Study of High-Temperature-Induced Morphological and Physiological Changes in Potato Using Nondestructive Plant Phenotyping
Abstract
:1. Introduction
2. Results
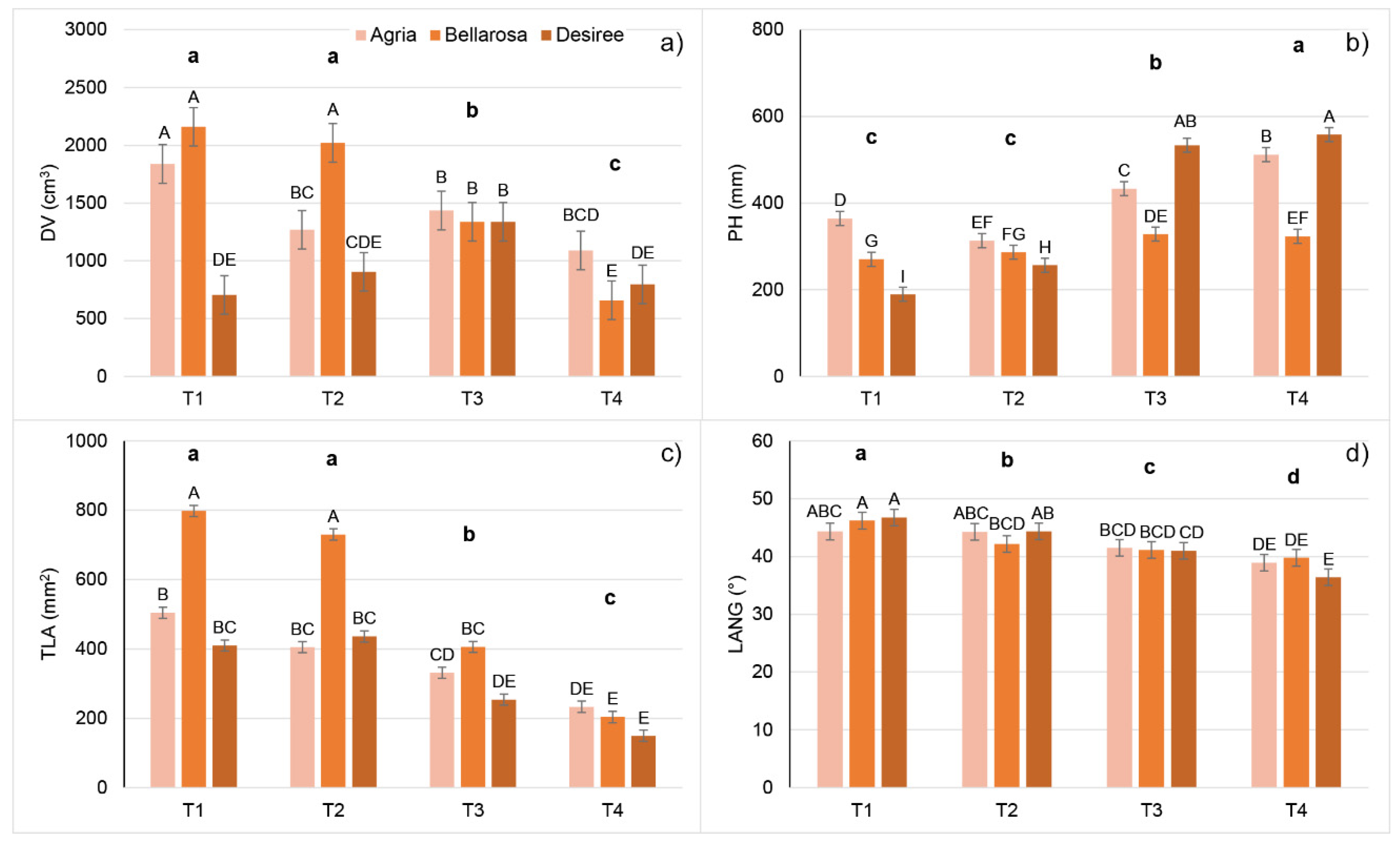
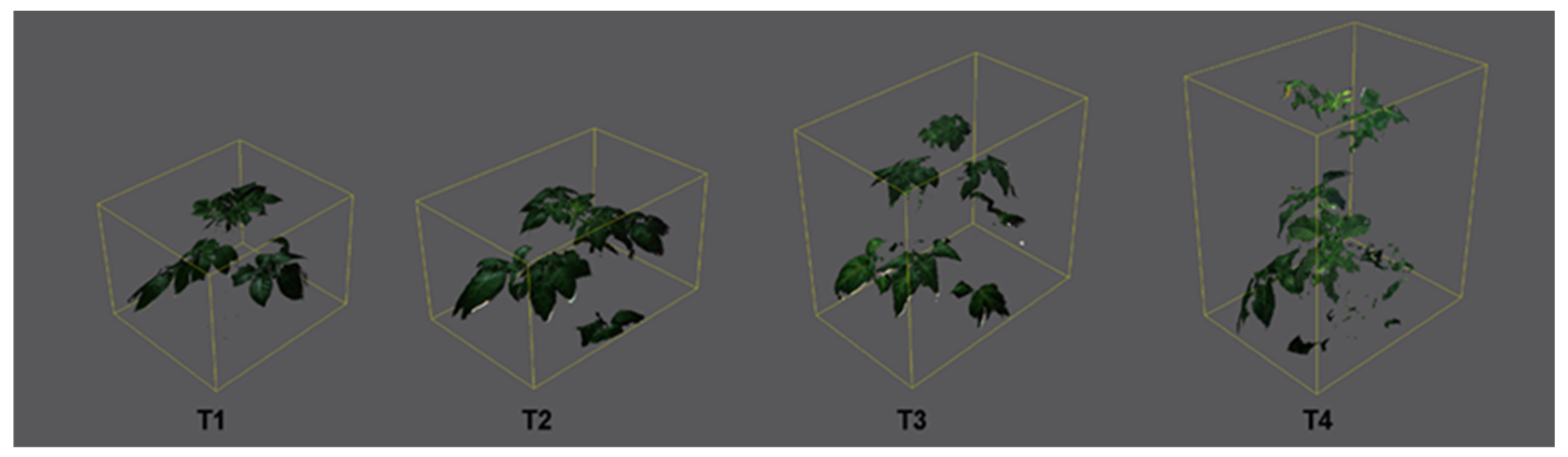
2.1. Effect of Increasing Temperatures on Potato Morphological Traits (MORPH)
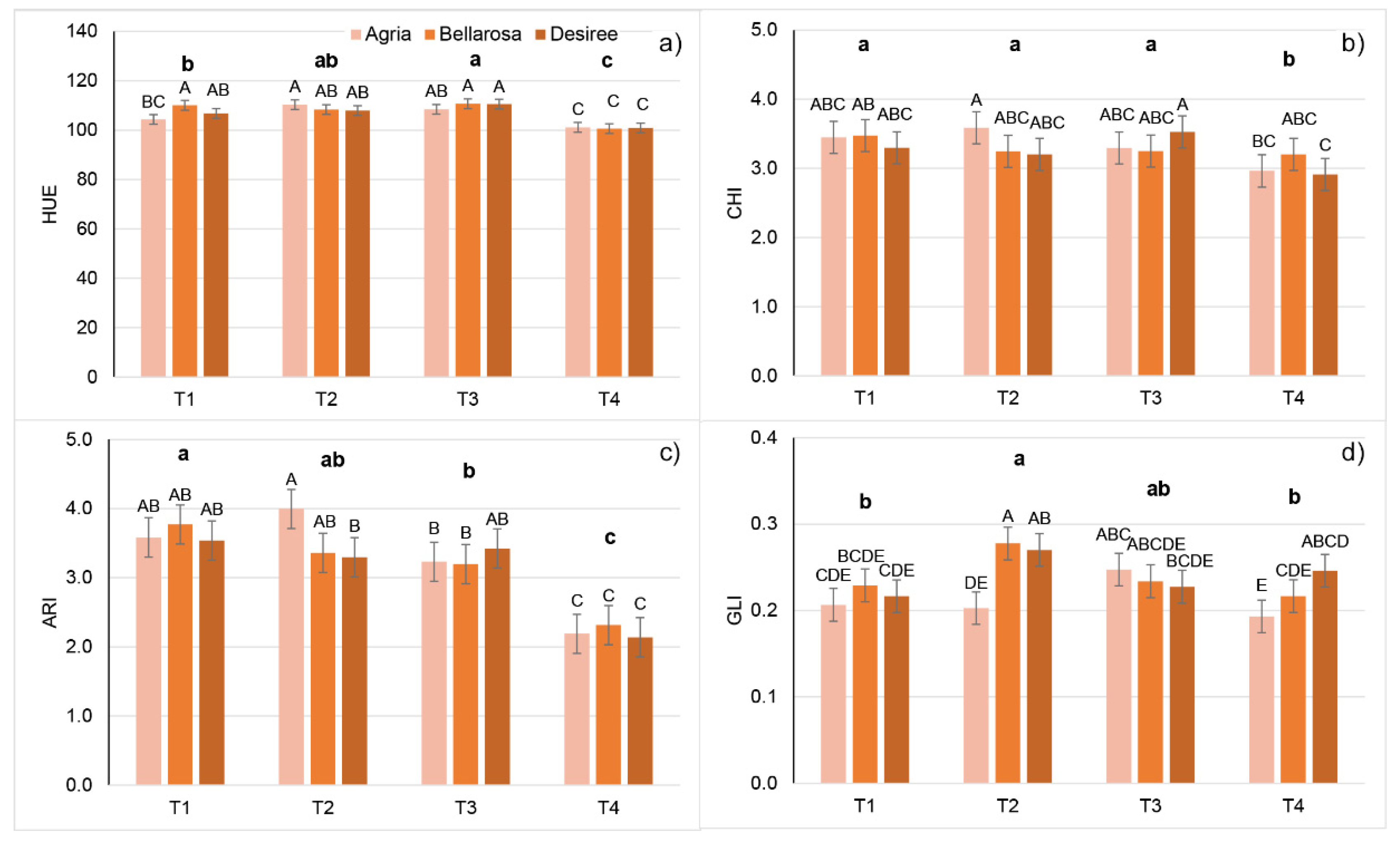
