Climate and Land-Cover Change Impacts and Extinction Risk Assessment of Rare and Threatened Endemic Taxa of Chelmos-Vouraikos National Park (Peloponnese, Greece)
Abstract
1. Introduction
2. Results
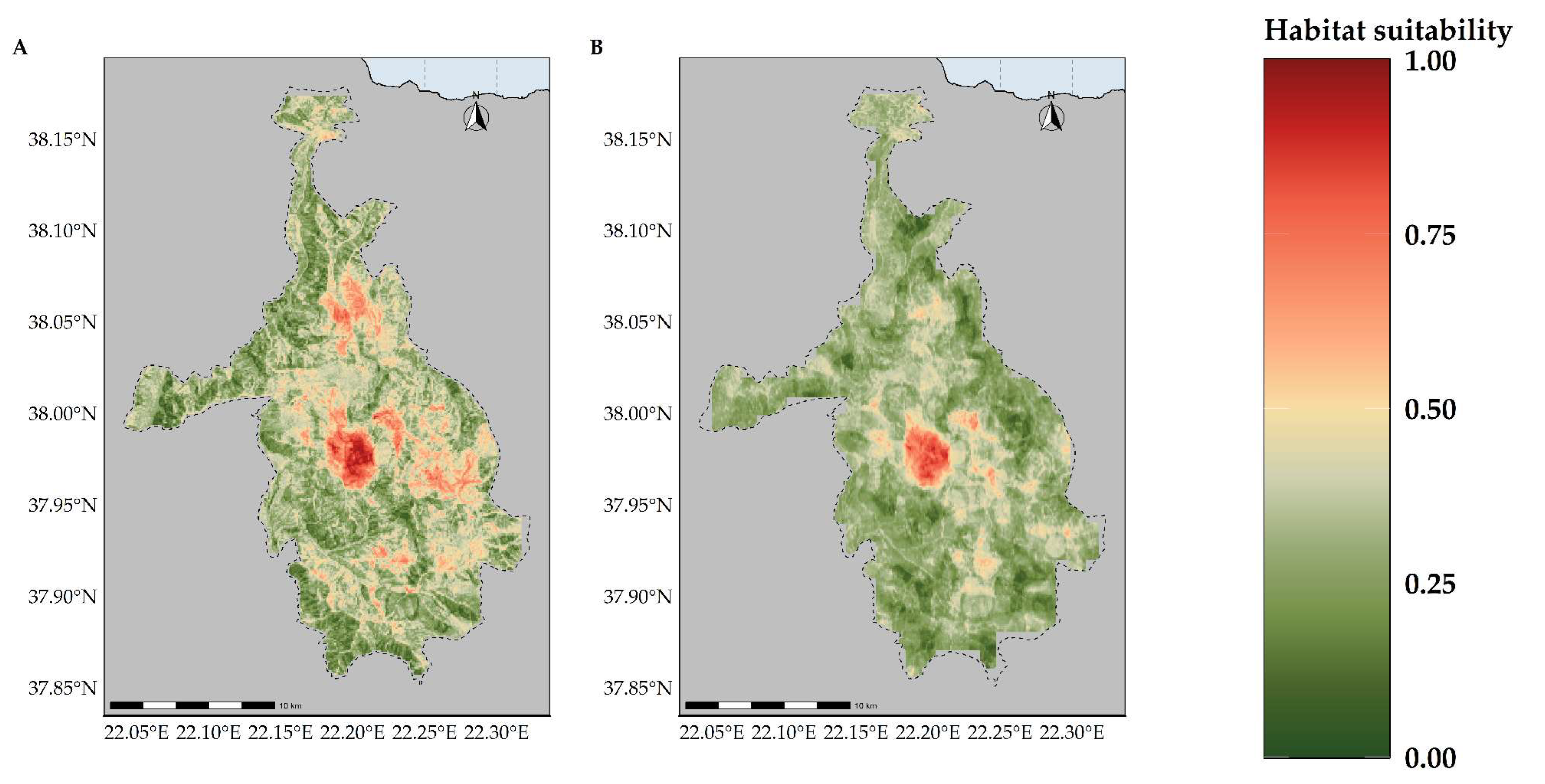
2.1. Species Distribution Models
2.2. Land Use and Land Cover Changes
2.3. Habitat Suitability Range Change
2.4. IUCN Extinction Risk Assessment
3. Discussion
4. Materials and Methods
4.1. Species Occurrence Data
4.2. Environmental Data
4.3. Land Use and Land Cover Changes
4.4. Species Distribution Models
4.5. Future IUCN Extinction Risk Assessment
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC) Report. United Nations Climate Change Widespread, Rapid, and Intensifying. Available online: https://www.ipcc.ch/2021/08/09/ar6-wg1-20210809-pr/ (accessed on 9 August 2021).
