Cell-Type-Specific Length and Cytosolic pH Response of Superficial Cells of Arabidopsis Root to Chronic Salinity
Abstract
1. Introduction
2. Results
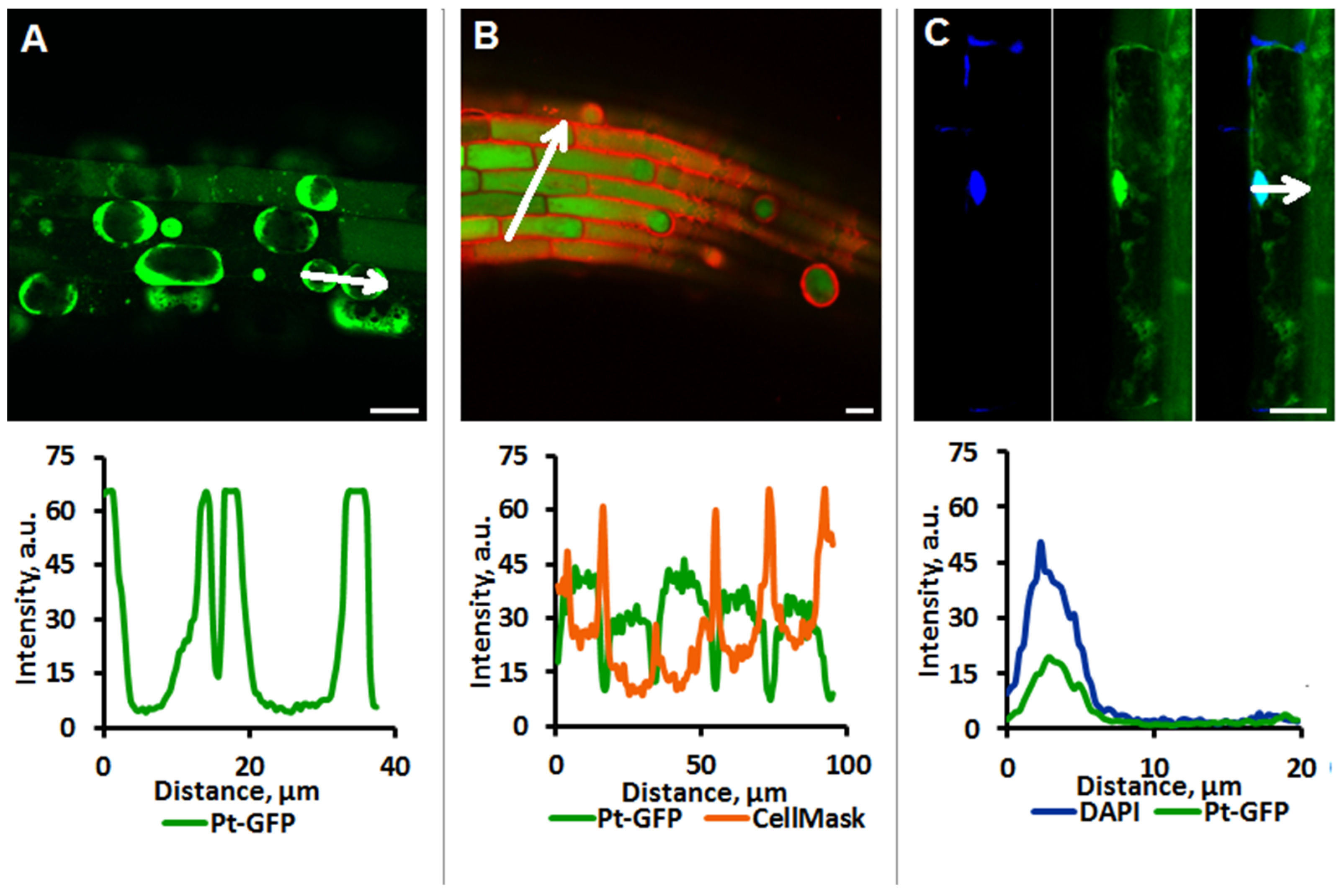
2.1. Localization of Pt-Gfp in Arabidopsis Root Cells
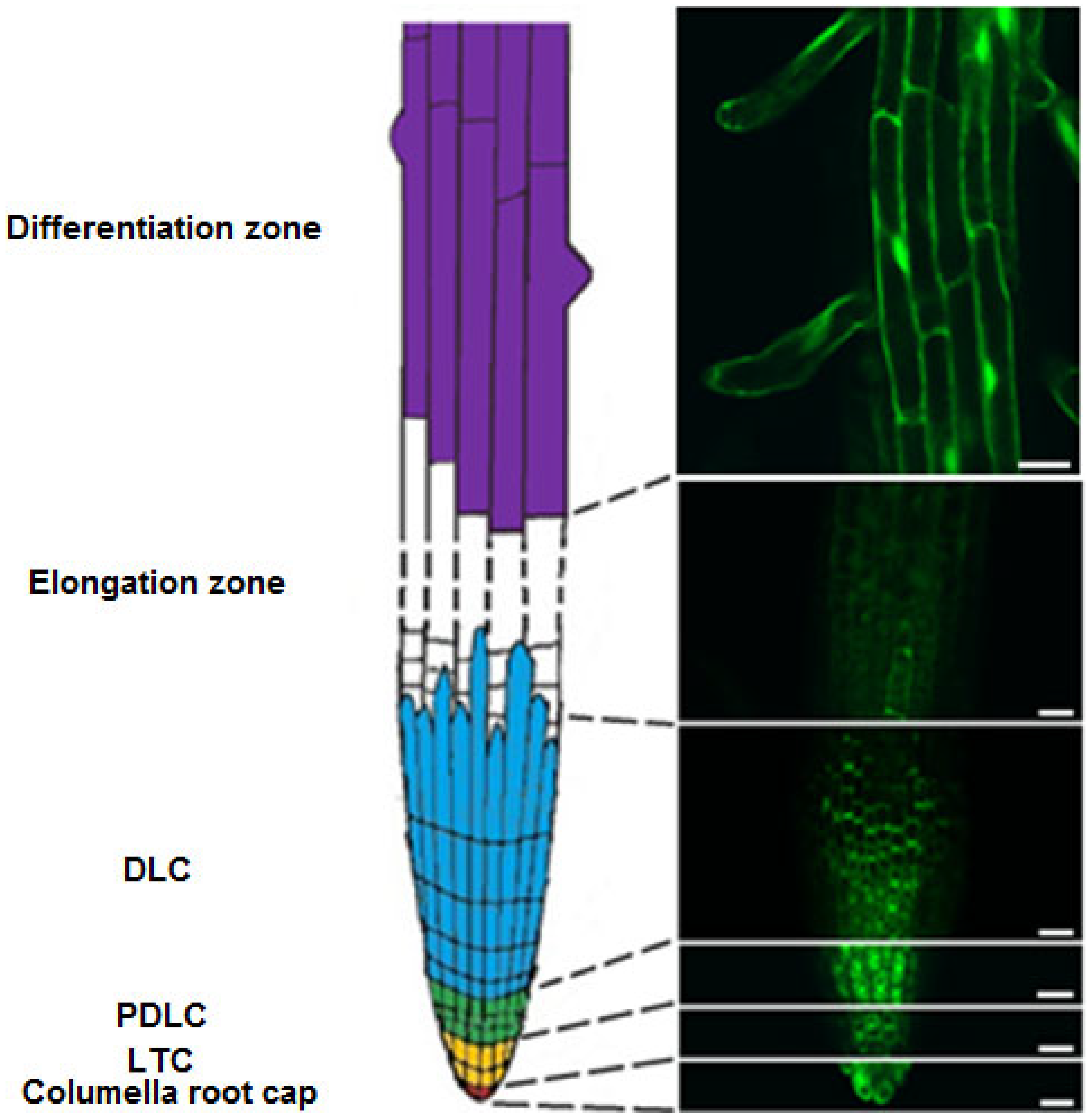
2.2. Identification of Different Zones of Root Cells
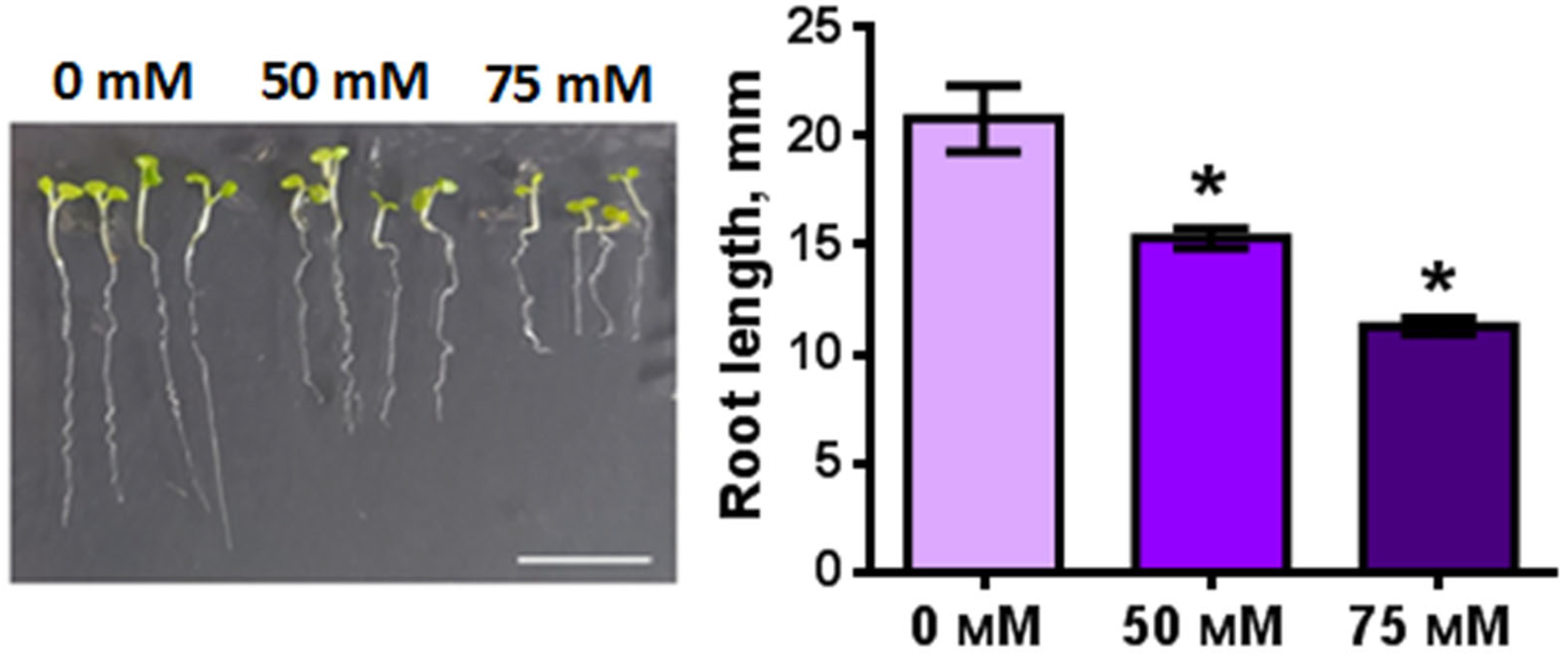
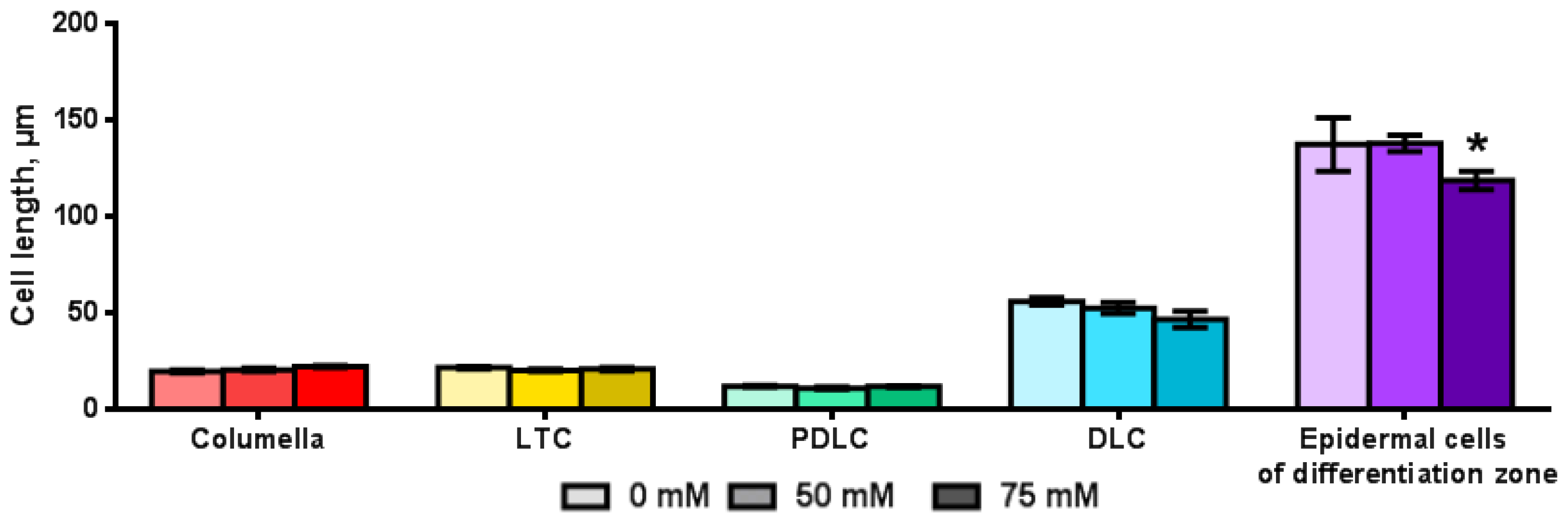
2.3. Influence of NaCl on the Length of the Root, Root Zones and Root Cells
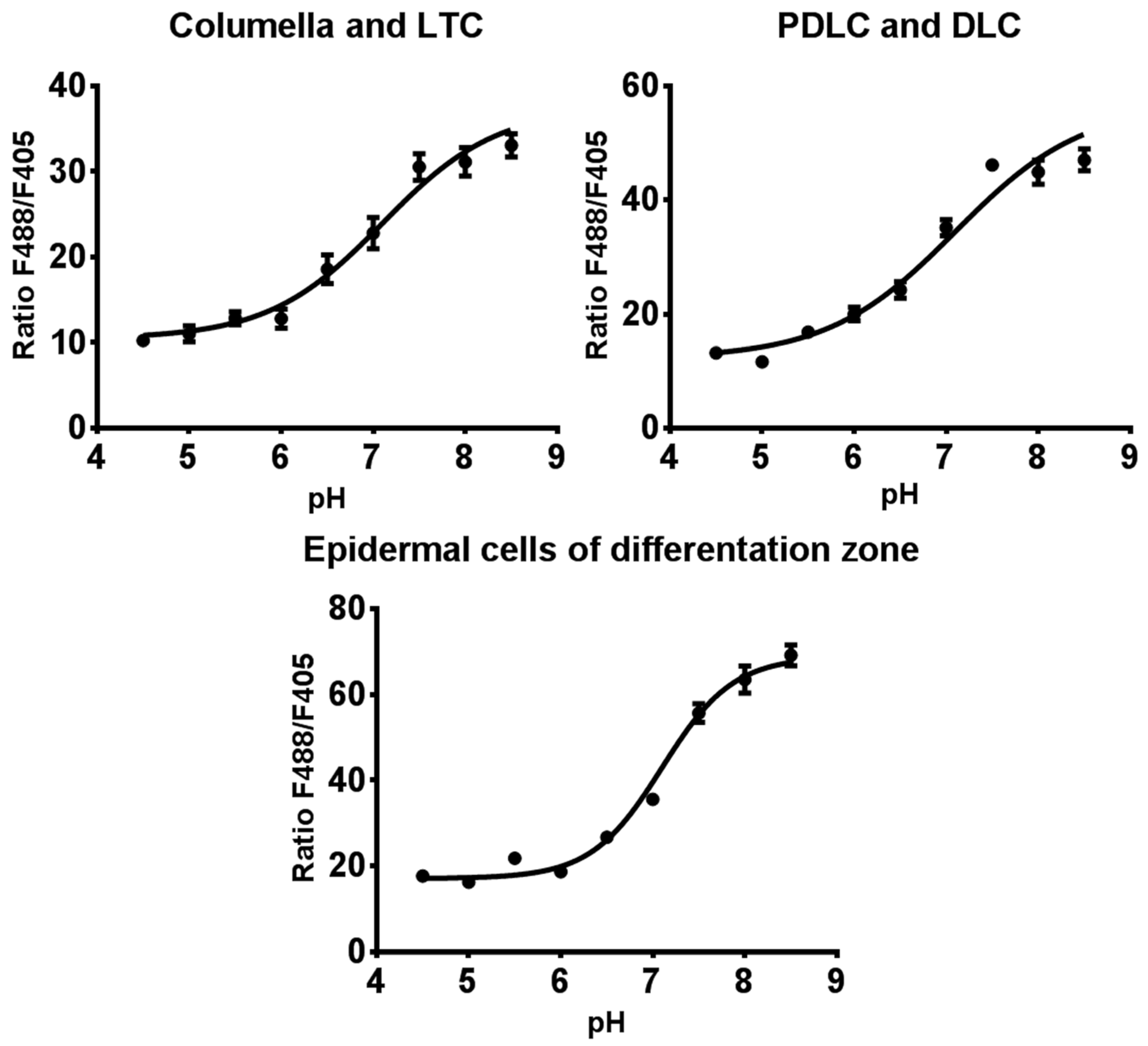
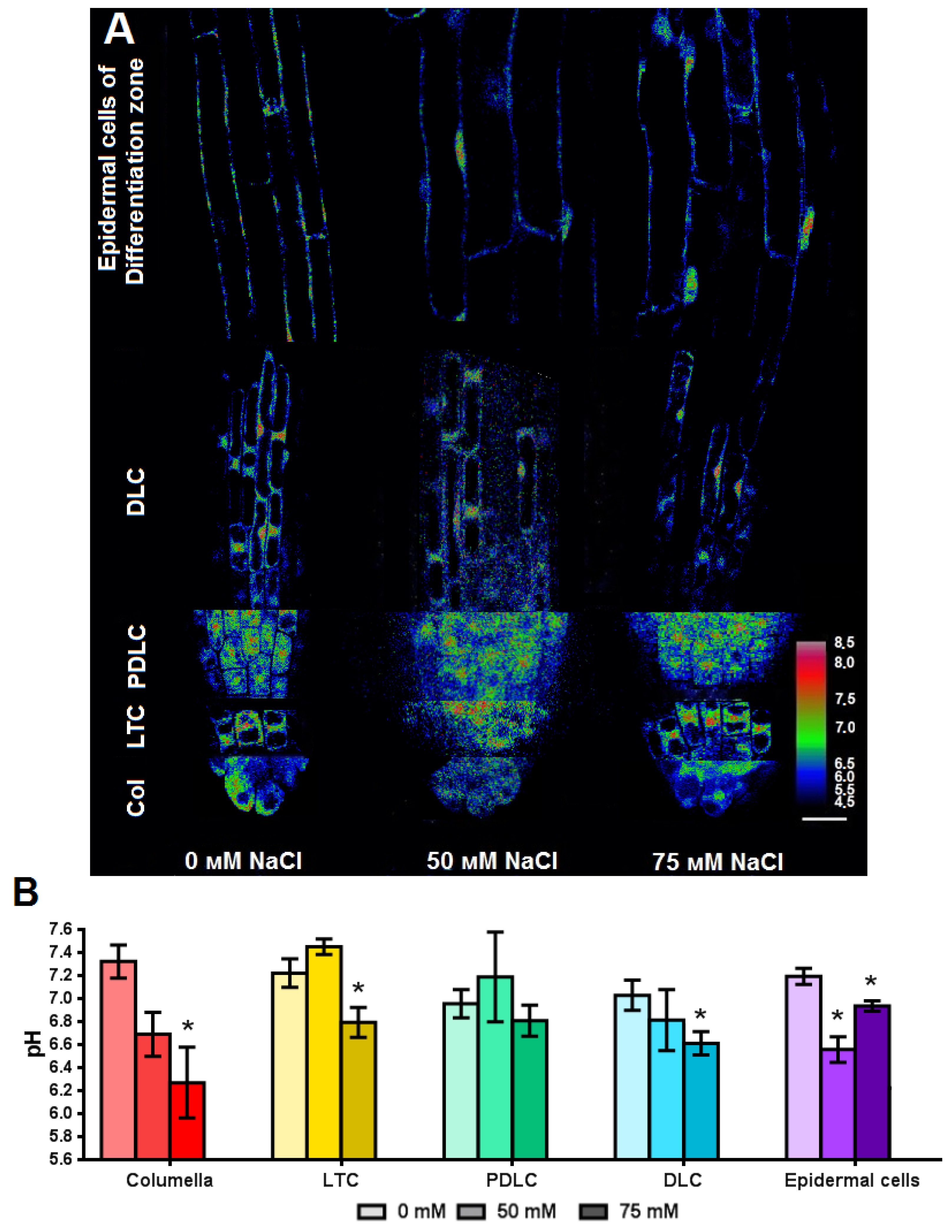
2.4. Study of Effect of NaCl on pH of Arabidopsis Root Cells
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Confocal Microscopy of Root Cells of Arabidopsis
4.3. Assessment of Morphological Parameters of Root
4.4. In Vivo Calibration of Pt-GFP Signal and Ratiometric Analysis in Arabidopsis Plants
4.5. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques, 1st ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 43–53. ISBN 978-3-319-96189-7. [Google Scholar]
- Haj-Amor, Z.; Araya, T.; Kim, D.-G.; Bouri, S.; Lee, J.; Ghiloufi, W.; Yang, Y.; Kang, H.; Jhariya, M.K.; Banerjee, A.; et al. Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: A review. Sci. Total Environ. 2022, 843, 156946. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Abbas, A.; Khan, S.; Hussain, N.; Hanjra, M.A.; Akbar, S. Characterizing soil salinity in irrigated agriculture using a remote sensing approach. Phys. Chem. Earth Parts ABC 2013, 55–57, 43–52. [Google Scholar] [CrossRef]
