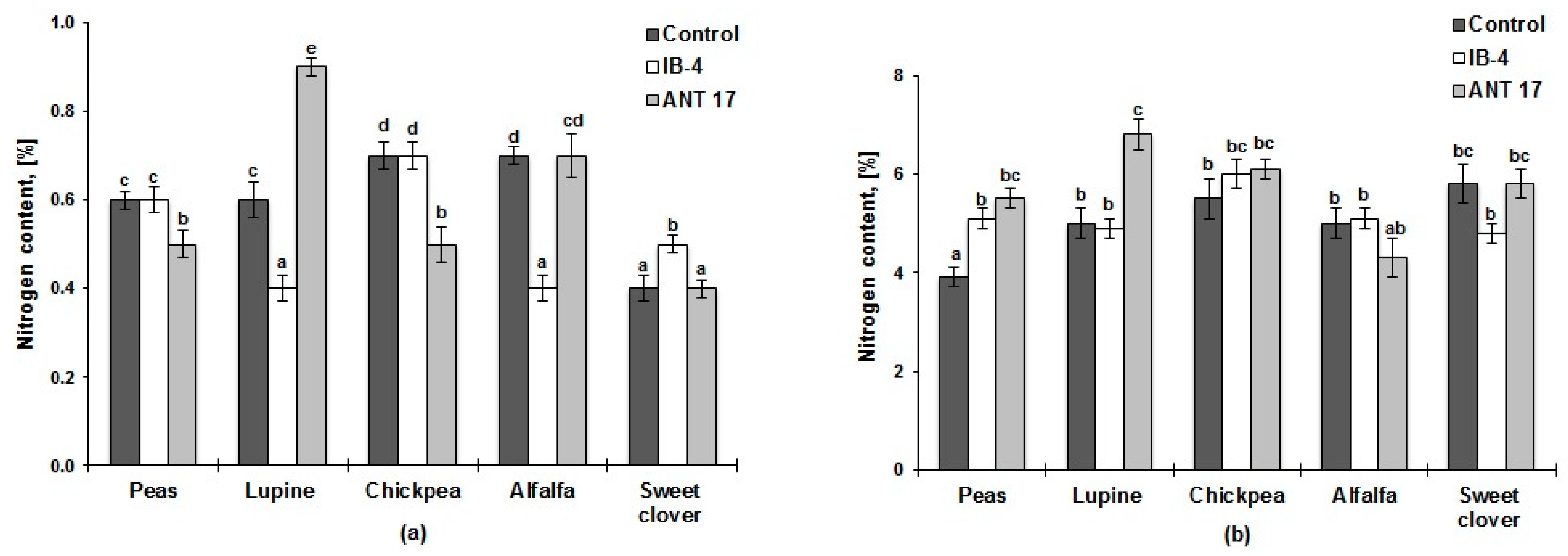

| Variants of Experiments | Germination (%) | Shoot Length (cm) | Length of the Main Root (cm) | Number of Leaves (pcs) | Number of Nodules (pcs Plant−1) | Shoot Length (cm) | Length of the Main Root (cm) | Number of Leaves (pcs) | Number of Nodules (pcs Plant−1) |
|---|---|---|---|---|---|---|---|---|---|
| 21 Days | 42 Days | ||||||||
| Peas | |||||||||
| Control | 93.3 ± 4.6 a | 32.3 ± 1.8 a | 11.0 ± 0.3 ab | 6.3 ± 0.2 a | 2.1 ± 0.1 a | 73.7 ± 2.9 a | 16.2 ± 0.9 a | 12.7 ± 0.5 a | 9.0 ± 0.3 a |
| IB-4 | 96.7 ± 3.7 ab | 38.0 ± 1.0 b | 10.8 ± 0.3 a | 6.7 ± 0.3 a | 1.8 ± 0.1 a | 85.5 ± 4.3 b | 18.2 ± 1.2 a | 15.4 ± 0.5 b | 20.4 ± 1.2 b |
| ANT 17 | 96.7 ± 3.7 ab | 36.8 ± 0.9 b | 12.4 ± 0.2 b | 5.9 ± 0.1 a | 12.0 ± 0.4 b | 78.7 ± 3.5 ab | 17.9 ± 1.0 a | 15.0 ± 0.6 b | 20.8 ± 1.2 b |
| Lupine | |||||||||
| Control | 90.0 ± 4.9 a | 14.7 ± 0.6 a | 8.3 ± 0.3 a | 4.0 ± 0.1 a | 0.3 ± 0.1 a | 29.1 ± 1.3 a | 14.5 ± 0.8 a | 10.5 ± 0.4 a | 10.2 ± 0.4 a |
| IB-4 | 93.3 ± 4.6 a | 18.5 ± 0.8 b | 8.8 ± 0.2 ab | 5.0 ± 0.1 a | - * | 34.3 ± 1.5 a | 14.6 ± 0.7 a | 11.7 ± 0.5 ab | 8.3 ± 0.4 a |
| ANT 17 | 90.0 ± 4.9 a | 18.8 ± 0.7 b | 10.2 ± 0.4 b | 5.0 ± 0.2 a | 0.5 ± 0.2 a | 35.3 ± 1.7 a | 18.9 ± 1.4 b | 12.8 ± 0.5 b | 16.1 ± 0.6 b |
| Chickpea | |||||||||
| Control | 86.7 ± 4.6 a | 28.5 ± 1.1 ab | 12.7 ± 0.5 a | 9.4 ± 0.4 a | - | 38.1 ± 2.1 a | 17.0 ± 1.1 a | 16.0 ± 0.7 ab | - |
| IB-4 | 90.0 ± 4.9 a | 27.0 ± 0.9 a | 15.3 ± 0.7 b | 9.7 ± 0.3 a | - | 44.0 ± 3.2 a | 22.5 ± 1.2 b | 14.7 ± 0.6 a | - |
| ANT 17 | 90.0 ± 4.9 a | 31.1 ± 1.3 b | 14.0 ± 0.5 ab | 10.6 ± 0.5 a | - | 45.2 ± 2.1 a | 22.7 ± 1.0 b | 17.4 ± 0.8 b | - |
| Alfalfa | |||||||||
| Control | 71.7 ± 1.8 a | 9.3 ± 0.3 a | 5.2 ± 0.1 a | 3.1 ± 0.1 a | 1.9 ± 0.1 a | 18.9 ± 0.9 a | 15.0 ± 0.6 a | 7.2 ± 0.2 a | 7.2 ± 0.2 a |