- le Roux, J.J.; Hui, C.; Castillo, M.L.; Iriondo, J.M.; Keet, J.H.; Khapugin, A.A.; Médail, F.; Rejmánek, M.; Theron, G.; Yannelli, F.A.; et al. Recent Anthropogenic Plant Extinctions Differ in Biodiversity Hotspots and Coldspots. Curr. Biol. 2019, 29, 2912–2918.e2. [Google Scholar] [CrossRef] [PubMed]
- Powers, R.P.; Jetz, W. Global Habitat Loss and Extinction Risk of Terrestrial Vertebrates under Future Land-Use-Change Scenarios. Nat. Clim. Chang. 2019, 9, 323–329. [Google Scholar] [CrossRef]
- Li, D.; Olden, J.D.; Lockwood, J.L.; Record, S.; McKinney, M.L.; Baiser, B. Changes in Taxonomic and Phylogenetic Diversity in the Anthropocene. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200777. [Google Scholar] [CrossRef] [PubMed]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Trigas, P.; Strid, A.; Dimopoulos, P. Spatial Phylogenetics, Biogeographical Patterns and Conservation Implications of the Endemic Flora of Crete (Aegean, Greece) under Climate Change Scenarios. Biology 2020, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W.; Lavergne, S.; Roquet, C.; Boulangeat, I.; Lafourcade, B.; Araujo, M.B. Consequences of Climate Change on the Tree of Life in Europe. Nature 2011, 470, 531–534. [Google Scholar] [CrossRef]
- Cronk, Q. Plant Extinctions Take Time. Science 2016, 353, 446–447. [Google Scholar] [CrossRef]
- Nic Lughadha, E.; Bachman, S.P.; Leão, T.C.C.; Forest, F.; Halley, J.M.; Moat, J.; Acedo, C.; Bacon, K.L.; Brewer, R.F.A.; Gâteblé, G.; et al. Extinction Risk and Threats to Plants and Fungi. Plants People Planet 2020, 2, 389–408. [Google Scholar] [CrossRef]
- Bongaarts, J. IPBES, 2019. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Popul. Dev. Rev. 2019, 45, 680–681. [Google Scholar] [CrossRef]
- Oliver, T.H.; Morecroft, M.D. Interactions between Climate Change and Land Use Change on Biodiversity: Attribution Problems, Risks, and Opportunities. WIREs Clim. Chang. 2014, 5, 317–335. [Google Scholar] [CrossRef]
- Cuttelod, A.; García, N.; Abdul Malak, D.; Temple, H.; Katariya, V. The Mediterranean: A Biodiversity Hotspot under Threat. In The 2008 Review of the IUCN Red List of Threatened Species; Vié, J.-C., Hilton-Taylor, C., Stuart, S.N., Eds.; IUCN: Gland, Switzerland, 2008. [Google Scholar]
- Médail, F.; Quézel, P. Hot-Spots Analysis for Conservation of Plant Biodiversity in the Mediterranean Basin. Ann. Mo. Bot. Gard. 1997, 84, 112. [Google Scholar] [CrossRef]
- Thompson, J.D. Plant Evolution in the Mediterranean: Insights for Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Myers, N.; Mittermeier, R.; Mittermeier, C.; da Fonseca, G.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Brooks, T.M.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Rylands, A.B.; Konstant, W.R.; Flick, P.; Pilgrim, J.; Oldfield, S.; Magin, G.; et al. Habitat Loss and Extinction in the Hotspots of Biodiversity. Conserv. Biol. 2002, 16, 909–923. [Google Scholar] [CrossRef]
- Cao, Y.; Tseng, T.-H.; Wang, F.; Jacobson, A.; Yu, L.; Zhao, J.; Carver, S.; Locke, H.; Zhao, Z.; Yang, R. Potential Wilderness Loss Could Undermine the Post-2020 Global Biodiversity Framework. Biol. Conserv. 2022, 275, 109753. [Google Scholar] [CrossRef]
- Pressey, R.L.; Mills, M.; Weeks, R.; Day, J.C. The Plan of the Day: Managing the Dynamic Transition from Regional Conservation Designs to Local Conservation Actions. Biol. Conserv. 2013, 166, 155–169. [Google Scholar] [CrossRef]
- Pfab, M.F.; Victor, J.E.; Armstrong, A.J. Application of the IUCN Red Listing System to Setting Species Targets for Conservation Planning Purposes. Biodivers. Conserv. 2011, 20, 1001–1012. [Google Scholar] [CrossRef]