- Hassani, A.; Azapagic, A.; Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef] [PubMed]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Bachani, J.; Mahanty, A.; Aftab, T.; Kumar, K. Insight into calcium signaling in salt stress response. S. Afr. J. Bot. 2022, 151, 1–8. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Guo, M.; Wang, X.-S.; Guo, H.-D.; Bai, S.-Y.; Khan, A.; Wang, X.-M.; Gao, Y.-M.; Li, J.-S. Tomato salt tolerance mechanisms and their potential applications for fighting salinity: A review. Front. Plant Sci. 2022, 13, 949541. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Shabala, L.; Zhang, J.; Pottosin, I.; Bose, J.; Zhu, M.; Fuglsang, A.T.; Velarde-Buendia, A.; Massart, A.; Hill, C.B.; Roessner, U.; et al. Cell-type-specific H+-ATPase activity in root tissues enables K+ retention and mediates acclimation of barley (Hordeum vulgare) to salinity stress. Plant Physiol. 2016, 172, 2445–2458. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lindberg, S.; Shabala, L.; Morgan, S.; Shabala, S.; Jacobsen, S.-E. A comparative analysis of cytosolic Na+ changes under salinity between halophyte quinoa (Chenopodium quinoa) and glycophyte pea (Pisum sativum). Environ. Exp. Bot. 2017, 141, 154–160. [Google Scholar] [CrossRef]
- Felle, H.H. pH: Signal and messenger in plant cells. Plant Biol. 2001, 3, 577–591. [Google Scholar] [CrossRef]
- Gjetting, S.K.; Ytting, C.K.; Schulz, A.; Fuglsang, A.T. Live imaging of intra- and extracellular pH in plants using phusion, a novel genetically encoded biosensor. J. Exp. Bot. 2012, 63, 3207–3218. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zeng, Y.; Zhuang, X.; Sun, L.; Yao, X.; Pimpl, P.; Jiang, L. Organelle pH in the Arabidopsis endomembrane system. Mol. Plant 2013, 6, 1419–1437. [Google Scholar] [CrossRef] [PubMed]
- Wegner, L.H.; Shabala, S. Biochemical pH clamp: The forgotten resource in membrane bioenergetics. New Phytol. 2020, 225, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Moseyko, N.; Feldman, L.J. Expression of pH-sensitive green fluorescent protein in Arabidopsis thaliana: pH-sensitive GFP in plants. Plant Cell Environ. 2001, 24, 557–563. [Google Scholar] [CrossRef]
