Cytotoxic Activities and Fingerprint Analysis of Triterpenes by HPTLC Technique for Distinguishing Ganoderma Species from Vietnam and other Asian Countries
Abstract
1. Introduction
2. Results
2.1. Selection of Extraction Method
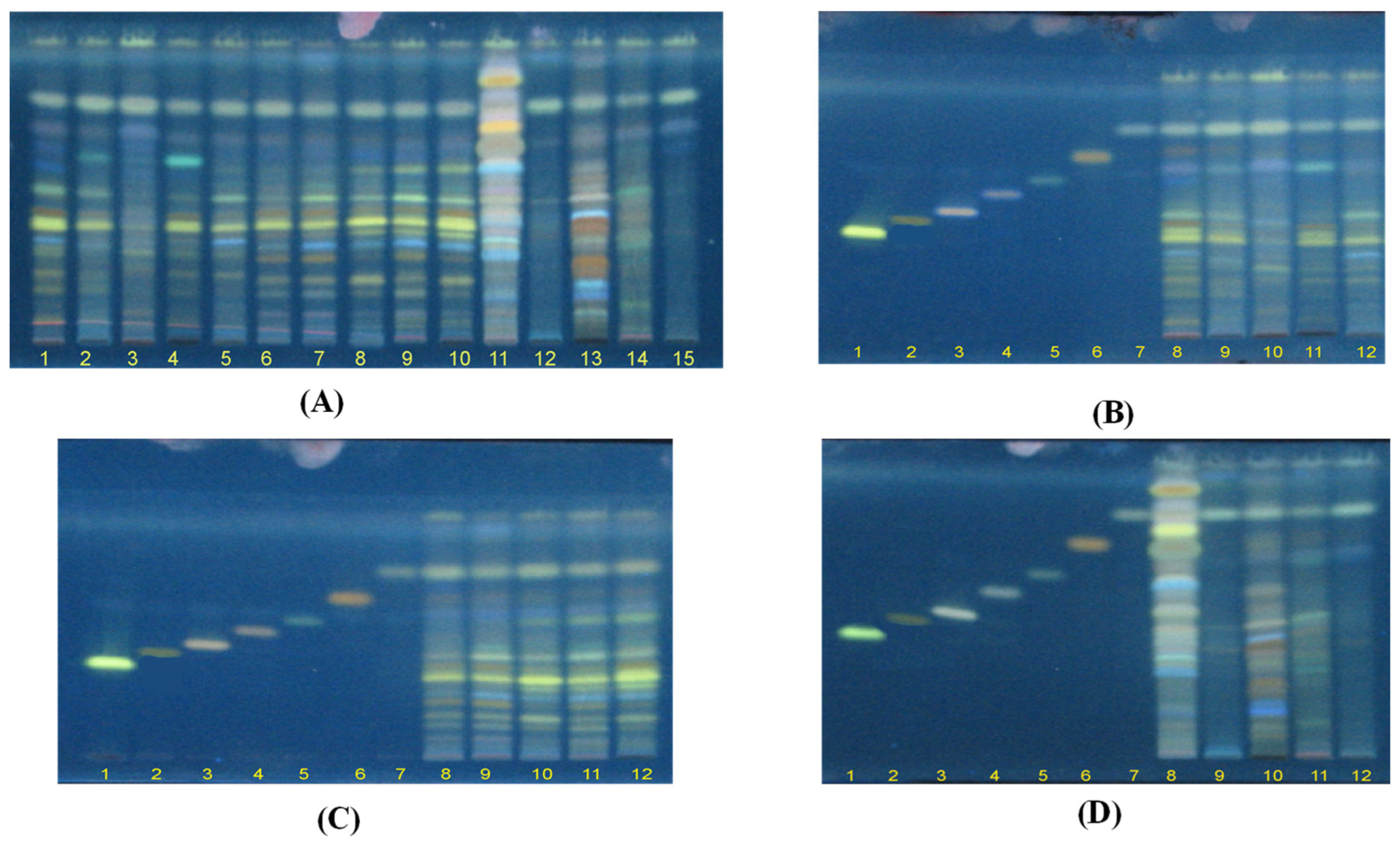
2.2. HPTLC Analysis
2.3. Fingerprint Profile of Different Ganoderma Species Extracts
2.4. In Vitro Cytotoxic Activity
3. Discussion
4. Materials and Methods
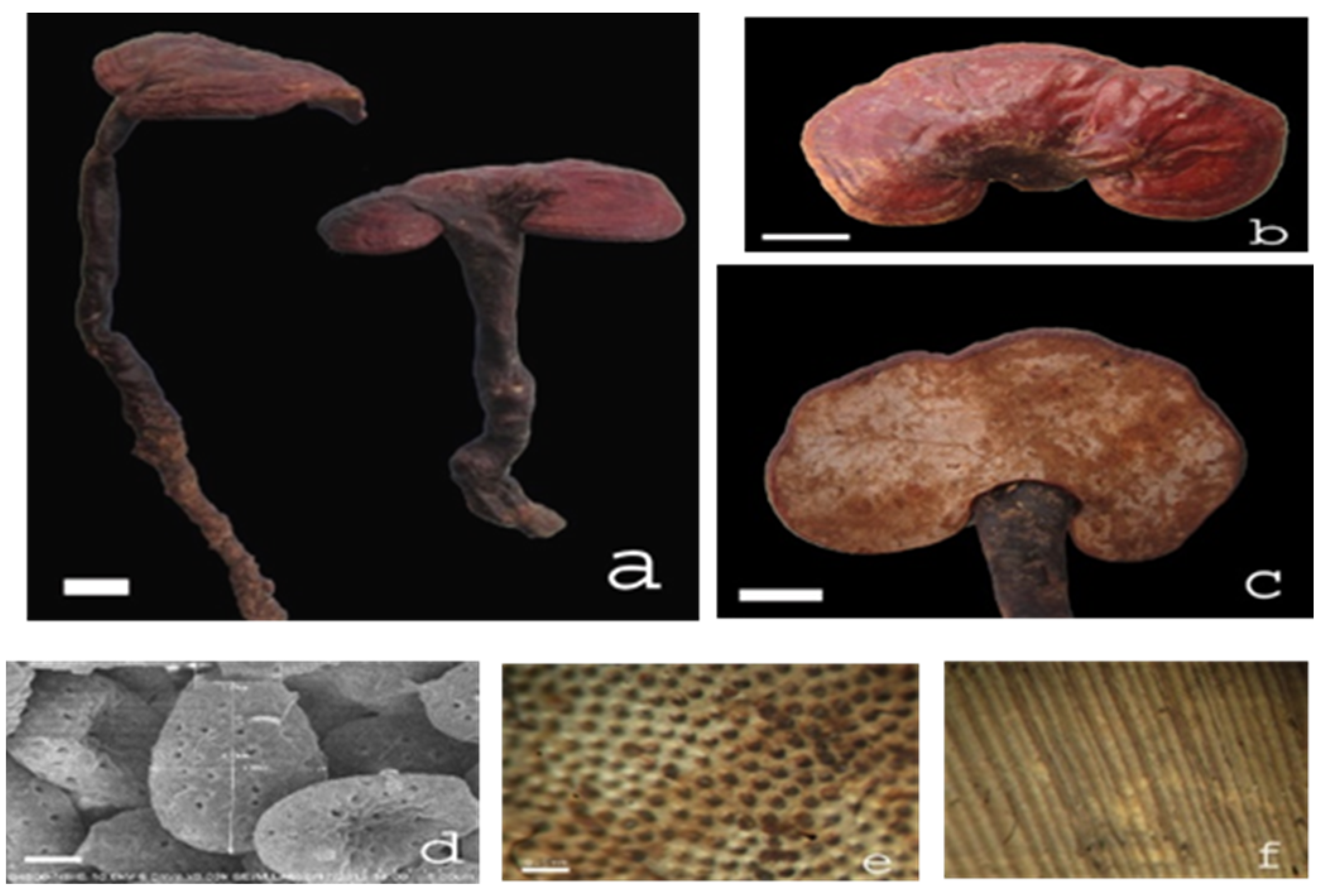
4.1. Plant Materials
4.2. Cell Lines, Chemicals, and Reagents
4.3. Instrumentations
4.4. Extract Preparations
4.4.1. Preparation of Extracts for Cytotoxic Test
4.4.2. Preparation of Sample Solution for HPTLC Analysis
4.4.3. Extraction and Isolation Marker Compounds
4.4.4. Preparation of Standard Compound Solution
4.5. MTT Assay
4.6. Chromatography
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selvi, S.; Polat, R.; Çakilcioğlu, U.; Celep, F.; Dirmenci, T.; Ertuğ, Z.F. An ethnobotanical review on medicinal plants of the Lamiaceae family in Turkey. Turk. J. Bot. 2022, 46, 283–332. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.F.; Aktumsek, A.; Jekő, J.; Cziáky, Z.; Rodrigues, M.J.; Custodio, L.; Polat, R.; Cakilcioglu, U.; Ayna, A.; et al. Chemical Profiling and Biological Evaluation of Nepeta baytopii Extracts and Essential Oil: An Endemic Plant from Turkey. Plants 2021, 10, 1176. [Google Scholar] [CrossRef] [PubMed]
- Long, S.C.; Birmingham, J.M. Medicinal benefits of the Mushroom Ganoderma. Adv. Appl. Microbiol. 1992, 37, 101–134. [Google Scholar]
- Boh, B.; Berovic, M.; Zhang, J.; Zhi-Bin, L. Ganoderma Lucidum and Its Pharmaceutically Active Compounds, 1st ed.; Biotechnology Annual Review; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Paterson, R.R.M. Ganoderma-A therapeutic fungal biofactory: A Review. Phytochemistry 2006, 87, 1985–2001. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, B.K. Biomedicinal triterpenoids of Ganoderma lucidum (Curt.: Fr.) P. Karst (Aphyllophoromycetidae). Int. J. Med. Mushrooms 1999, 1, 121–138. [Google Scholar] [CrossRef]