| IB-4 | 86.7 ± 2.3 b | 9.5 ± 0.5 a | 5.9 ± 0.2 a | 3.3 ± 0.1 a | 3.8 ± 0.2 b | 17.8 ± 0.8 a | 14.2 ± 0.6 a | 8.1 ± 0.3 a | 11.1 ± 0.6 b |
| ANT 17 | 81.7 ± 1.8 b | 8.5 ± 0.3 a | 4.8 ± 0.1 a | 3.0 ± 0.1 a | 3.8 ± 0.2 b | 18.4 ± 0.9 a | 14.5 ± 0.5 a | 8.1 ± 0.2 a | 10.4 ± 0.6 b |
| Sweet clover | |||||||||
| Control | 83.3 ± 2.3 a | 7.2 ± 0.2 ab | 5.0 ± 0.2 a | 2.9 ± 0.1 a | 1.5 ± 0.1 a | 14.0 ± 0.6 a | 14.3 ± 0.7 ab | 5.6 ± 0.2 a | 12.5 ± 0.6 a |
| IB-4 | 90.0 ± 2.8 b | 8.3 ± 0.2 b | 5.6 ± 0.2 a | 2.7 ± 0.2 a | 3.0 ± 0.1 b | 14.0 ± 0.8 a | 12.3 ± 0.5 a | 6.0 ± 0.3 a | 13.6 ± 0.6 a |
| ANT 17 | 86.7 ± 3.7 ab | 6.8 ± 0.3 a | 4.9 ± 0.2 a | 3.4 ± 0.2 a | 2.3 ± 0.1 ab | 16.5 ± 0.8 a | 15.0 ± 0.7 b | 7.2 ± 0.3 a | 16.6 ± 0.8 b |
| Variants of Experiments | Peas | Lupine | Chickpea | Alfalfa | Sweet Clover | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 21 Days | 42 Days | 21 Days | 42 Days | 21 Days | 42 Days | 21 Days | 42 Days | 21 Days | 42 Days | |
| Heterotrophic microorganisms, ×107 | ||||||||||
| Control | (5.4 ± 0.3) a | (4.4 ± 0.2) b | (3.5 ± 0.2) a | (5.8 ± 0.3) b | (5.3 ± 0.3) b | (3.9 ± 0.2) c | (2.7 ± 0.1) b | (0.9 ± 0.1) a | (1.0 ± 0.2) a | (1.9 ± 0.1) a |
| IB-4 | (4.3 ± 0.2) a | (1.7 ± 0.1) a | (4.3 ± 0.2) a | (3.1 ± 1.2) ab | (3.3 ± 0.2) a | (1.3 ± 0.1) b | (0.6 ± 0.1) a | (2.0 ± 0.1) a | (0.5 ± 0.1) a | (3.1 ± 0.2) a |
| ANT 17 | (5.4 ± 0.2) a | (6.4 ± 0.3) c | (3.2 ± 0.1) a | (1.9 ± 0.1) a | (4.2 ± 0.2) ab | (0.7 ± 0.1) a | (0.7 ± 0.1) a | (2.0 ± 0.1) a | (1.0 ± 0.1) a | (2.9 ± 0.2) a |
| Oligonitrophilic microorganisms, ×107 | ||||||||||
| Control | (1.2 ± 0.1) a | (2.0 ± 0.1) a | (1.6 ± 0.1) a | (1.2 ± 0.1) a | (2.8 ± 0.1) a | (1.1 ± 0.1) a | (1.2 ± 0.1) a | (1.7 ± 0.1) a | (0.8 ± 0.1) a | (1.3 ± 0.1) a |