2.2. Effect of Increasing Temperatures on Potato Multispectral Traits (MSTs)
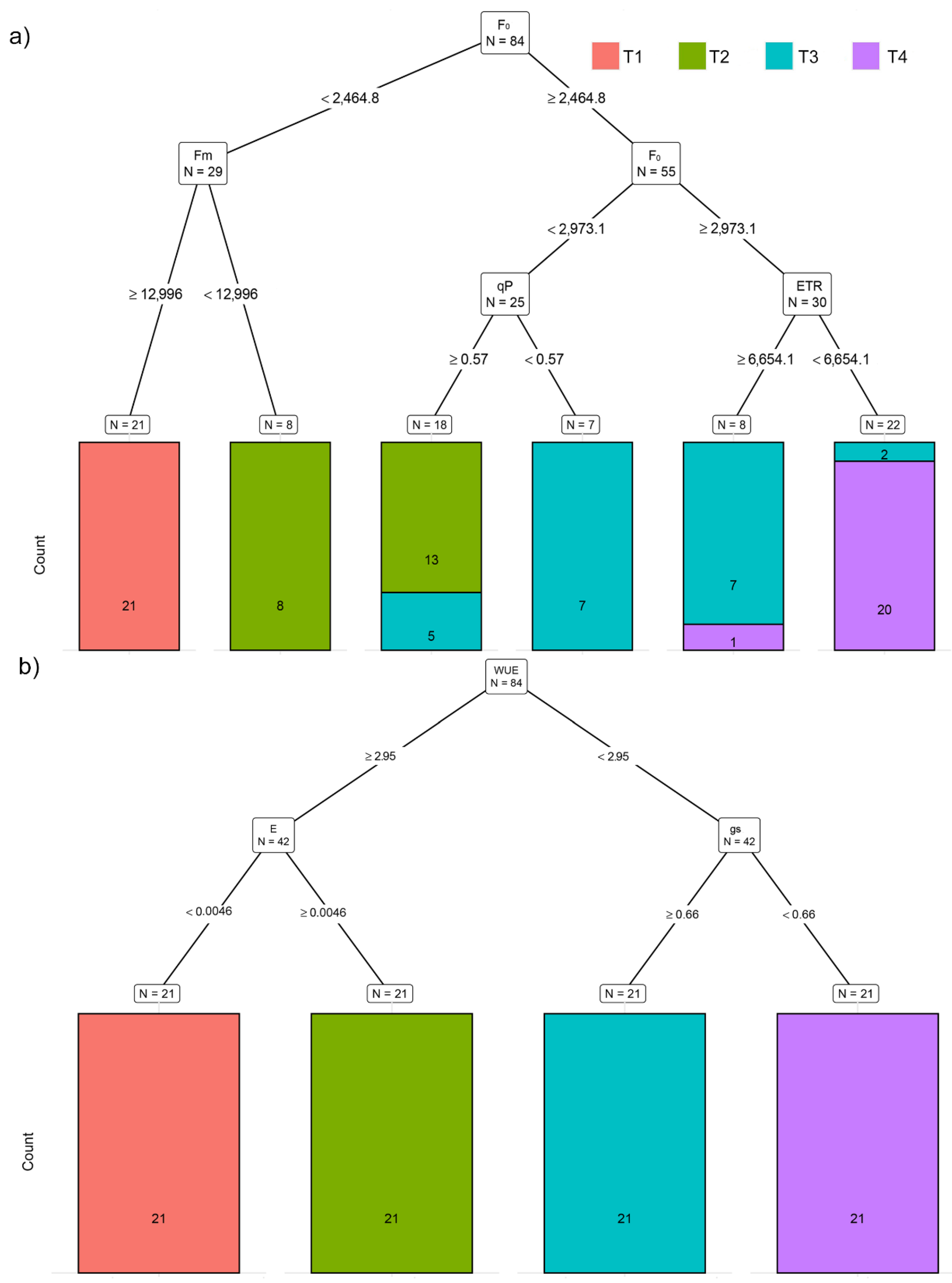
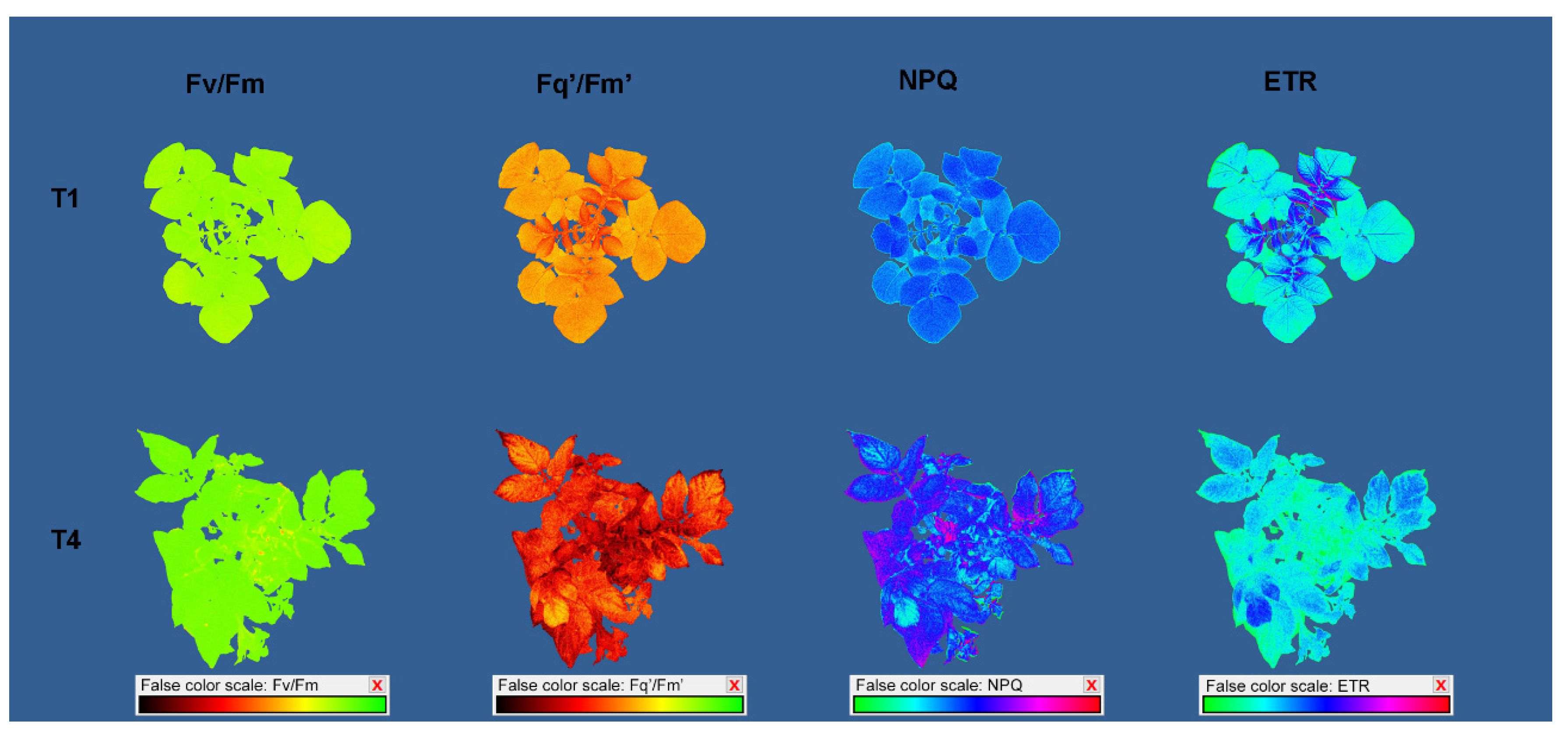
2.3. Effect of Increasing Temperatures on Potato Chlorophyll Fluorescence Traits (CFTs)
2.4. Effect of Increasing Temperatures on Potato Gas Exchange Traits (GEXTs)
3. Discussion
4. Materials and Methods