- Kobuta, T.; Asaka, Y.; Miura, I.; Mori, H. Structure of Ganoderic acid A and, B., two new lanostane type bitter triterpenes from Ganoderma lucidum (FR.) KARST. Helv. Chim. Acta 1982, 65, 611–619. [Google Scholar] [CrossRef]
- Kodora, Y.; Shimizu, M.; Sonoda, Y.; Sato, Y. Ganoderic acid and its derivatives as cholesterol synthesis inhibitors. Chem. Pharm. Bull. 1990, 38, 1359–1364. [Google Scholar]
- Lin, Z.B.; Zhang, H.N. Anti-tumor and immunoregulatory activities of Ganoderma lucidum and its possible mechanisms. Acta Pharmacol. Sin. 2004, 25, 1387–1395. [Google Scholar] [PubMed]
- Yuen, J.W.; Gohel, M.D. Anticancer effects of Ganoderma lucidum: A review of scientific evidence. Nutr. Cancer 2005, 53, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.W.; Man, R.Y.; Siow, Y.L.; Choy, P.C.; Wan, E.W.; Lau, C.S. Ganoderma lucidum inhibits inducible nitric oxide synthase expression in macrophages. Mol. Cell. Biochem. 2005, 275, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Rhee, H.M. Cardiovascular effects of mycelium extract of Ganoderma lucidum: Inhibition of sympathetic outflow as a mechanism of its hypotensive action. Chem. Pharm. Bull. 1990, 38, 1359–1364. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, J.; He, H.; Guo, H.; Zhang, S. Hepatoprotective effects of Ganoderma lucidum peptides against D-galactosamine-induced liver injury in mice. J. Ethnopharmacol. 2008, 117, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Huang, M.; Lin, Z.-B.; Zhou, S. Hepatoprotective activity and the mechanisms of action of Ganoderma lucidum (Curt.:Fr.) P. Karst. (Ling Zhi, Reishi Mushroom) (Aphyllophoromycetideae). Int. J. Med. Mushrooms 2003, 5, 113–133. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Nguyen, M.K.; Phan, N.T.; Duong, M.T.; Tran, V.H.; Do, T.H. Lanostane Triterpenoids from Ganoderma Tropicum Collected in Vietnam and Their Nitroblue Tetrazolium Reductive Activity In Vitro. Nat. Prod. Sci. 2020, 26, 334–339. [Google Scholar] [CrossRef]