- Perbal, G.; Driss-Ecole, D. Mechanotransduction in gravisensing cells. Trends Plant Sci. 2003, 8, 498–504. [Google Scholar] [CrossRef]
- Schulte, A.; Lorenzen, I.; Böttcher, M.; Plieth, C. A novel fluorescent pH probe for expression in plants. Plant Methods 2006, 2, 7. [Google Scholar] [CrossRef]
- Barbez, E.; Dünser, K.; Gaidora, A.; Lendl, T.; Busch, W. Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2017, 114, E4884–E4893. [Google Scholar] [CrossRef] [PubMed]
- Fasano, J.M.; Swanson, S.J.; Blancaflor, E.B.; Dowd, P.E.; Kao, T.; Gilroy, S. Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell 2001, 13, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Couldwell, D.L.; Dunford, R.; Kruger, N.J.; Lloyd, D.C.; Ratcliffe, R.G.; Smith, A.M.O. Response of cytoplasmic pH to anoxia in plant tissues with altered activities of fermentation enzymes: Application of methyl phosphonate as an NMR pH probe. Ann. Bot. 2009, 103, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Kurkdjian, A.; Guern, J. Intracellular pH: Measurement and importance in cell activity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 271–303. [Google Scholar] [CrossRef]
- Martinière, A.; Gibrat, R.; Sentenac, H.; Dumont, X.; Gaillard, I.; Paris, N. Uncovering pH at both sides of the root plasma membrane interface using noninvasive imaging. Proc. Natl. Acad. Sci. USA 2018, 115, 6488–6493. [Google Scholar] [CrossRef]
- Diéguez-Uribeondo, J.; Förster, H.; Adaskaveg, J.E. Visualization of localized pathogen-induced pH modulation in almond tissues infected by Colletotrichum acutatum using confocal scanning laser microscopy. Phytopathology 2008, 98, 1171–1178. [Google Scholar] [CrossRef][Green Version]