4.1. Experimental Setup and Growth Conditions
4.2. Morphological Measurements
4.3. Chlorophyll Fluorescence Measurements
- Maximum quantum yield of PSII (Fv/Fm) = (Fm − F0)/Fm [39].
- Effective quantum yield of PSII (Fq’/Fm’) = (Fm’ − Fs’)/Fm’ [39].
- Electron transport rate (ETR) = Fq’/Fm’ × PPFD × 0.5 × 0.83 [39].
- Non-photochemical quenching (NPQ) = (Fm − Fm’)/Fm’ [40].
- Coefficient of Photochemical Quenching (qP) = (Fm’ − Fs)/Fv [41].
- Coefficient of Non-photochemical Quenching (qN) = 1 − (Fm’ − F0’)/(Fm − F0) [41].
- Estimation of ‘open’ Reaction Centers based on a Lake Model (qL) = ((Fm’ − Fs’) × F0’))/((Fm’ − F0’) × Fs’)) [42].
- Quantum Yield of Non-regulated Non-Photochemical Energy Loss in PSII (ɸnq) = 1/(NPQ + 1 + qL(Fm/F0 − 1)) [43].
- Quantum yield of Regulated Non-Photochemical Energy Loss in PSII (ɸnpq) = 1 − ɸpsII − 1/(NPQ + 1 + qL(Fm/F0 − 1)) [43].
4.4. Multispectral Measurements
4.5. Gas Exchange Measurements
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. FAOSTAT. 2020. Available online: https://www.fao.org/faostat/en/#data/SCL (accessed on 23 November 2022).
