Impact of Temperature on Phenolic and Osmolyte Contents in In Vitro Cultures and Micropropagated Plants of Two Mediterranean Plant Species, Lavandula viridis and Thymus lotocephalus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of Photosynthetic Pigments and Oxidative Stress Markers
2.2. Primary Metabolites as Osmoprotectants
2.3. Secondary Metabolism Keys
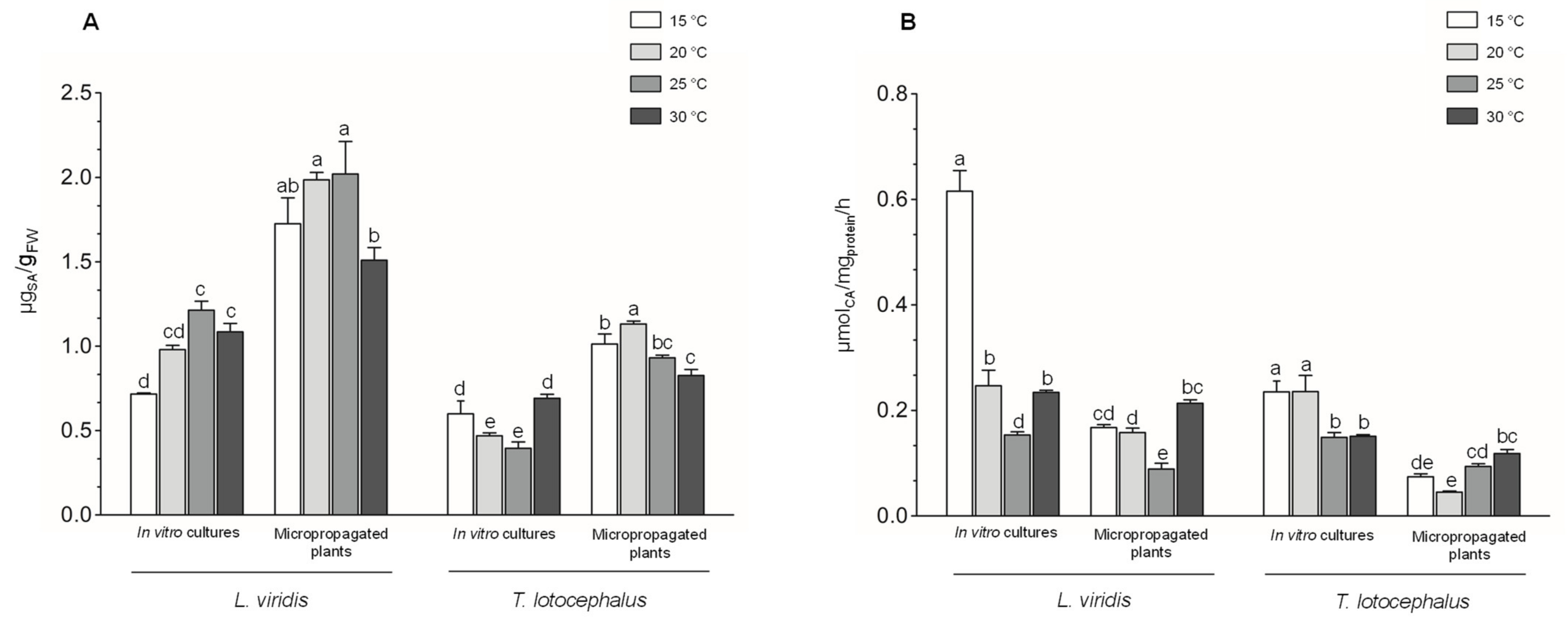
2.3.1. Shikimic Acid and PAL Activity
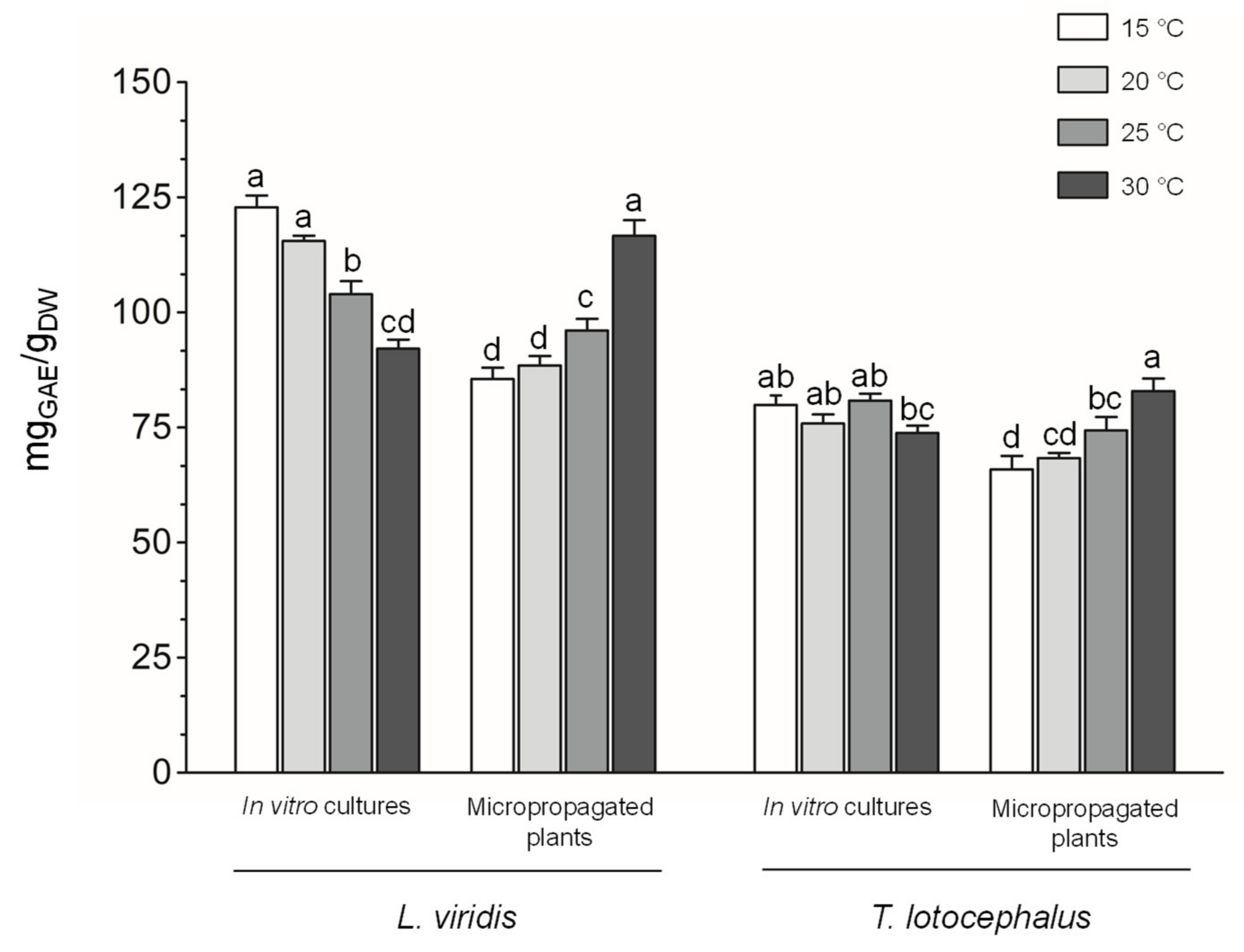
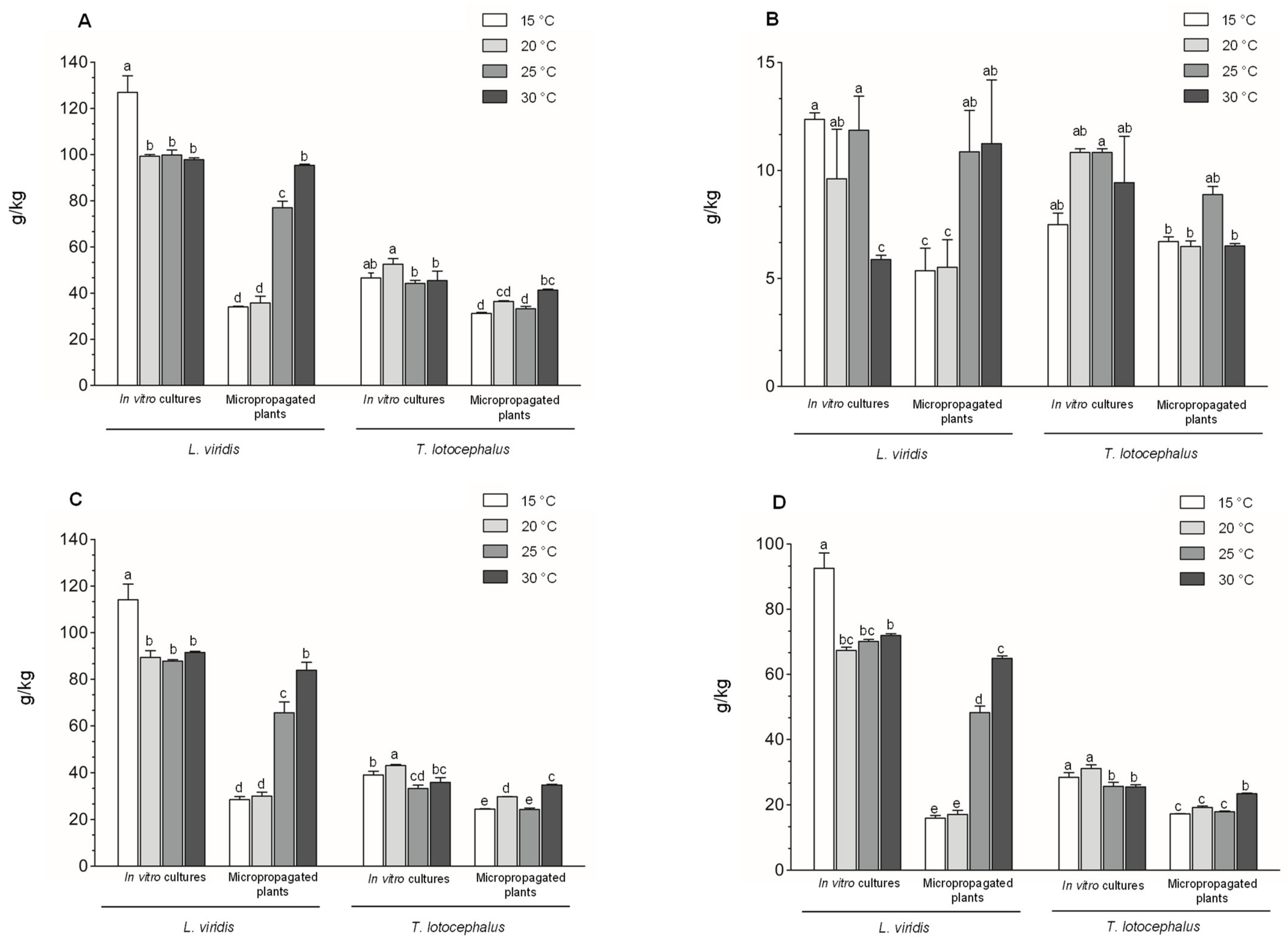
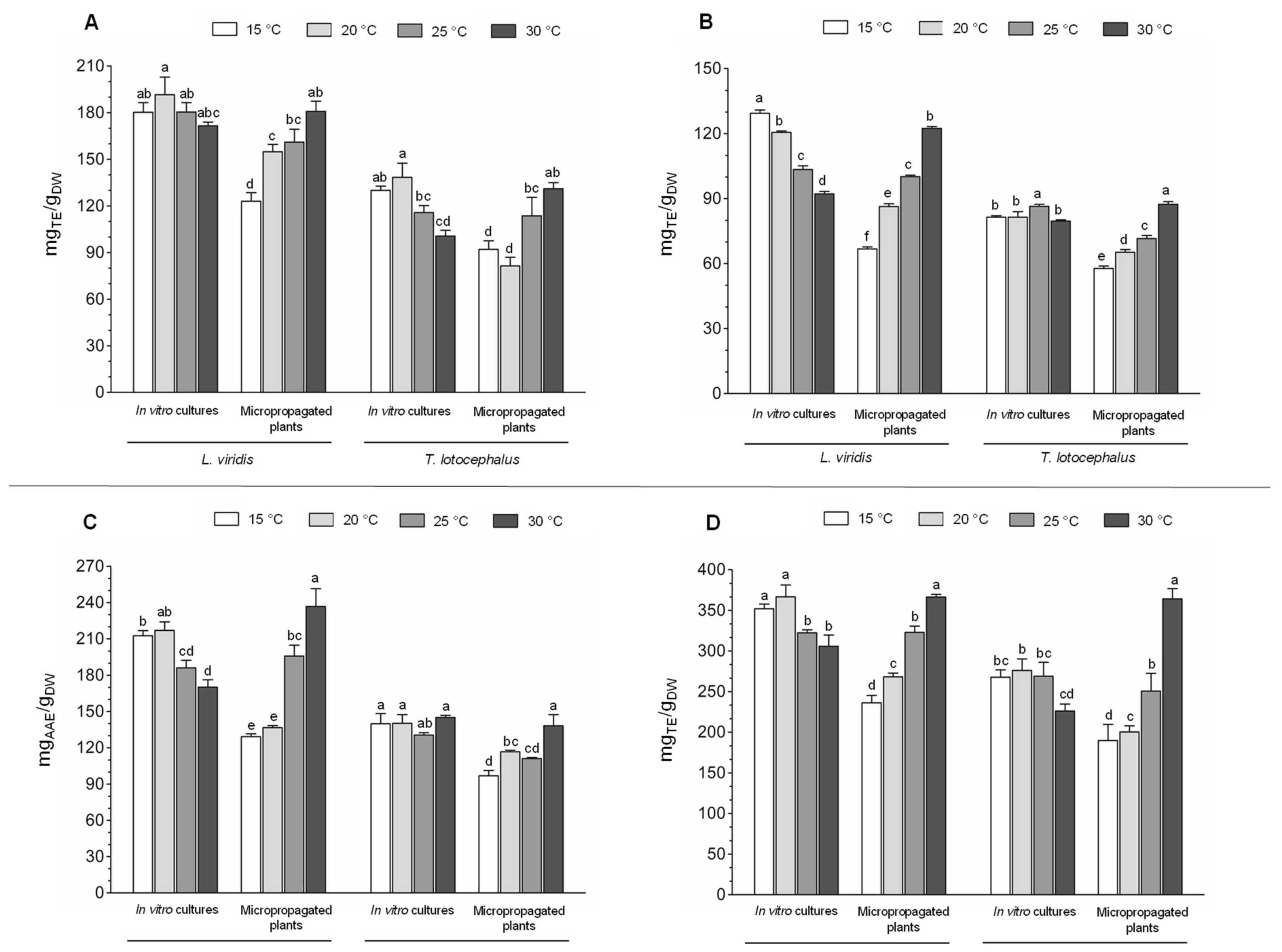
2.3.2. Phenolic Contents, Phenolic Profile and Antioxidant Activity of the Extracts
2.4. Pearson Correlations
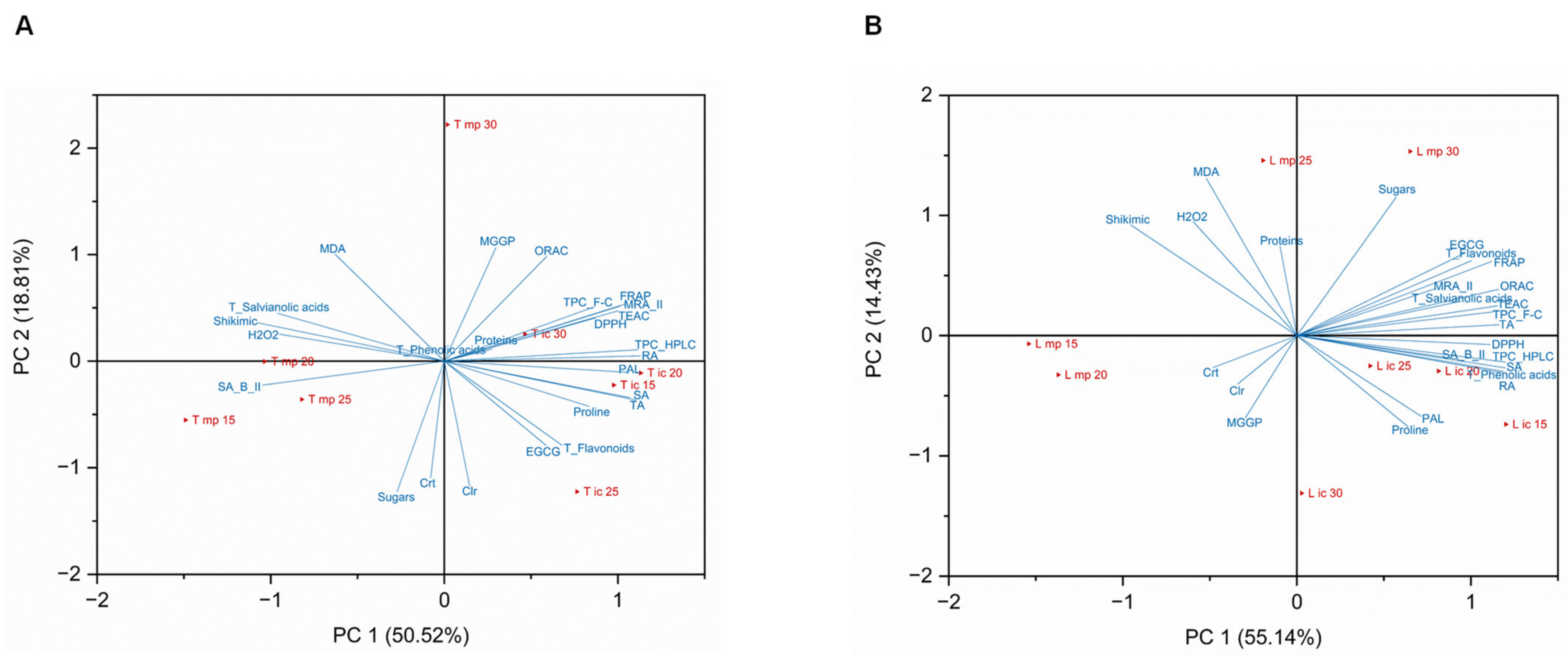
2.5. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Micropropagation and Temperature Experiments
3.2.1. Culture Conditions and Micropropagation
3.2.2. Temperature Experiments
3.3. Photosynthetic Pigments Determination
3.4. Oxidative Stress Markers
3.5. Osmoprotectants Determination
3.6. Secondary Metabolism Keys
3.6.1. Shikimic Acid Content
3.6.2. Phenylalanine Ammonia Lyase (PAL) Activity
3.6.3. Phenolic Compounds Extraction and Quantification
Ultrasound-Assisted Extraction
Phenolics Quantification
Antioxidant Activity
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costa, P.; Goncalves, S.; Grosso, C.; Andrade, P.B.; Valentao, P.; Gabriela Bernardo-Gil, M.; Romano, A. Chemical Profiling and Biological Screening of Thymus lotocephalus Extracts Obtained by Supercritical Fluid Extraction and Hydrodistillation. Ind. Crops Prod. 2012, 36, 246–256. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Andrade, P.B.; Valentão, P.; Romano, A. Inhibitory Effect of Lavandula Viridis on Fe(2+)-Induced Lipid Peroxidation, Antioxidant and Anti-Cholinesterase Properties. Food Chem. 2011, 126, 1779–1786. [Google Scholar] [CrossRef]
- Hawrył, A.; Hawrył, M.; Waksmundzka-Hajnos, M. Liquid Chromatography Fingerprint Analysis and Antioxidant Activity of Selected Lavender Species with Chemometric Calculations. PLoS ONE 2019, 14, e0218974. [Google Scholar] [CrossRef]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Duarte, H.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Response of Thymus Lotocephalus In Vitro Cultures to Drought Stress and Role of Green Extracts in Cosmetics. Antioxidants 2022, 11, 1475. [Google Scholar] [CrossRef]