- Barnes, A.C.; Benning, C.; Roston, R.L. Chloroplast membrane remodeling during freezing stress is accompanied by cytoplasmic acidification activating SENSITIVE TO FREEZING2. Plant Physiol. 2016, 171, 2140–2149. [Google Scholar] [CrossRef]
- Pecherina, A.; Grinberg, M.; Ageyeva, M.; Zdobnova, T.; Ladeynova, M.; Yudintsev, A.; Vodeneev, V.; Brilkina, A. Whole-plant measure of temperature-induced changes in the cytosolic pH of potato plants using genetically encoded fluorescent sensor Pt-GFP. Agriculture 2021, 11, 1131. [Google Scholar] [CrossRef]
- Gao, D.; Knight, M.R.; Trewavas, A.J.; Sattelmacher, B.; Plieth, C. Self-reporting Arabidopsis expressing pH and [Ca2+] indicators unveil ion dynamics in the cytoplasm and in the apoplast under abiotic stress. Plant Physiol. 2004, 134, 898–908. [Google Scholar] [CrossRef]
- Martinière, A.; Desbrosses, G.; Sentenac, H.; Paris, N. Development and properties of genetically encoded pH sensors in plants. Front. Plant Sci. 2013, 4, 523. [Google Scholar] [CrossRef]
- Martinière, A.; Bassil, E.; Jublanc, E.; Alcon, C.; Reguera, M.; Sentenac, H.; Blumwald, E.; Paris, N. In vivo intracellular pH measurements in tobacco and Arabidopsis reveal an unexpected pH gradient in the endomembrane system. Plant Cell 2013, 25, 4028–4043. [Google Scholar] [CrossRef] [PubMed]
- Fendrych, M.; Van Hautegem, T.; Van Durme, M.; Olvera-Carrillo, Y.; Huysmans, M.; Karimi, M.; Lippens, S.; Guérin, C.J.; Krebs, M.; Schumacher, K.; et al. Programmed cell death controlled by ANAC033/SOMBRERO determines root cap organ size in Arabidopsis. Curr. Biol. 2014, 24, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Dolan, L.; Janmaat, K.; Willemsen, V.; Linstead, P.; Poethig, S.; Roberts, K.; Scheres, B. Cellular