- Canturk, U.; Kulaç, Ş. The Effects of Climate Change Scenarios on Tilia Ssp. in Turkey. Environ. Monit. Assess. 2021, 193, 1–15. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction Risk Assessment of the Greek Endemic Flora. Biology 2021, 10, 195. [Google Scholar] [CrossRef]
- Eliades, N.G.H.; Andreou, M.; Laguna, E.; Kounnamas, C.; Georghiou, K.; Costantinou, C.; Kouzali, I.; Thanos, C.A.; Kadis, C. Integrated Conservation of Important Plant Taxa through the Improvement of the Original Plant Micro-Reserve (PMR) Approach: The Intensive PMR Monitoring Case of Ophrys Kotschyi. J. Environ. Manag. 2021, 280, 111731. [Google Scholar] [CrossRef]
- Médail, F. The Specific Vulnerability of Plant Biodiversity and Vegetation on Mediterranean Islands in the Face of Global Change. Reg. Environ. Chang. 2017, 17, 1775–1790. [Google Scholar] [CrossRef]
- Pacifici, M.; Foden, W.B.; Visconti, P.; Watson, J.E.M.; Butchart, S.H.M.; Kovacs, K.M.; Scheffers, B.R.; Hole, D.G.; Martin, T.G.; Akçakaya, H.R.; et al. Assessing Species Vulnerability to Climate Change. Nat. Clim. Chang. 2015, 5, 215–224. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Kallimanis, A.; Strid, A.; Dimopoulos, P. Plant Endemism Centres and Biodiversity Hotspots in Greece. Biology 2021, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Charitonidou, M.; Kougioumoutzis, K.; Halley, J.M. An Orchid in Retrograde: Climate-Driven Range Shift Patterns of Ophrys helenae in Greece. Plants 2021, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Fassou, G.; Kougioumoutzis, K.; Iatrou, G.; Trigas, P.; Papasotiropoulos, V. Genetic Diversity and Range Dynamics of Helleborus odorus subsp. cyclophyllus under Different Climate Change Scenarios. Forests 2020, 11, 620. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kaloveloni, A.; Petanidou, T. Assessing Climate Change Impacts on Island Bees: The Aegean Archipelago. Biology 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Kougioumoutzis, K.; Kotsakiozi, P.; Stathi, E.; Trigas, P.; Parmakelis, A. Conservation Genetics of Four Critically Endangered Greek Endemic Plants: A Preliminary Assessment. Diversity 2021, 13, 152. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Papanikolaou, A.; Kokkoris, I.P.; Strid, A.; Dimopoulos, P.; Panitsa, M. Climate Change Impacts and Extinction Risk Assessment of Nepeta Representatives (Lamiaceae) in Greece. Sustainability 2022, 14, 4269. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Strid, A.; Raus, T.; Dimopoulos, P. Climate-Change Impacts on the Southernmost Mediterranean Arctic-Alpine Plant Populations. Sustainability 2021, 13, 13778. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Trigas, P.; Strid, A.; Dimopoulos, P. Plant Diversity Patterns and Conservation Implications under Climate-Change Scenarios in the Mediterranean: The Case of Crete (Aegean, Greece). Diversity 2020, 12, 270. [Google Scholar] [CrossRef]
- Stathi, E.; Kougioumoutzis, K.; Abraham, E.M.; Trigas, P.; Ganopoulos, I.; Avramidou, E.V.; Tani, E. Population Genetic Variability and Distribution of the Endangered Greek Endemic Cicer Graecum under Climate Change Scenarios. AoB Plants 2021, 12, plaa007. [Google Scholar] [CrossRef]
- Minachilis, K.; Kougioumoutzis, K.; Petanidou, T. Climate Change Effects on Multi-Taxa Pollinator Diversity and Distribution along the Elevation Gradient of Mount Olympus, Greece. Ecol. Indic. 2021, 132, 108335. [Google Scholar] [CrossRef]
- Fyllas, N.M.; Koufaki, T.; Sazeides, C.I.; Spyroglou, G.; Theodorou, K. Potential Impacts of Climate Change on the Habitat Suitability of the Dominant Tree Species in Greece. Plants 2022, 11, 1616. [Google Scholar] [CrossRef] [PubMed]