| IB-4 | (1.6 ± 0.1) a | (1.5 ± 0.1) a | (2.0 ± 0.1) a | (0.8 ± 0.1) a | (3.0 ± 0.2) a | (1.2 ± 0.1) a | (0.5 ± 0.1) a | (1.7 ± 0.1) a | (0.9 ± 0.2) a | (2.5 ± 0.2) a |
| ANT 17 | (1.1 ± 0.1) a | (1.5 ± 0.1) a | (1.7 ± 0.1) a | (2.1 ± 0.1) a | (2.6 ± 0.1) a | (0.8 ± 0.1) a | (0.9 ± 0.1) a | (1.8 ± 0.1) a | (0.8 ± 0.1) a | (1.5 ± 0.1) a |
| Micromycetes, ×104 | ||||||||||
| Control | (8.6 ± 0.5) c | (4.2 ± 0.1) b | (35.0 ± 1.8) b | (3.4 ± 0.2) b | (13.3 ± 0.7) c | (1.4 ± 0.1) a | (11.3 ± 0.7) c | (2.5 ± 0.1) a | (9.8 ± 0.5) c | (2.9 ± 0.2) ab |
| IB-4 | (3.2 ± 0.1) a | (2.5 ± 0.1) a | (2.0 ± 0.1) a | (2.6 ± 0.1) ab | (2.1 ± 0.1) a | (1.8 ± 0.1) a | (0.8 ± 0.1) a | (2.3 ± 0.1) a | (0.5 ± 0.1) a | (3.9 ± 0.2) b |
| ANT 17 | (4.7 ± 0.2) b | (5.0 ± 0.3) b | (2.8 ± 0.1) a | (1.1 ± 0.1) a | (4.9 ± 0.3) b | (1.4 ± 0.1) a | (2.3 ± 0.2) b | (3.6 ± 0.2) a | (1.6 ± 0.1) b | (2.2 ± 0.1) a |
| Variants of Experiments | Dry Mass of Shoots (mg) | Dry Mass of Roots (mg) | Root Mass/ Shoot Mass | Number of Nodules (pcs Plant−1) |
|---|---|---|---|---|
| Chickpea | ||||
| Control | 54.3 ± 2.3 a | 28.5 ± 2.1 a | 0.52 a | -* |
| Oil | 56.6 ± 1.8 a | 33.3 ± 3.3 ab | 0.59 ab | - |
| Oil + IB-4 | 66.8 ± 2.5 b | 33.7 ± 4.5 ab | 0.50 a | - |
| Oil + ANT 17 | 62.3 ± 2.6 b | 38.6 ± 2.6 b | 0.62 b | - |
| Alfalfa | ||||
| Control | 9.75 ± 0.86 c | 1.18 ± 0.21 a | 0.12 a | 6.7 ± 0.2 b |
| Oil | 2.41 ± 0.19 a | 1.79 ± 0.14 a | 0.74 c | 2.8 ± 0.2 a |
| Oil + IB-4 | 4.09 ± 0.29 b | 2.24 ± 0.19 a | 0.55 b | 3.7 ± 0.4 a |
| Oil + ANT 17 | 3.56 ± 0.47 ab | 1.80 ± 0.06 a | 0.51 b | 3.7 ± 0.4 a |
| Variants of Experiments | Heterotrophic Microorganisms, ×107 | Oligonitrophilic Microorganisms, ×106 | Micromycetes, ×104 | Petroleum-Degrading Microorganisms, ×105 |
|---|---|---|---|---|
| Chickpea | ||||
| Control | (3.4 ± 0.7) a | (8.0 ± 0.6) b | (5.3 ± 0.8) bc | (4.4 ± 0.9) a |