organisation of the Arabidopsis thaliana root. Development 1993, 119, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Moe-Lange, J.; Hennet, L.; Feldman, L.J. Salt stress affects the redox status of Arabidopsis root meristems. Front. Plant Sci. 2016, 7, 81. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Y.; Testerink, C. Root dynamic growth strategies in response to salinity. Plant Cell Environ. 2022, 45, 695–704. [Google Scholar] [CrossRef]
- Kumpf, R.P.; Nowack, M.K. The root cap: A short story of life and death. J. Exp. Bot. 2015, 66, 5651–5662. [Google Scholar] [CrossRef]
- Zhu, T.; Rost, T.L. Directional cell-to-cell communication in the Arabidopsis root apical meristem III. Plasmodesmata turnover and apoptosis in meristem and root cap cells during four weeks after germination. Protoplasma 2000, 213, 99–107. [Google Scholar] [CrossRef]
- Wenzel, C.L.; Rost, T.L. Cell division patterns of the protoderm and root cap in theroot apical meristem of Arabidopsis thaliana. Protoplasma 2001, 218, 203–213. [Google Scholar] [CrossRef]
- Haruta, M.; Sussman, M.R. The effect of a genetically reduced plasma membrane protonmotive force on vegetative growth of Arabidopsis. Plant Physiol. 2012, 158, 1158–1171. [Google Scholar] [CrossRef]
- Cosse, M.; Seidel, T. Plant proton pumps and cytosolic pH-homeostasis. Front. Plant Sci. 2021, 12, 672873. [Google Scholar] [CrossRef]
- Wang, Y.; Blatt, M.R.; Chen, Z. Ion transport at the plant plasma membrane. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2018; p. a0001307. ISBN 9780470015902. [Google Scholar] [CrossRef]