- Tsakiri, M.; Koumoutsou, E.; Kokkoris, I.P.; Trigas, P.; Iliadou, E.; Tzanoudakis, D.; Dimopoulos, P.; Iatrou, G. National Park and Unesco Global Geopark of Chelmos-Vouraikos (Greece): Floristic Diversity, Ecosystem Services and Management Implications. Land 2022, 11, 33. [Google Scholar] [CrossRef]
- Solomou, A.D.; Karetsos, G.; Trigas, P.; Proutsos, N.; Avramidou, E.V.; Korakaki, E.; Kougioumoutzis, K.; Goula, A.; Pavlidis, G.; Stamouli, S.; et al. Vascular Plants of Oiti and Parnassos National Parks of Greece, as Important Components of Biodiversity and Touring Experiences. In CEUR Workshop Proceedings of the 9th International Conference on Information and Communication Technologies in Agriculture, Food & Environment (HAICTA), Thessaloniki, Greece, 24–27 September 2020; Volume 2761, pp. 542–548. [Google Scholar]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Scheffers, B.R.; de Meester, L.; Bridge, T.C.L.; Hoffmann, A.A.; Pandolfi, J.M.; Corlett, R.T.; Butchart, S.H.M.; Pearce-Kelly, P.; Kovacs, K.M.; Dudgeon, D.; et al. The Broad Footprint of Climate Change from Genes to Biomes to People. Science 2016, 354, aaf7671. [Google Scholar] [CrossRef] [PubMed]
- Fois, M.; Bacchetta, G.; Cogoni, D.; Fenu, G. Current and Future Effectiveness of the Natura 2000 Network for Protecting Plant Species in Sardinia: A Nice and Complex Strategy in Its Raw State? J. Environ. Plan. Manag. 2018, 61, 332–347. [Google Scholar] [CrossRef]
- Fois, M.; Cuena-Lombraña, A.; Fenu, G.; Bacchetta, G. Using Species Distribution Models at Local Scale to Guide the Search of Poorly Known Species: Review, Methodological Issues and Future Directions. Ecol. Model. 2018, 385, 124–132. [Google Scholar] [CrossRef]
- Stévart, T.; Dauby, G.; Lowry, P.; Blach-Overgaard, A.; Droissart, V.; Harris, D.J.; Mackinder, A.B.; Schatz, G.E.; Sonké, B.; Sosef, M.S.M.; et al. A Third of the Tropical African Flora Is Potentially Threatened with Extinction. Sci. Adv. 2019, 5, eaax9444. [Google Scholar] [CrossRef]
- Burrows, M.T.; Schoeman, D.S.; Richardson, A.J.; Molinos, J.G.; Hoffmann, A.; Buckley, L.B.; Moore, P.J.; Brown, C.J.; Bruno, J.F.; Duarte, C.M.; et al. Geographical Limits to Species-Range Shifts Are Suggested by Climate Velocity. Nature 2014, 507, 492–495. [Google Scholar] [CrossRef]
- Erfanian, M.B.; Sagharyan, M.; Memariani, F.; Ejtehadi, H. Predicting Range Shifts of Three Endangered Endemic Plants of the Khorassan-Kopet Dagh Floristic Province under Global Change. Sci. Rep. 2021, 11, 9159. [Google Scholar] [CrossRef]
- Elsen, P.R.; Monahan, W.B.; Dougherty, E.R.; Merenlender, A.M. Keeping Pace with Climate Change in Global Terrestrial Protected Areas. Sci. Adv. 2020, 6, eaay0814. [Google Scholar] [CrossRef]
- Watson, J.E.M.; Dudley, N.; Segan, D.B.; Hockings, M. The Performance and Potential of Protected Areas. Nature 2014, 515, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Michalak, J.L.; Lawler, J.J.; Gross, J.E.; Agne, M.C.; Emmet, R.L.; Hsu, H.-W.; Griffey, V. Climate-Change Vulnerability Assessments of Natural Resources in U.S. National Parks. Conserv. Sci. Pract. 2022, 4, e12703. [Google Scholar] [CrossRef]
- Dube, K.; Nhamo, G. Evidence and Impact of Climate Change on South African National Parks. Potential Implications for Tourism in the Kruger National Park. Environ. Dev. 2020, 33, 100485. [Google Scholar] [CrossRef]
- Abe, H.; Mitsui, S.; Yamano, H. Conservation of the Coral Community and Local Stakeholders’ Perceptions of Climate Change Impacts: Examples and Gap Analysis in Three Japanese National Parks. Ocean Coast. Manag. 2022, 218, 106042. [Google Scholar] [CrossRef]