- Admad, S. Oxidative Stress and Anti-Oxidant Defense in Biology; Chapman and Hall: New York, NY, USA, 1995. [Google Scholar]
- Kimura, S.; Tamura, T.J. Dietary effects of Ganoderma lucidum mushroom on blood pressure and lipid levels in spontaneously hypotensive rats (SHR). J. Nutr. Sci. Vitaminol. 1998, 34, 433–438. [Google Scholar]
- Kim, D.H.; Shim, S.B.; Kim, N.J.; Jang, J.S. Beta-glucuronidase inhibitory activity and hepatoprotective effect of Ganoderma lucidum. Biol. Pharm. Bull. 1999, 22, 162–164. [Google Scholar] [CrossRef]
- Su, C.Y.; Shiao, M.S.; Wang, C.T. Differential effects of ganodermic acid S on the bromthoxane A2-signaling pathways in human platelets. Biochem. Pharmacol. 1999, 58, 587–595. [Google Scholar] [CrossRef]
- Wang, X.M.; Yang, M.; Guan, S.H.; Liu, R.X.; Xia, J.M.; Bi, K.S.; Guo, D.A. Quantitative determination of six major triterpenoids in Ganoderma lucidum and related species by high performance liquid chromatography. J. Pharm. Biomed. Anal. 2006, 41, 838–844. [Google Scholar] [CrossRef]
- The Pharmacopoeia Commission of the Ministry of Health. In Pharmacopoeia of the People’s Republic of China; The Pharmacopoeia Commission: Beijing, China, 2005.
- Joseph, S.; Sabulal, B.; George, V.; Smina, T.P.; Janardhanan, K.K. Antioxidative and anti-inflammatory activities of the chloroform extract of Ganoderma lucidum found in south India. Sci. Pharm. 2009, 77, 111–121. [Google Scholar] [CrossRef]
- Salteralli, R.; Ceccaroli, P.; Lotti, M.; Zambonelli, A.; Buffalini, M.; Casadei, L. Biochemical characterization and antioxidant activity of mycelium of Ganoderma lucidum from central Italy. Food Chem. 2009, 116, 143–151. [Google Scholar] [CrossRef]