- Walid, S.; Faiçal, B. Ion transporters and their molecular regulation mechanism in plants. J. Plant Sci. Phytopathol. 2021, 5, 28–43. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, Y.; Chen, S.; Ning, N.; Hu, H. Arabidopsis IAR4 modulates primary root growth under salt stress through ROS-mediated modulation of auxin distribution. Front. Plant Sci. 2019, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, X.; Chang, N.; Nan, W.; Wang, S.; Ruan, M.; Sun, L.; Li, S.; Bi, Y. Cytosolic glucose-6-phosphate dehydrogenase is involved in seed germination and root growth under salinity in Arabidopsis. Front. Plant Sci. 2019, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zayed, O.; Zeng, F.; Liu, C.; Zhang, L.; Zhu, P.; Hsu, C.; Tuncil, Y.E.; Tao, W.A.; Carpita, N.C.; et al. Arabinose biosynthesis is critical for salt stress tolerance in Arabidopsis. New Phytol. 2019, 224, 274–290. [Google Scholar] [CrossRef]
- West, G.; Inzé, D.; Beemster, G.T.S. Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol. 2004, 135, 1050–1058. [Google Scholar] [CrossRef]
- Galvan-Ampudia, C.S.; Julkowska, M.M.; Darwish, E.; Gandullo, J.; Korver, R.A.; Brunoud, G.; Haring, M.A.; Munnik, T.; Vernoux, T.; Testerink, C. Halotropism is a response of plant roots to avoid a saline environment. Curr. Biol. 2013, 23, 2044–2050. [Google Scholar] [CrossRef]
- Fasano, J.M.; Massa, G.D.; Gilroy, S. Ionic signaling in plant responses to gravity and touch. J. Plant Growth Regul. 2002, 21, 71–88. [Google Scholar] [CrossRef]
- Wegner, L.H.; Li, X.; Zhang, J.; Yu, M.; Shabala, S.; Hao, Z. Biochemical and biophysical pH clamp controlling net H+ efflux across the plasma membrane of plant cells. New Phytol. 2021, 230, 408–415. [Google Scholar] [CrossRef]
- Padmanaban, S.; Lin, X.; Perera, I.; Kawamura, Y.; Sze, H. Differential expression of vacuolar H+-ATPase subunit c genes in tissues active in membrane trafficking and their roles in plant growth as revealed by RNAi. Plant Physiol. 2004, 134, 1514–1526. [Google Scholar] [CrossRef]