- Wiens, J.A.; Seavy, N.E.; Jongsomjit, D. Protected Areas in Climate Space: What Will the Future Bring? Biol. Conserv. 2011, 144, 2119–2125. [Google Scholar] [CrossRef]
- Tan, T.; Iatrou, G. Endemic Plants of Greece the Peloponnese; Gads Forlag: Copenhagen, Denmark, 2001; Volume 28. [Google Scholar]
- Tsakiri, M.; Kokkoris, I.P.; Trigas, P.; Tzanoudakis, D.; Iatrou, G. Contribution to the Vascular Flora of Chelmos-Vouraikos National Park (N Peloponnese, Greece). Phytol. Balc. 2020, 26, 523–536. [Google Scholar]
- Ma, Y.; Chen, G.; Edward Grumbine, R.; Dao, Z.; Sun, W.; Guo, H. Conserving Plant Species with Extremely Small Populations (PSESP) in China. Biodivers. Conserv. 2013, 22, 803–809. [Google Scholar] [CrossRef]
- Laguna, E. The Micro-Reserves as a Tool for Conservation of Threatened Plants in Europe; Nature and Environment 121; Council of Europe Publishing: Strasbourg, France, 2001. [Google Scholar]
- Laguna, E.; Fos, S.; Jiménez, J.; Volis, S. Role of Micro-Reserves in Conservation of Endemic, Rare and Endangered Plants of the Valencian Region (Eastern Spain). Isr. J. Plant Sci. 2016, 63, 320–332. [Google Scholar] [CrossRef]
- Fos, S.; Laguna, E.; Jiménez, J.; Gómez-Serrano, M.Á. Plant Micro-Reserves in Valencia (E. Spain): A Model to Preserve Threatened Flora in China? Plant Divers. 2017, 39, 383–389. [Google Scholar] [CrossRef]
- Fenu, G.; Bacchetta, G.; Christodoulou, C.S.; Cogoni, D.; Fournaraki, C.; del Galdo Gian Pietro, G.; Gotsiou, P.; Kyratzis, A.; Piazza, C.; Vicens, M.; et al. A Common Approach to the Conservation of Threatened Island Vascular Plants: First Results in the Mediterranean Basin. Diversity 2020, 12, 157. [Google Scholar] [CrossRef]
- Cogoni, D.; Fenu, G.; Dessì, C.; Deidda, A.; Giotta, C.; Piccitto, M.; Bacchetta, G. Importance of Plants with Extremely Small Populations (Psesps) in Endemic-Rich Areas, Elements Often Forgotten in Conservation Strategies. Plants 2021, 10, 1504. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, G.; Fenu, G.; Mattana, E. A Checklist of the Exclusive Vascular Flora of Sardinia with Priority Rankings for Conservation. An. Del Jard. Bot. De Madr. 2012, 69, 81–89. [Google Scholar] [CrossRef]
- Gauthier, P.; Debussche, M.; Thompson, J.D. Regional Priority Setting for Rare Species Based on a Method Combining Three Criteria. Biol. Conserv. 2010, 143, 1501–1509. [Google Scholar] [CrossRef]
- Schatz, B.; Gauthier, P.; Debussche, M.; Thompson, J.D. A Decision Tool for Listing Species for Protection on Different Geographic Scales and Administrative Levels. J. Nat. Conserv. 2014, 22, 75–83. [Google Scholar] [CrossRef]
- Laguna, E.; Deltoro, V.I.; Pèrez-Botella, J.; Pèrez-Rovira, P.; Serra, L.; Olivares, A.; Fabregat, C. The Role of Small Reserves in Plant Conservation in a Region of High Diversity in Eastern Spain. Biol. Conserv. 2004, 119, 421–426. [Google Scholar] [CrossRef]
- Laguna, E. Origin, Concept and Evolution of Plant MicroReserves: The Pilot Network of the Valencian Community (Spain). In A Pilot Network of Small Protected Sites for Conservation of Rare Plants in Bulgaria; Vladimirov, V., Ed.; IBER—BAS & MoEW: Sofia, Bulgaria, 2014; pp. 14–23. [Google Scholar]
- Laguna, E.; Thanos, C.; Fournaraki, C.; Kadis, C.; Bou Dagher Kharrat, M. Plant Micro-Reserves in the Mediterranean Area. In Conserving Wild Plants in the South and East Mediterranean Region; Valderrabano, M., Gil, T., Heywood, V., de Montmollin, B., Eds.; IUCN: Gland, Switzerland, 2018; pp. 106–107. [Google Scholar]
- Cadis, C.; Thanos, K.; Laguna, E. Plant Micro-Reserves: From Theory to Practise. Experiences Gained from EU LIFE and Other Related Projects; UTOPIA Publishing: Athens, Greece, 2013. [Google Scholar]