- Keypour, S.; Rafati, H.; Riahi, H.; Mirzajani, F.; Moradali, M.F. Qualitative analysis of ganoderic acids in Ganoderma lucidum from Iran and China by RP-HPLC and electrospray ionisation-mass spectrometry (ESI-MS). Food Chem. 2010, 119, 1704–1708. [Google Scholar] [CrossRef]
- Moradali, M.F.; Hedjaroude, G.H.A.; Mostafavi, H.; Abbasi, M.; Ghods, S.H.; Tehrani, A.S. The genus Ganoderma (Basidiomycota) in Iran. Mycotaxon 2007, 99, 251–269. [Google Scholar]
- Nguyen, P.D.N.; Do, H.T.; Le, B.D. Characteristics of ecological factors and their distribution of Ganodermataceae Donk. in Highlands of Vietnam. J. Biol. 2013, 35, 198–205. [Google Scholar]
- Dam, N.; Nguyen, G.C.; Nguyen, B.; Trinh, T.K. Ganoderma species in Vietnam. J. Med. Mater. -Hanoi 1997, 2, 10–13. [Google Scholar]
- Do, T.L. Medicinals and Herbs in Vietnam; Medical Publishing House: Hanoi, Vietnam, 2004; pp. 831–833. [Google Scholar]
- Ha, D.T.; Loan, L.T.; Hung, T.M.; Han, L.V.; Khoi, N.M.; Dung, L.V.; Min, B.S.; Nguyen, N.P. An Improved HPLC-DAD Method for Quantitative Comparisons of Triterpenes in Ganoderma Lucidum and Its Five Related Species Originating from Vietnam. Molecules 2015, 20, 1059–1077. [Google Scholar] [CrossRef]
- Srivastava, M.M.E. High-Performance Thin-Layer Chromatography (HPTLC), 1st ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Koll, K.; Reich, E.; Blatter, A.; Veit, M. Validation of Standardized High-Performance Thin-Layer Chromatographic Methods for Quality Control and Stability Testing of Herbals. J. AOAC Int. 2003, 86, 909–915. [Google Scholar] [CrossRef]
- Dušanka, M.-O.; Petar, R.; Filip, A.; Jelena, T. Planar Chromatographic Systems in Pattern Recognition and Fingerprint Analysis. Chromatographia 2013, 76, 1239–1247. [Google Scholar]
- Boik, J. Natural Compounds in Cancer Therapy: Promising Nontoxic Antitumor Agents From Plants & Other Natural Sources; Oregon Medical Press: Princeton, MN, USA, 2001. [Google Scholar]