- Ueno, K.; Kinoshita, T.; Inoue, S.; Emi, T.; Shimazaki, K. Biochemical Characterization of plasma membrane H+-ATPase activation in guard cell protoplasts of Arabidopsis thaliana in response to blue light. Plant Cell Physiol. 2005, 46, 955–963. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, D.; Song, T.; Xu, F.; Lin, S.; Xu, W.; Li, Q.; Zhu, Y.; Liang, J.; Zhang, J. Arabidopsis plasma membrane H+-ATPase genes AHA2 and AHA7 have distinct and overlapping roles in the modulation of root tip H+ Efflux in response to low-phosphorus stress. J. Exp. Bot. 2017, 68, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zhou, Y.; Jiang, X. Structure, function, and regulation of the plasma membrane Na+/H+ antiporter salt overly sensitive 1 in plants. Front. Plant Sci. 2022, 13, 866265. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, M.; Tanudjaja, E.; Uozumi, N. Diverse physiological functions of cation proton antiporters across bacteria and plant cells. Int. J. Mol. Sci. 2020, 21, 4566. [Google Scholar] [CrossRef]
- Keisham, M.; Mukherjee, S.; Bhatla, S. Mechanisms of sodium transport in plants—Progresses and challenges. Int. J. Mol. Sci. 2018, 19, 647. [Google Scholar] [CrossRef]
- Shi, H.; Quintero, F.J.; Pardo, J.M.; Zhu, J.-K. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na + transport in plants. Plant Cell 2002, 14, 465–477. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Sa, G.; Zhang, Y.; Deng, J.; Deng, S.; Wang, M.; Zhang, H.; Yao, J.; Ma, X.; et al. Populus euphratica J3 mediates root K+/Na+ homeostasis by activating plasma membrane H+-ATPase in transgenic Arabidopsis under NaCl salinity. Plant Cell Tissue Organ Cult. 2017, 131, 75–88. [Google Scholar] [CrossRef]