- Gonçalves, S.; Mansinhos, I.; Rodríguez-Solana, R.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Romano, A. Impact of Metallic Nanoparticles on In Vitro Culture, Phenolic Profile and Biological Activity of Two Mediterranean Lamiaceae Species: Lavandula viridis L’Hér and Thymus lotocephalus G. López and R. Morales. Molecules 2021, 26, 6427. [Google Scholar] [CrossRef]
- Gonçalves, S.; Mansinhos, I.; Rodríguez-Solana, R.; Pérez-Santín, E.; Coelho, N.; Romano, A. Elicitation Improves Rosmarinic Acid Content and Antioxidant Activity in Thymus lotocephalus Shoot Cultures. Ind. Crops Prod. 2019, 137, 214–220. [Google Scholar] [CrossRef]
- Dias, M.I.; Sousa, M.J.; Alves, R.C.; Ferreira, I.C.F.R. Exploring Plant Tissue Culture to Improve the Production of Phenolic Compounds: A Review. Ind. Crops Prod. 2016, 82, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Isah, T. Stress and Defense Responses in Plant Secondary Metabolites Production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, S.; Romano, A. Production of Plant Secondary Metabolites by Using Biotechnological Tools. Second. Metab.-Sources Appl. 2018, 5, 81–99. [Google Scholar] [CrossRef] [Green Version]
- Ancillotti, C.; Bogani, P.; Biricolti, S.; Calistri, E.; Checchini, L.; Ciofi, L.; Gonnelli, C.; Del Bubba, M. Changes in Polyphenol and Sugar Concentrations in Wild Type and Genetically Modified Nicotiana Langsdorffii Weinmann in Response to Water and Heat Stress. Plant Physiol. Biochem. 2015, 97, 52–61. [Google Scholar] [CrossRef]
- Manukyan, A. Secondary Metabolites and Their Antioxidant Capacity of Caucasian Endemic Thyme (Thymus transcaucasicus Ronn.) as Affected by Environmental Stress. J. Appl. Res. Med. Aromat. Plants 2019, 13, 100209. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Gupta, P. Chapter 8—Plant Secondary Metabolites for Preferential Targeting among Various Stressors of Metabolic Syndrome. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 71, pp. 221–261. [Google Scholar]
- Guo, J.; Zhou, X.; Wang, T.; Wang, G.; Cao, F. Regulation of Flavonoid Metabolism in Ginkgo Leaves in Response to Different Day-Night Temperature Combinations. Plant Physiol. Biochem. 2020, 147, 133–140. [Google Scholar] [CrossRef]
- Abd Elbar, O.H.; Elkelish, A.; Niedbała, G.; Farag, R.; Wojciechowski, T.; Mukherjee, S.; Abou-Hadid, A.F.; El-Hennawy, H.M.; Abou El-Yazied, A.; Abd El-Gawad, H.G.; et al. Protective Effect of γ-Aminobutyric Acid against Chilling Stress during Reproductive Stage in Tomato Plants Through Modulation of Sugar Metabolism, Chloroplast Integrity, and Antioxidative Defense Systems. Front. Plant Sci. 2021, 12, 663750. [Google Scholar] [CrossRef]