- Saldaña, A.; Amich, F.; Fernández-González, P.E.; Rico, E. Plant Micro-Reserves in Castilla y Leόn (Spain). In Plant Micro-Reserves: From Theory to Practice. Experiences Gained from EU LIFE and Other Related Projects; Kadis, C., Thanos, C.A., Laguna Lumbreras, E., Eds.; Utopia Publishing: Athens, Greece, 2013; pp. 97–100. [Google Scholar]
- Rubio, M.Á. Micro-Reserves: A Useful Tool for the Conservation Strategy of the Natural Environment in Castilla-La Mancha (Spain). In Plant Micro-Reserves: From Theory to Practice. Experiences Gained from EU LIFE and Other Related Projects; Kadis, C., Thanos, C.A., Laguna Lumbreras, E., Eds.; Utopia Publishing: Athens, Greece, 2013; pp. 79–82. [Google Scholar]
- Thanos, C.A.; Fournaraki, C.; Georghiou, K.; Dimopoulos, P. PMRs in Western Crete. In Plant Micro-Reserves: From Theory to Practice. Experiences Gained from EU LIFE and Other Related Projects. PlantNet CY Project Beneficiaries; Kadis, C., Thanos, C.A., Laguna Lumbreras, E., Eds.; Utopia Publishing: Athens, Greece, 2013; pp. 27–36. [Google Scholar]
- Thanos, C.A.; Fournaraki, C.; Georghiou, K.; Dimopoulos, P.; Bergmeier, E. The Establishment, Monitoring and Management of a Pilot Network of Micro-Reserves in Western Crete for the Conservation of European Threatened Plants (CRETAPLANT Project, EU-LIFE). In Proceedings of the MEDECOS XI, the International Mediterranean Ecosystems Conference, Perth, WA, Australia, 2–5 September 2007; pp. 249–250. [Google Scholar]
- Goranova, V.; Pee, D. The Project ‘A Pilot Network of Small Protected Sites for Plant Species in Bulgaria Using the Plant Micro-Reserve Model’. In A Pilot Network of Small Protected Sites for Conservation of Rare Plants in Bulgaria; Vladimirov, V., Ed.; IBER—BAS & MoEW: Sofia, Bulgaria, 2014; pp. 25–30. [Google Scholar]
- Bancheva, S.; Goranova, V.; Pedashenko, H.; Vladimirov, V. Bulgarian National Network of Small Protected Sites: “Plant Micro-Reserves”. In A Pilot Network of Small Protected Sites for Conservation of Rare Plants in Bulgaria; Vladimirov, V., Ed.; IBER—BAS & MoEW: Sofia, Bulgaria, 2014. [Google Scholar]
- Fos Martín, S.; Laguna Lumbreras, E.; Jiménez Pérez, J. Plant Micro-Reserves in the Valencian Region (E of Spain): Are We Achieving the Expected Results? Passive Conservation of Relevant Vascular Plant Species. Flora Mediterr. 2014, 24, 153–162. [Google Scholar] [CrossRef]
- Carrión, M.A.; García, J.; Guerra, J.; Sánchez-Gómez, P. Plant Micro-Reserves in the Region of Murcia. In Plant Micro-Reserves: From Theory to Practice. Experiences Gained from EU LIFE and Other Related Projects; Kadis, C., Thanos, C.A., Laguna Lumbreras, E., Eds.; Utopia Publishing: Athens, Greece, 2013; pp. 101–104. [Google Scholar]
- Natcheva, R.; Bancheva, S.; Vladimirov, V.; Goranova, V. A Pilot Network of Small Protected Sites for Plant Species in Bulgaria Using the Plant Micro-Reserve Model. In Plant Micro-Reserves: From Theory to Practice. Experiences Gained from EU LIFE and Other Related Projects; Kadis, C., Thanos, C.A., Laguna Lumbreras, E., Eds.; Utopia Publishing: Athens, Greece, 2013; pp. 53–64. [Google Scholar]
- Troia, A. Proposals of Plant Micro-Reserves in Sicily (Italy). In Plant Micro-Reserves: From Theory to Practice. Experiences Gained from EU LIFE and Other Related Projects; Kadis, C., Thanos, C.A., Laguna Lumbreras, E., Eds.; Utopia Publishing: Athens, Greece, 2013; pp. 83–85. [Google Scholar]
- Santos, M.J.; Smith, A.B.; Dekker, S.C.; Eppinga, M.B.; Leitão, P.J.; Moreno-Mateos, D.; Morueta-Holme, N.; Ruggeri, M. The Role of Land Use and Land Cover Change in Climate Change Vulnerability Assessments of Biodiversity: A Systematic Review. Landsc. Ecol. 2021, 36, 3367–3382. [Google Scholar] [CrossRef]