- Fan, Y.; Wan, S.; Jiang, Y.; Xia, Y.; Chen, X.; Gao, M.; Cao, Y.; Luo, Y.; Zhou, Y.; Jiang, X. Over-expression of a plasma membrane H+-ATPase SpAHA1 conferred salt tolerance to transgenic Arabidopsis. Protoplasma 2018, 255, 1827–1837. [Google Scholar] [CrossRef]
- Shi, H.; Ishitani, M.; Kim, C.; Zhu, J.-K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6896–6901. [Google Scholar] [CrossRef]
- Peelle, B.; Gururaja, T.L.; Payan, D.G.; Anderson, D.C. Characterization and use of green fluorescent proteins from Renilla mulleri and Ptilosarcus guernyi for the human cell display of functional peptides. J. Protein Chem. 2001, 20, 507–519. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- ImageJ. 2011. Available online: https://imagej.nih.gov/ij/index.html (accessed on 15 September 2021).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ageyeva, M.; Veselov, A.; Vodeneev, V.; Brilkina, A. Cell-Type-Specific Length and Cytosolic pH Response of Superficial Cells of Arabidopsis Root to Chronic Salinity. Plants 2022, 11, 3532. https://doi.org/10.3390/plants11243532
Ageyeva M, Veselov A, Vodeneev V, Brilkina A. Cell-Type-Specific Length and Cytosolic pH Response of Superficial Cells of Arabidopsis Root to Chronic Salinity. Plants. 2022; 11(24):3532. https://doi.org/10.3390/plants11243532
Chicago/Turabian StyleAgeyeva, Maria, Alexander Veselov, Vladimir Vodeneev, and Anna Brilkina. 2022. "Cell-Type-Specific Length and Cytosolic pH Response of Superficial Cells of Arabidopsis Root to Chronic Salinity" Plants 11, no. 24: 3532. https://doi.org/10.3390/plants11243532
APA StyleAgeyeva, M., Veselov, A., Vodeneev, V., & Brilkina, A. (2022). Cell-Type-Specific Length and Cytosolic pH Response of Superficial Cells of Arabidopsis Root to Chronic Salinity. Plants, 11(24), 3532. https://doi.org/10.3390/plants11243532