- Osório, M.L.; Osório, J.; Romano, A. Photosynthesis, Energy Partitioning, and Metabolic Adjustments of the Endangered Cistaceae Species Tuberaria Major under High Temperature and Drought. Photosynthetica 2013, 51, 75–84. [Google Scholar] [CrossRef]
- Puniran-Hartley, N.; Hartley, J.; Shabala, L.; Shabala, S. Salinity-Induced Accumulation of Organic Osmolytes in Barley and Wheat Leaves Correlates with Increased Oxidative Stress Tolerance: In Planta Evidence for Cross-Tolerance. Plant Physiol. Biochem. 2014, 83, 32–39. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Barbanti, L.; Aamer, M.; Iqbal, M.M.; Nawaz, M.; Mahmood, A.; Ali, A.; et al. Heat Stress in Cultivated Plants: Nature, Impact, Mechanisms, and Mitigation Strategies—A Review. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2021, 155, 211–234. [Google Scholar] [CrossRef]
- Mellacheruvu, S.; Talakayala, A.; Garladinne, M. Chapter 7—Crop Improvement of Cereals Through Manipulation of Signaling Pathways in Response to Drought Stress. In Plant Signaling Molecules; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 125–139. ISBN 978-0-12-816451-8. [Google Scholar]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Chapter 12—Phenolic Compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 253–271. ISBN 978-0-12-813278-4. [Google Scholar]
- Lin, K.-H.; Jhou, Y.-J.; Wu, C.-W.; Chang, Y.-S. Growth, Physiological, and Antioxidant Characteristics in Green and Red Perilla Frutescens Varieties as Affected by Temperature- and Water-Stressed Conditions. Sci. Hortic. 2020, 274, 109682. [Google Scholar] [CrossRef]
- Rani, R.; Khan, M.A.; Kayani, W.K.; Ullah, S.; Naeem, I.; Mirza, B. Metabolic Signatures Altered by in Vitro Temperature Stress in Ajuga bracteosa Wall. Ex. Benth. Acta Physiol. Plant 2017, 39, 97. [Google Scholar] [CrossRef]
- Luis, J.C.; Martín, R.; Frías, I.; Valdés, F. Enhanced Carnosic Acid Levels in Two Rosemary Accessions Exposed to Cold Stress Conditions. J. Agric. Food Chem. 2007, 55, 8062–8066. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Sun, Y.; Zheng, S.; Wang, J.; Zhang, T. Hydrogen Peroxide Is Involved in Strigolactone Induced Low Temperature Stress Tolerance in Rape Seedlings (Brassica rapa L.). Plant Physiol. Biochem. 2020, 157, 402–415. [Google Scholar] [CrossRef]