- Hijmans, R.J. The Effect of Climate Change on Global Potato Production. Am. J. Potato Res. 2003, 80, 271–279. [Google Scholar] [CrossRef]
- Birch, P.R.J.; Bryan, G.; Fenton, B.; Gilroy, E.M.; Hein, I.; Jones, J.T.; Prashar, A.; Taylor, M.A.; Torrance, L.; Toth, I.K. Crops That Feed the World 8: Potato: Are the Trends of Increased Global Production Sustainable? Food Secur. 2012, 4, 477–508. [Google Scholar] [CrossRef]
- FAO. 2020. Available online: http://www.fao.org/3/ca9692en/online/ca9692en.html#chapter-1_1 (accessed on 20 September 2022).
- 2021 Tied for 6th Warmest Year in Continued Trend, NASA Analysis Shows|NASA. Available online: https://www.nasa.gov/press-release/2021-tied-for-6th-warmest-year-in-continued-trend-nasa-analysis-shows (accessed on 23 November 2022).
- IPCC. Summary for Policymakers. In Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 1–30. [Google Scholar]
- Levy, D.; Veilleux, R.E. Adaptation of Potato to High Temperatures and Salinity—A Review. Am. J. Potato Res. 2007, 84, 487–506. [Google Scholar] [CrossRef]
- Struik, P.C. Responses of the Potato Plant to the Temperature. In Potato Biology and Biotechnology; Vreugdenhil, D., Bradshaw, J., Gebhardt, C., Govers, F., Taylor, M., MacKerron, D., Ross, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 367–393. [Google Scholar]
- Fleisher, D.H.; Timlin, D. Modeling Expansion of Individual Leaves in the Potato Canopy. Agric. Meteorol. 2006, 139, 84–93. [Google Scholar] [CrossRef]
- Lee, Y.H.; Sang, W.G.; Baek, J.K.; Kim, J.H.; Shin, P.; Seo, M.C.; Cho, J.I. The Effect of Concurrent Elevation in CO2 and Temperature on the Growth, Photosynthesis, and Yield of Potato Crops. PLoS ONE 2020, 15, e0241081. [Google Scholar] [CrossRef]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature Response of Photosynthesis in C3, C4, and CAM Plants: Temperature Acclimation and Temperature Adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef]
- Prange, R.K.; Mcrae, K.B.; Midmore, D.J.; Deng, R. Reduction in Potato Growth at High Temperature: Role of Photosynthesis and Dark Respiration. Am. Potato J. 2008, 67, 357–369. [Google Scholar] [CrossRef]
- Midmore, D.J. Potato (Solanum spp.) in the Hot Tropics I. Soil Temperature Effects on Emergence, Plant Development and Yield. Field Crops Res. 1984, 8, 255–271. [Google Scholar] [CrossRef]
- Benoit, G.R.; Stanley, C.D.; Grant, W.J.; Torrey, D.B. Potato Top Growth as Influenced by Temperatures. Am. Potato J. 1983, 60, 489–501. [Google Scholar] [CrossRef]
- Ewing, E.E.; Struik, P.C. Tuber Formation in Potato: Induction, Initiation, and Growth. Hortic. Rev. (Am. Soc. Hortic. Sci.) 2010, 14, 89–198. [Google Scholar]
- Hancock, R.D.; Morris, W.L.; Ducreux, L.J.M.; Morris, J.A.; Usman, M.; Verrall, S.R.; Fuller, J.; Simpson, C.G.; Zhang, R.; Hedley, P.E.; et al. Physiological, Biochemical and Molecular Responses of the Potato (Solanum tuberosum L.) Plant to Moderately Elevated Temperature. Plant Cell Environ. 2014, 37, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Dahal, K.; Li, X.Q.; Tai, H.; Creelman, A.; Bizimungu, B. Improving Potato Stress Tolerance and Tuber Yield under a Climate Change Scenario—A Current Overview. Front. Plant Sci. 2019, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Third Synthesis Report on Technology Needs Identified by Parties Not Included in Annex I to the Convention. Draft Conclusions Proposed by the Chair|UNFCCC. Available online: https://unfccc.int/documents/7997 (accessed on 23 November 2022).