- Foden, W.B.; Butchart, S.H.M.; Stuart, S.N.; Vié, J.C.; Akçakaya, H.R.; Angulo, A.; DeVantier, L.M.; Gutsche, A.; Turak, E.; Cao, L.; et al. Identifying the World’s Most Climate Change Vulnerable Species: A Systematic Trait-Based Assessment of All Birds, Amphibians and Corals. PLoS ONE 2013, 8, e65427. [Google Scholar] [CrossRef]
- Lannuzel, G.; Balmot, J.; Dubos, N.; Thibault, M.; Fogliani, B. High-Resolution Topographic Variables Accurately Predict the Distribution of Rare Plant Species for Conservation Area Selection in a Narrow-Endemism Hotspot in New Caledonia. Biodivers. Conserv. 2021, 30, 963–990. [Google Scholar] [CrossRef]
- Meineri, E.; Hylander, K. Fine-Grain, Large-Domain Climate Models Based on Climate Station and Comprehensive Topographic Information Improve Microrefugia Detection. Ecography 2017, 40, 1003–1013. [Google Scholar] [CrossRef]
- Tomlinson, S.; Lewandrowski, W.; Elliott, C.P.; Miller, B.P.; Turner, S.R. High-Resolution Distribution Modeling of a Threatened Short-Range Endemic Plant Informed by Edaphic Factors. Ecol. Evol. 2020, 10, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting Pseudo-Absences for Species Distribution Models: How, Where and How Many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Sirami, C.; Caplat, P.; Popy, S.; Clamens, A.; Arlettaz, R.; Jiguet, F.; Brotons, L.; Martin, J.L. Impacts of Global Change on Species Distributions: Obstacles and Solutions to Integrate Climate and Land Use. Glob. Ecol. Biogeogr. 2017, 26, 385–394. [Google Scholar] [CrossRef]
- Martin, Y.; van Dyck, H.; Dendoncker, N.; Titeux, N. Testing Instead of Assuming the Importance of Land Use Change Scenarios to Model Species Distributions under Climate Change. Glob. Ecol. Biogeogr. 2013, 22, 1204–1216. [Google Scholar] [CrossRef]
- Larson, D.W.; Matthes, U.; Kelly, P. Cliff Ecology: Pattern and Process in Cliff Ecosystems; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Dullinger, S.; Willner, W.; Plutzar, C.; Englisch, T.; Schratt-Ehrendorfer, L.; Moser, D.; Ertl, S.; Essl, F.; Niklfeld, H. Post-Glacial Migration Lag Restricts Range Filling of Plants in the European Alps. Glob. Ecol. Biogeogr. 2012, 21, 829–840. [Google Scholar] [CrossRef]
- Abolmaali, S.M.R.; Tarkesh, M.; Bashari, H. MaxEnt Modeling for Predicting Suitable Habitats and Identifying the Effects of Climate Change on a Threatened Species, Daphne Mucronata, in Central Iran. Ecol. Inform. 2018, 43, 116–123. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.; Wang, H. Potential Geographical Distribution of Populus Euphratica in China under Future Climate Change Scenarios Based on Maxent Model. Shengtai Xuebao 2020, 40, 6552–6563. [Google Scholar] [CrossRef]
- Han, L.; Zuo, Y.; He, X.; Hou, Y.; Li, M.; Li, B. Plant Identity and Soil Variables Shift the Colonisation and Species Composition of Dark Septate Endophytes Associated with Medicinal Plants in a Northern Farmland in China. Appl. Soil Ecol. 2021, 167, 104042. [Google Scholar] [CrossRef]
- Feng, L.; Sun, J.; Wang, T.; Tian, X.; Wang, W.; Guo, J.; Feng, H.; Guo, H.; Deng, H.; Wang, G. Predicting suitable habitats of Ginkgo biloba L. fruit forests in China. Clim. Risk Manag. 2021, 34, 100364. [Google Scholar] [CrossRef]
- Lenoir, J.; Svenning, J.C. Climate-Related Range Shifts—A Global Multidimensional Synthesis and New Research Directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Steinbauer, M.J.; Grytnes, J.A.; Jurasinski, G.; Kulonen, A.; Lenoir, J.; Pauli, H.; Rixen, C.; Winkler, M.; Bardy-Durchhalter, M.; Barni, E.; et al. Accelerated Increase in Plant Species Richness on Mountain Summits Is Linked to Warming. Nature 2018, 556, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.M.; Diez, J.M.; Levine, J.M. Novel Competitors Shape Species’ Responses to Climate Change. Nature 2015, 525, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Panitsa, M.; Kokkoris, I.P.; Kougioumoutzis, K.; Kontopanou, A.; Bazos, I.; Strid, A.; Dimopoulos, P. Linking Taxonomic, Phylogenetic and Functional Plant Diversity with Ecosystem Services of Cliffs and Screes in Greece. Plants 2021, 10, 992. [Google Scholar] [CrossRef] [PubMed]