- Hanif, S.; Saleem, M.F.; Sarwar, M.; Irshad, M.; Shakoor, A.; Wahid, M.A.; Khan, H.Z. Biochemically Triggered Heat and Drought Stress Tolerance in Rice by Proline Application. J. Plant Growth Regul. 2021, 40, 305–312. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; El-Yazied, A.A.; Alabdallah, N.M.; Hikal, M.; Mohamed, M.H.M.; Ibrahim, M.F.M.; et al. Melatonin-Mediated Photosynthetic Performance of Tomato Seedlings under High-Temperature Stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Mahmud, J.-A.; Suzuki, T.; Fujita, M. Insights into Spermine-Induced Combined High Temperature and Drought Tolerance in Mung Bean: Osmoregulation and Roles of Antioxidant and Glyoxalase System. Protoplasma 2017, 254, 445–460. [Google Scholar] [CrossRef]
- Pistelli, L.; Tonelli, M.; Pellegrini, E.; Cotrozzi, L.; Pucciariello, C.; Trivellini, A.; Lorenzini, G.; Nali, C. Accumulation of Rosmarinic Acid and Behaviour of ROS Processing Systems in Melissa officinalis L. under Heat Stress. Ind. Crops Prod. 2019, 138, 111469. [Google Scholar] [CrossRef]
- Kalisz, A.; Sękara, A.; Pokluda, R.; Jezdinský, A.; Neugebauerová, J.; Grabowska, A.; Jurkow, R.; Slezák, K.A. Physio-Biochemical Responses of Sage Genotypes to Chilling. Hortic. Sci. 2020, 47, 158–168. [Google Scholar] [CrossRef]
- Qian, R.; Hu, Q.; Ma, X.; Zhang, X.; Ye, Y.; Liu, H.; Gao, H.; Zheng, J. Comparative Transcriptome Analysis of Heat Stress Responses of Clematis Lanuginosa and Clematis Crassifolia. BMC Plant Biol. 2022, 22, 138. [Google Scholar] [CrossRef]
- Batková, P.; Pospíšilová, J.; Synková, H. Production of Reactive Oxygen Species and Development of Antioxidative Systems during in Vitro Growth and Ex Vitro Transfer. Biol. Plant. 2008, 52, 413–422. [Google Scholar] [CrossRef]
- Takkis, K.; Tscheulin, T.; Petanidou, T. Differential Effects of Climate Warming on the Nectar Secretion of Early- and Late-Flowering Mediterranean Plants. Front. Plant Sci. 2018, 9, 874. [Google Scholar] [CrossRef] [Green Version]
- Mollo, L.; Martins, M.; Oliveira, V.F.; Nievola, C.C.; LFigueiredo-Ribeiro, R.D. Effects of Low Temperature on Growth and Non-Structural Carbohydrates of the Imperial Bromeliad Alcantarea imperialis Cultured in Vitro. Plant Cell Tissue Organ Cult. 2011, 107, 141. [Google Scholar] [CrossRef]