- Romero, A.P.; Alarcón, A.; Valbuena, R.I.; Galeano, C.H. Physiological Assessment of Water Stress in Potato Using Spectral Information. Front. Plant Sci. 2017, 8, 1608. [Google Scholar] [CrossRef] [PubMed]
- Prinzenberg, A.E.; Víquez-Zamora, M.; Harbinson, J.; Lindhout, P.; van Heusden, S. Chlorophyll Fluorescence Imaging Reveals Genetic Variation and Loci for a Photosynthetic Trait in Diploid Potato. Physiol. Plant. 2018, 164, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Zhang, Y.; Du, J.; Guo, X.; Wen, W.; Gu, S.; Wang, J.; Fan, J. Crop Phenomics: Current Status and Perspectives. Front. Plant Sci. 2019, 10, 714. [Google Scholar] [CrossRef] [Green Version]
- Fleisher, D.H.; Wang, Q.; Timlin, D.J.; Chun, J.A.; Reddy, V.R. Response of Potato Gas Exchange and Productivity to Phosphorus Deficiency and Carbon Dioxide Enrichment. Crop Sci. 2012, 52, 1803–1815. [Google Scholar] [CrossRef]
- Ray, S.S.; Das, G.; Singh, J.P.; Panigrahy, S. Evaluation of Hyperspectral Indices for LAI Estimation and Discrimination of Potato Crop under Different Irrigation Treatments. Int. J. Remote Sens. 2006, 27, 5373–5387. [Google Scholar] [CrossRef]
- Prashar, A.; Yildiz, J.; McNicol, J.W.; Bryan, G.J.; Jones, H.G. Infra-Red Thermography for High Throughput Field Phenotyping in Solanum Tuberosum. PLoS ONE 2013, 8, e65816. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Xu, X.; Han, J.; Zhang, L.; Bian, C.; Jin, L.; Liu, J. The Estimation of Crop Emergence in Potatoes by UAV RGB Imagery. Plant Methods 2019, 15, 15. [Google Scholar] [CrossRef] [Green Version]
- de Jesus Colwell, F.; Souter, J.; Bryan, G.J.; Compton, L.J.; Boonham, N.; Prashar, A. Development and Validation of Methodology for Estimating Potato Canopy Structure for Field Crop Phenotyping and Improved Breeding. Front. Plant Sci. 2021, 12, 612843. [Google Scholar] [CrossRef]
- Tang, R.; Niu, S.; Zhang, G.; Chen, G.; Haroon, M.; Yang, Q.; Rajora, O.P.; Li, X.-Q.; Niu, S.; Yang, Q.; et al. Physiological and Growth Responses of Potato Cultivars to Heat Stress. Botany 2018, 96, 897–912. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I. Visible and Near-Infrared Reflectance Techniques for Diagnosing Plant Physiological Status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Solovchenko, A.E.; Pogosyan, S.I. Application of Reflectance Spectroscopy for Analysis of Higher Plant Pigments. Russ. J. Plant Physiol. 2003, 50, 704–710. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, G.; Lee, S.; Zhu, J.Y.; Paik, I.; Nguyen, T.T.; Kim, J.; Oh, E. High Ambient Temperature Represses Anthocyanin Biosynthesis through Degradation of HY5. Front. Plant Sci. 2017, 8, 1787. [Google Scholar] [CrossRef] [Green Version]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Havaux, M. Rapid Photosynthetic Adaptation to Heat Stress Triggered in Potato Leaves by Moderately Elevated Temperatures. Plant Cell Environ. 1993, 16, 461–467. [Google Scholar] [CrossRef]
- Evers, D.; Lefevre, I.; Legay, S.; Lamoureux, D.; Hausman, J.F.; Rosales, R.O.G.; Marca, L.R.T.; Hoffmann, L.; Bonierbale, M.; Schafleitner, R. Identification of drought-responsive compounds in potato through a combined transcriptomic and targeted metabolite approach. J. Exp. Bot. 2010, 61, 2327–2343. [Google Scholar] [CrossRef] [Green Version]
- Rykaczewska, K. The effect of high temperature occurring in subsequent stages of plant development on potato yield and tuber physiological defects. Am. J. Potato Res. 2015, 92, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [Green Version]
- Lazarević, B.; Šatović, Z.; Nimac, A.; Vidak, M.; Gunjača, J.; Politeo, O.; Carović-Stanko, K. Application of Phenotyping Methods in Detection of Drought and Salinity Stress in Basil (Ocimum basilicum L.). Front Plant Sci 2021, 12, 629441. [Google Scholar] [CrossRef] [PubMed]