| Oil | (8.5 ± 0.7) b | (0.8 ± 0.3) a | (1.6 ± 0.5) a | (18.9 ± 2.3) b |
| Oil + IB-4 | (15.2 ± 0.9) c | (1.3 ± 0.4) a | (4.5 ± 0.8) ab | (16.3 ± 1.9) b |
| Oil + ANT 17 | (20.4 ± 1.5) d | (1.8 ± 0.4) a | (7.7 ± 0.8) c | (20.8 ± 1.6) b |
| Alfalfa | ||||
| Control | (1.1 ± 0.1) a | (3.7 ± 0.3) b | (3.1 ± 0.2) b | (1.3 ± 0.1) a |
| Oil | (2.4 ± 0.1) ab | (0.4 ± 0.1) a | (1.1 ± 0.1) a | (5.0 ± 0.4) b |
| Oil + IB-4 | (6.5 ± 0.3) c | (0.7 ± 0.1) a | (3.5 ± 0.3) b | (5.3 ± 0.3) b |
| Oil + ANT 17 | (4.6 ± 0.3) b | (0.9 ± 0.1) a | (3.5 ± 0.2) b | (4.9 ± 0.3) b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzina, E.; Mukhamatdyarova, S.; Sharipova, Y.; Makhmutov, A.; Belan, L.; Korshunova, T. Influence of Bacteria of the Genus Pseudomonas on Leguminous Plants and Their Joint Application for Bioremediation of Oil Contaminated Soils. Plants 2022, 11, 3396. https://doi.org/10.3390/plants11233396
Kuzina E, Mukhamatdyarova S, Sharipova Y, Makhmutov A, Belan L, Korshunova T. Influence of Bacteria of the Genus Pseudomonas on Leguminous Plants and Their Joint Application for Bioremediation of Oil Contaminated Soils. Plants. 2022; 11(23):3396. https://doi.org/10.3390/plants11233396
Chicago/Turabian StyleKuzina, Elena, Svetlana Mukhamatdyarova, Yuliyana Sharipova, Ainur Makhmutov, Larisa Belan, and Tatyana Korshunova. 2022. "Influence of Bacteria of the Genus Pseudomonas on Leguminous Plants and Their Joint Application for Bioremediation of Oil Contaminated Soils" Plants 11, no. 23: 3396. https://doi.org/10.3390/plants11233396
APA StyleKuzina, E., Mukhamatdyarova, S., Sharipova, Y., Makhmutov, A., Belan, L., & Korshunova, T. (2022). Influence of Bacteria of the Genus Pseudomonas on Leguminous Plants and Their Joint Application for Bioremediation of Oil Contaminated Soils. Plants, 11(23), 3396. https://doi.org/10.3390/plants11233396