- Chaves, I.; Passarinho, J.A.P.; Capitão, C.; Chaves, M.M.; Fevereiro, P.; Ricardo, C.P.P. Temperature Stress Effects in Quercus suber Leaf Metabolism. J. Plant Physiol. 2011, 168, 1729–1734. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd_Allah, E.F.; Gucel, S.; Tran, L.-S.P. Nitric Oxide Mitigates Salt Stress by Regulating Levels of Osmolytes and Antioxidant Enzymes in Chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Nishimura, Y.; Fukumoto, Y.; Li, J. Effect of High Temperature on Active Oxygen Species, Senescence and Photosynthetic Properties in Cucumber Leaves. Environ. Exp. Bot. 2011, 70, 212–216. [Google Scholar] [CrossRef]
- Lehmann, S.; Funck, D.; Szabados, L.; Rentsch, D. Proline Metabolism and Transport in Plant Development. Amino Acids 2010, 39, 949–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scalschi, L.; Fernández-Crespo, E.; Pitarch-Marin, M.; Llorens, E.; González-Hernández, A.I.; Camañes, G.; Vicedo, B.; García-Agustín, P. Response of Tomato-Pseudomonas Pathosystem to Mild Heat Stress. Horticulturae 2022, 8, 174. [Google Scholar] [CrossRef]
- Asayesh, Z.M.; Vahdati, K.; Aliniaeifard, S. Investigation of Physiological Components Involved in Low Water Conservation Capacity of in Vitro Walnut Plants. Sci. Hortic. 2017, 224, 1–7. [Google Scholar] [CrossRef]
- Alzandi, A.A.; Naguib, D.M. Effect of Hydropriming on Trigonella Foenum Callus Growth, Biochemical Traits and Phytochemical Components under PEG Treatment. Plant Cell Tissue Organ Cult. 2020, 141, 179–190. [Google Scholar] [CrossRef]
- Riikonen, J.; Kontunen-Soppela, S.; Ossipov, V.; Tervahauta, A.; Tuomainen, M.; Oksanen, E.; Vapaavuori, E.; Heinonen, J.; Kivimäenpää, M. Needle Metabolome, Freezing Tolerance and Gas Exchange in Norway Spruce Seedlings Exposed to Elevated Temperature and Ozone Concentration. Tree Physiol. 2012, 32, 1102–1112. [Google Scholar] [CrossRef] [Green Version]
- Woznicki, T.L.; Sønsteby, A.; Aaby, K.; Martinsen, B.K.; Heide, O.M.; Wold, A.-B.; Remberg, S.F. Ascorbate Pool, Sugars and Organic Acids in Black Currant (Ribes nigrum L.) Berries Are Strongly Influenced by Genotype and Post-Flowering Temperature. J. Sci. Food Agric. 2017, 97, 1302–1309. [Google Scholar] [CrossRef]
- Yu, J.; Du, H.; Xu, M.; Huang, B. Metabolic Responses to Heat Stress under Elevated Atmospheric CO2 Concentration in a Cool-Season Grass Species. J. Am. Soc. Hortic. Sci. 2012, 137, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Løvdal, T.; Olsen, K.M.; Slimestad, R.; Verheul, M.; Lillo, C. Synergetic Effects of Nitrogen Depletion, Temperature, and Light on the Content of Phenolic Compounds and Gene Expression in Leaves of Tomato. Phytochemistry 2010, 71, 605–613. [Google Scholar] [CrossRef]