- Brestic, M.; Zivcak, M. PSII Fluorescence Techniques for Measurement of Drought and High Temperature Stress Signal in Crop Plants: Protocols and Applications. In Molecular Stress Physiology of Plants; Rout, G.R., Das, A.B., Eds.; Springer: Delhi, India, 2013; pp. 87–131. ISBN 978-81-322-0807-5. [Google Scholar]
- Genty, B.; Briantais, J.M.; Baker, N.R. The Relationship between the Quantum Yield of Photosynthetic Electron Transport and Quenching of Chlorophyll Fluorescence. Biochim. Biophys. Acta (BBA) Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the Xanthophyll Cycle in Photoprotection Elucidated by Measurements of Light-Induced Absorbance Changes, Fluorescence and Photosynthesis in Leaves of Hedera Canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous Recording of Photochemical and Non-Photochemical Chlorophyll Fluorescence Quenching with a New Type of Modulation Fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New Fluorescence Parameters for the Determination of QA Redox State and Excitation Energy Fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Genty, B.; Harbinson, J.; Cailly, A.L.; Rizza, F. Fate of excitation at PS II in leaves: The non-photochemical side. In Proceedings of the Third BBSRC Robert Hill Symposium on Photosynthesis, University of Sheffield, Department of Molecular Biology and Biotechnology, Western Bank, Sheffield, UK, 31 March–3 April 1996. Abstract no. P28. [Google Scholar]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS; Goddard Space Flight Center 3d ERTS-1 Symp.; NASA: Washington, DC, USA, 1973; Volume 1, Sect. A; pp. 309–317. [Google Scholar]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between Leaf Chlorophyll Content and Spectral Reflectance and Algorithms for Nondestructive Chlorophyll Assessment in Higher Plant Leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical Properties and Nondestructive Estimation of Anthocyanin Content in Plant Leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Gobron, N.; Pinty, B.; Verstraete, M.M.; Widlowski, J.L. Advanced Vegetation Indices Optimized for Up-Coming Sensors: Design, Performance, and Applications. IEEE Trans. Geosci. Remote Sens. 2000, 38, 2489–2505. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Taylor & Francis: Wadsworth, OH, USA, 1983; pp. 246–280. [Google Scholar]
- Therneau, T.M.; Atkinson, E.J. rpart: Recursive Partitioning and Regression Trees; An Introduction to Recursive Partitioning Using the RPART Routines; R package Version 4.1.16. 2022. Available online: https://CRAN.R-project.org/package=rpart (accessed on 5 September 2022).




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarević, B.; Carović-Stanko, K.; Safner, T.; Poljak, M. Study of High-Temperature-Induced Morphological and Physiological Changes in Potato Using Nondestructive Plant Phenotyping. Plants 2022, 11, 3534. https://doi.org/10.3390/plants11243534
Lazarević B, Carović-Stanko K, Safner T, Poljak M. Study of High-Temperature-Induced Morphological and Physiological Changes in Potato Using Nondestructive Plant Phenotyping. Plants. 2022; 11(24):3534. https://doi.org/10.3390/plants11243534
Chicago/Turabian StyleLazarević, Boris, Klaudija Carović-Stanko, Toni Safner, and Milan Poljak. 2022. "Study of High-Temperature-Induced Morphological and Physiological Changes in Potato Using Nondestructive Plant Phenotyping" Plants 11, no. 24: 3534. https://doi.org/10.3390/plants11243534
APA StyleLazarević, B., Carović-Stanko, K., Safner, T., & Poljak, M. (2022). Study of High-Temperature-Induced Morphological and Physiological Changes in Potato Using Nondestructive Plant Phenotyping. Plants, 11(24), 3534. https://doi.org/10.3390/plants11243534