- Jing, Y.; Yiwen, C.; Nga-I, L.; Jing, Z.; Jin-Ao, D.; Yu-Ping, T.; Shao-Ping, L. Quality evaluation of different products derived from Ganoderma. J. Med. Plants Res. 2012, 6, 1969–1974. [Google Scholar]
- Gao, J.L.; Leung, K.S.; Wang, Y.T.; Lai, C.M.; Li, S.P.; Hu, L.F.; Lu, G.H.; Jiang, Z.H.; Yu, Z.L. Qualitative and quantitative analyses of nucleosides and nucleobases in Ganoderma spp. by HPLC-DAD-MS. J. Pharm. Biomed. Anal. 2007, 44, 807–811. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ishizuka, T.; Yaoita, Y.; Kikuchi, M. Sterol constituents from the fruit bodies of Grifola frondosa (FR) S.F.Gray. Chem. Pharm. Bull. 1997, 45, 1756–1760. [Google Scholar] [CrossRef]
- Arisawa, M.; Fujita, A.; Hayashi, T.; Shimizu, M.; Morita, N.; Kikuchi, T.; Kadota, S.; Tezuka, Y. Revision of 1H- and 13C-nmr assignments of lanostanoids from Ganoderma lucidum by 2D-NMR studies. J. Nat. Prod. 1998, 51, 54–59. [Google Scholar] [CrossRef]
- Nishitoba, T.; Oda, K.; Sato, H.; Sakamura, S. Novel triterpenoids from the fungus Ganoderma lucidum (Organic Chemistry). Agric. Biol. Chem. 1988, 52, 367–372. [Google Scholar]
- Lin, C.N.; Tome, W.P.; Won, S.J. A lanostanoid of formosan Ganoderma lucidum. Phytochemistry 1990, 29, 673–675. [Google Scholar] [CrossRef]
- Tang, W.; Gu, T.; Zhong, J.J. Separation of targeted ganoderic acids from Ganoderma lucidum by reversed phase liquid chromatography with ultraviolet and mass spectrometry detections. Biochem. Eng. J. 2006, 32, 205–210. [Google Scholar] [CrossRef]
- Wu, T.S.; Shi, L.S.; Kuo, S.C. Cytotoxicity of Ganoderma lucidum triterpenes. J. Nat. Prod. 2001, 64, 1121–1122. [Google Scholar] [CrossRef] [PubMed]
- “ICH Q2(R2) Validation of Analytical Procedures—Scientific Guideline | European Medicines Agency.” n.d. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 11 November 2022).