- Kumar, R.; Joshi, R.; Kumari, M.; Thakur, R.; Kumar, D.; Kumar, S. Elevated CO2 and Temperature Influence Key Proteins and Metabolites Associated with Photosynthesis, Antioxidant and Carbon Metabolism in Picrorhiza kurroa. J. Proteom. 2020, 219, 103755. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Romano, A. Accumulation of Phenolic Compounds in in Vitro Cultures and Wild Plants of Lavandula viridis L’Hér and Their Antioxidant and Anti-Cholinesterase Potential. Food Chem. Toxicol. 2013, 57, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, R.S.; Slimmon, T.; McAuley, C.Y.; Kott, L.S. Heat Stress Reduces the Accumulation of Rosmarinic Acid and the Total Antioxidant Capacity in Spearmint (Mentha spicata L). J. Sci. Food Agric. 2005, 85, 2429–2436. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Algieri, F.; Rodriguez-Nogales, A.; Gálvez, J.; Segura-Carretero, A. Phytochemical Profiling of Anti-Inflammatory Lavandula Extracts via RP-HPLC-DAD-QTOF-MS and -MS/MS: Assessment of Their Qualitative and Quantitative Differences. Electrophoresis 2018, 39, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.L.; Pereira, E.; Soković, M.; Carvalho, A.M.; Barata, A.M.; Lopes, V.; Rocha, F.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Phenolic Composition and Bioactivity of Lavandula pedunculata (Mill.) Cav. Samples from Different Geographical Origin. Molecules 2018, 23, 1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Coelho, N.; Goncalves, S.; Elena Gonzalez-Benito, M.; Romano, A. Establishment of an in Vitro Propagation Protocol for Thymus lotocephalus, a Rare Aromatic Species of the Algarve (Portugal). Plant Growth Regul. 2012, 66, 69–74. [Google Scholar] [CrossRef]
- Dias, M.C.; Almeida, R.; Romano, A. Rapid Clonal Multiplication of Lavandula viridis L’Hér through in Vitro Axillary Shoot Proliferation. Plant Cell Tissue Organ Cult. 2002, 68, 99–102. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Loreto, F.; Velikova, V. Isoprene Produced by Leaves Protects the Photosynthetic Apparatus against Ozone Damage, Quenches Ozone Products, and Reduces Lipid Peroxidation of Cellular Membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Martins, N.; Gonçalves, S.; Palma, T.; Romano, A. The Influence of Low PH on in Vitro Growth and Biochemical Parameters of Plantago almogravensis and P. algarbiensis. Plant Cell Tissue Organ Cult. 2011, 107, 113–121. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Abid, G.; Ouertani, R.N.; Muhovski, Y.; Jebara, S.H.; Hidri, Y.; Ghouili, E.; Abdelkarim, S.; Chaieb, O.; Souissi, F.; Zribi, F.; et al. Variation in Antioxidant Metabolism of Faba Bean (Vicia faba) under Drought Stress Induced by Polyethylene Glycol Reveals Biochemical Markers Associated with Antioxidant Defense. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2021, 155, 797–806. [Google Scholar] [CrossRef]
- Zhan, L.; Li, Y.; Hu, J.; Pang, L.; Fan, H. Browning Inhibition and Quality Preservation of Fresh-Cut Romaine Lettuce Exposed to High Intensity Light. Innov. Food Sci. Emerg. Technol. 2012, 14, 70–76. [Google Scholar] [CrossRef]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Ultrasonic-Assisted Extraction and Natural Deep Eutectic Solvents Combination: A Green Strategy to Improve the Recovery of Phenolic Compounds from Lavandula pedunculata Subsp. lusitanica (Chaytor) Franco. Antioxidants 2021, 10, 582. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin–Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]


| Treatment | Cltotal (mg/gFW) | Crt (mg/gFW) | MDA (nmol/gFW) | H2O2 (µmol/gFW) | Soluble Sugars (mg/gFW) | Soluble Proteins (mg/gFW) | Proline (µmol/gFW) |
|---|---|---|---|---|---|---|---|
| L. viridis | |||||||
| In vitro cultures | |||||||
| 15 °C | 1.34 ± 0.06 a | 0.30 ± 0.01 a | 23.7 ± 1.42 e | 2.83 ± 0.11 e | 31.7 ± 0.20 de | 7.92 ± 0.06 b | 0.99 ± 0.09 b |
| 20 °C | 0.88 ± 0.02 c | 0.22 ± 0.01 b | 25.5 ± 1.35 ef | 1.10 ± 0.13 f | 28.6 ± 1.53 e | 9.90 ± 0.13 a | 0.31 ± 0.03 d |
| 25 °C | 0.61 ± 0.02 d | 0.14 ± 0.00 c | 32.6 ± 1.99 de | 2.19 ± 0.06 e | 64.5 ± 1.22 a | 9.97 ± 0.05 a | 1.53 ± 0.11 a |
| 30 °C | 0.35 ± 0.01 e | 0.09 ± 0.00 d | 35.6 ± 2.10 cd | 2.77 ± 0.14 e | 54.1 ± 0.85 b | 6.10 ± 0.33 c | 1.43 ± 0.08 a |
| Micropropagated plants | |||||||
| 15 °C | 1.01 ± 0.04 b | 0.29 ± 0.02 a | 45.6 ± 2.58 b | 9.63 ± 0.18 a | 50.6 ± 0.63 b | 8.33 ± 0.14 b | 0.11 ± 0.01 de |
| 20 °C | 1.31 ± 0.07 a | 0.30 ± 0.01 a | 42.3 ± 2.34 bc | 3.80 ± 0.21 d | 37.6 ± 0.97 c | 9.65 ± 0.07 a | 0.08 ± 0.01 e |
| 25 °C | 0.86 ± 0.04 c | 0.21 ± 0.01 b | 58.8 ± 6.42 a | 5.77 ± 0.37 c | 35.6± 2.82 cd | 10.2 ± 0.05 a | 0.23 ± 0.08 de |
| 30 °C | 0.25 ± 0.00 e | 0.10 ± 0.00 d | 56.9 ± 3.00 a | 8.44 ± 0.47 b | 32.8 ± 1.35 de | 8.31 ± 0.55 b | 0.53 ± 0.04 c |