| No. | Scientific Name | Origin | Yield (%) |
|---|---|---|---|
| G1 | G. lucidum | Cultivated by Linh chi Vina Company, Vietnam, Japanese Linhzhi strain. | 4.05 ± 0.21 |
| G2 | G. lucidum | Cultivated by Linh chi Vina Company, Vietnam, Chinese Linhzhi strain. | 4.22 ± 0.15 |
| G3 | G. lucidum | Cultivated by Linh chi Vina Company, Vietnam, Vietnamese Linhzhi strain. | 3.61 ± 0.17 |
| G4 | G. lucidum | Cultivated by Longevity Linhzhi Farm, Chungnam Province, Korea, Korean Linhzhi strain. | 3.58 ± 0.23 |
| G5 | G. lucidum | Cultivated by Vietnam Academy of Agricultural Sciences. | 3.98 ± 0.18 |
| G6 | G. lucidum | Wild collected in Quang Nam-Da Nang, Vietnam, supported by Quang Nam Department of Health 2010. | 4.09 ± 0.20 |
| G7 | G. lucidum | Wild collected in Quang Nam-Da Nang, Vietnam (grown in the dead wood of Erythrophleum fordii) | 4.21 ± 0.32 |
| G8 | G. lucidum | 4.05 ± 0.46 | |
| G9 | G. lucidum | 4.15 ± 0.36 | |
| G10 | G. lucidum | 4.18 ± 0.29 | |
| G11 | G. applanatum | Cultivated by Linh chi Vina Company, Vietnam. | 3.79 ± 0.22 |
| G12 | G. clossum | Cultivated by Linh chi Vina Company, Vietnam. | 3.65 ± 0.34 |
| G13 | G. subresinosum | Cultivated by Linh chi Vina Company, Vietnam. | 4.15 ± 0.37 |
| G14 | Ganoderma sp. | Cultivated by Linh chi Vina Company, Vietnam. | 4.11 ± 0.40 |
| G15 | G. australe | Cultivated by Linh chi Vina Company, Vietnam. | 3.26 ± 0.29 |
| Samples | IC50 (µg/mL) | |||
|---|---|---|---|---|
| A549 | MCF7 | PC3 | HepG2 | |
| G1 | 29.3 ± 2.3 | > 50 | 10.0 ± 2.1 | 10.6 ± 1.5 |
| G2 | 16.8 ± 1.9 | > 50 | 10.6 ± 1.4 | 24.7 ± 2.7 |
| G3 | > 50 | 33.8 ± 3.4 | > 50 | 27.6 ± 3.4 |
| G4 | 18.6 ± 1.3 | > 50 | 13.5 ± 1.9 | 21.2 ± 2.3 |
| G6 | 9.12 ± 1.5 | 43.6 ± 2.9 | 11.6 ± 1.9 | 21.3 ± 1.9 |
| G11 | 46.3 ± 2.0 | > 50 | > 50 | > 50 |
| G12 | 24.8 ± 3.7 | > 50 | 23.6 ± 3.2 | 23.5 ± 2.2 |
| G13 | 15.6 ± 3.0 | 18.4 ± 2.9 | 27.8 ± 1.9 | > 50 |
| G14 | 17.7 ± 2.1 | 18.7 ± 3.1 | 27.7 ± 3.1 | 20.2 ± 2.5 |
| G15 | > 50 | 30.7 ± 2.8 | 32.1 ± 2.3 | > 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viet Hung, T.; Thang, P.N.T.; Hien, H.M.; Diep, V.T.; Thu, N.T.; Tan, D.M.; Pham, D.T.; Thi Ha, D.; Huynh, D.T.M. Cytotoxic Activities and Fingerprint Analysis of Triterpenes by HPTLC Technique for Distinguishing Ganoderma Species from Vietnam and other Asian Countries. Plants 2022, 11, 3397. https://doi.org/10.3390/plants11233397
Viet Hung T, Thang PNT, Hien HM, Diep VT, Thu NT, Tan DM, Pham DT, Thi Ha D, Huynh DTM. Cytotoxic Activities and Fingerprint Analysis of Triterpenes by HPTLC Technique for Distinguishing Ganoderma Species from Vietnam and other Asian Countries. Plants. 2022; 11(23):3397. https://doi.org/10.3390/plants11233397
Chicago/Turabian StyleViet Hung, Tran, Phan Nguyen Truong Thang, Ha Minh Hien, Vu Thi Diep, Nguyen Thi Thu, Duong Minh Tan, Duy Toan Pham, Do Thi Ha, and Duyen Thi My Huynh. 2022. "Cytotoxic Activities and Fingerprint Analysis of Triterpenes by HPTLC Technique for Distinguishing Ganoderma Species from Vietnam and other Asian Countries" Plants 11, no. 23: 3397. https://doi.org/10.3390/plants11233397
APA StyleViet Hung, T., Thang, P. N. T., Hien, H. M., Diep, V. T., Thu, N. T., Tan, D. M., Pham, D. T., Thi Ha, D., & Huynh, D. T. M. (2022). Cytotoxic Activities and Fingerprint Analysis of Triterpenes by HPTLC Technique for Distinguishing Ganoderma Species from Vietnam and other Asian Countries. Plants, 11(23), 3397. https://doi.org/10.3390/plants11233397