| T. lotocephalus | |||||||
| In vitro cultures | |||||||
| 15 °C | 1.23 ± 0.02 a | 0.32 ± 0.01 a | 18.6 ± 0.54 e | 1.16 ± 0.05 d | 50.7 ± 0.58 a | 7.92 ± 0.57 b | 6.05 ± 0.31 a |
| 20 °C | 1.03 ± 0.02 c | 0.27 ± 0.01 b | 16.9 ± 0.47 d | 0.93 ± 0.15 d | 36.0 ± 0.81 cd | 6.52 ± 0.18 c | 1.18 ± 0.24 d |
| 25 °C | 1.17 ± 0.03 ab | 0.26 ± 0.00 b | 11.7 ± 0.51 e | 0.69 ± 0.15 d | 42.6 ± 1.49 b | 6.45 ± 0.22 c | 3.76 ± 0.21 c |
| 30 °C | 0.44 ± 0.03 e | 0.12 ± 0.00 c | 30.9 ± 2.02 b | 1.13 ± 0.06 d | 28.9 ± 0.12 e | 10.1 ± 0.81 a | 4.51 ± 0.18 b |
| Micropropagated plants | |||||||
| 15 °C | 0.78 ± 0.00 d | 0.24 ± 0.01 b | 30.5 ± 2.17 b | 7.99 ± 0.66 a | 42.3 ± 1.28 b | 6.31 ± 0.47 c | n.d. |
| 20 °C | 1.12 ± 0.06 bc | 0.31 ± 0.01 a | 24.7 ± 1.84 c | 2.24 ± 0.22 c | 33.5 ± 0.35 d | 8.00 ± 0.40 b | n.d. |
| 25 °C | 1.01 ± 0.05 c | 0.27 ± 0.01 b | 41.2 ± 1.77 a | 5.41 ± 0.48 b | 49.9 ± 1.14 a | 5.81 ± 0.23 c | 0.05 ± 0.01 e |
| 30 °C | 0.23 ± 0.03 f | 0.09 ± 0.00 d | 44.5 ± 0.78 a | 4.72 ± 0.24 b | 38.5 ± 0.39 c | 6.38 ± 0.44 c | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Impact of Temperature on Phenolic and Osmolyte Contents in In Vitro Cultures and Micropropagated Plants of Two Mediterranean Plant Species, Lavandula viridis and Thymus lotocephalus. Plants 2022, 11, 3516. https://doi.org/10.3390/plants11243516
Mansinhos I, Gonçalves S, Rodríguez-Solana R, Ordóñez-Díaz JL, Moreno-Rojas JM, Romano A. Impact of Temperature on Phenolic and Osmolyte Contents in In Vitro Cultures and Micropropagated Plants of Two Mediterranean Plant Species, Lavandula viridis and Thymus lotocephalus. Plants. 2022; 11(24):3516. https://doi.org/10.3390/plants11243516
Chicago/Turabian StyleMansinhos, Inês, Sandra Gonçalves, Raquel Rodríguez-Solana, José Luis Ordóñez-Díaz, José Manuel Moreno-Rojas, and Anabela Romano. 2022. "Impact of Temperature on Phenolic and Osmolyte Contents in In Vitro Cultures and Micropropagated Plants of Two Mediterranean Plant Species, Lavandula viridis and Thymus lotocephalus" Plants 11, no. 24: 3516. https://doi.org/10.3390/plants11243516
APA StyleMansinhos, I., Gonçalves, S., Rodríguez-Solana, R., Ordóñez-Díaz, J. L., Moreno-Rojas, J. M., & Romano, A. (2022). Impact of Temperature on Phenolic and Osmolyte Contents in In Vitro Cultures and Micropropagated Plants of Two Mediterranean Plant Species, Lavandula viridis and Thymus lotocephalus. Plants, 11(24), 3516. https://doi.org/10.3390/plants11243516