- Cotto, O.; Wessely, J.; Georges, D.; Klonner, G.; Schmid, M.; Dullinger, S.; Thuiller, W.; Guillaume, F. A Dynamic Eco-Evolutionary Model Predicts Slow Response of Alpine Plants to Climate Warming. Nat. Commun. 2017, 8, 15399. [Google Scholar] [CrossRef] [PubMed]
- di Nuzzo, L.; Vallese, C.; Benesperi, R.; Giordani, P.; Chiarucci, A.; di Cecco, V.; di Martino, L.; di Musciano, M.; Gheza, G.; Lelli, C.; et al. Contrasting Multitaxon Responses to Climate Change in Mediterranean Mountains. Sci. Rep. 2021, 11, 4438. [Google Scholar] [CrossRef]
- Rota, F.; Casazza, G.; Genova, G.; Midolo, G.; Prosser, F.; Bertolli, A.; Wilhalm, T.; Nascimbene, J.; Wellstein, C. Topography of the Dolomites Modulates Range Dynamics of Narrow Endemic Plants under Climate Change. Sci. Rep. 2022, 12, 1398. [Google Scholar] [CrossRef]
- Newbold, T.; Oppenheimer, P.; Etard, A.; Williams, J.J. Tropical and Mediterranean Biodiversity Is Disproportionately Sensitive to Land-Use and Climate Change. Nat. Ecol. Evol. 2020, 4, 1630–1638. [Google Scholar] [CrossRef]
- Kiziridis, D.A.; Mastrogianni, A.; Pleniou, M.; Karadimou, E.; Tsiftsis, S.; Xystrakis, F.; Tsiripidis, I. Acceleration and Relocation of Abandonment in a Mediterranean Mountainous Landscape: Drivers, Consequences, and Management Implications. Land 2022, 11, 406. [Google Scholar] [CrossRef]
- Navarro, L.M.; Pereira, H.M. Rewilding Abandoned Landscapes in Europe. Ecosystems 2012, 15, 900–912. [Google Scholar] [CrossRef]
- Plieninger, T.; Hui, C.; Gaertner, M.; Huntsinger, L. The Impact of Land Abandonment on Species Richness and Abundance in the Mediterranean Basin: A Meta-Analysis. PLoS ONE 2014, 9, e98355. [Google Scholar] [CrossRef]
- Médail, F.; Baumel, A. Using Phylogeography to Define Conservation Priorities: The Case of Narrow Endemic Plants in the Mediterranean Basin Hotspot. Biol. Conserv. 2018, 224, 258–266. [Google Scholar] [CrossRef]
- Chevin, L.M.; Lande, R.; Mace, G.M. Adaptation, Plasticity, and Extinction in a Changing Environment: Towards a Predictive Theory. PLoS Biol. 2010, 8, e1000357. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant Phenotypic Plasticity in a Changing Climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Silcock, J.L.; Simmons, C.L.; Monks, L.; Dillon, R.; Reiter, N.; Jusaitis, M.; Vesk, P.A.; Byrne, M.; Coates, D.J. Threatened Plant Translocation in Australia: A Review. Biol. Conserv. 2019, 236, 211–222. [Google Scholar] [CrossRef]
- Ferretti-Gallon, K.; Griggs, E.; Shrestha, A.; Wang, G. National Parks Best Practices: Lessons from a Century’s Worth of National Parks Management. Int. J. Geoheritage Park. 2021, 9, 335–346. [Google Scholar] [CrossRef]
- Robertson, M.P.; Visser, V.; Hui, C. Biogeo: An R Package for Assessing and Improving Data Quality of Occurrence Record Datasets. Ecography 2016, 39, 394–401. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. SpThin: An R Package for Spatial Thinning of Species Occurrence Records for Use in Ecological Niche Models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- van Proosdij, A.S.J.; Sosef, M.S.M.; Wieringa, J.J.; Raes, N. Minimum Required Number of Specimen Records to Develop Accurate Species Distribution Models. Ecography 2016, 39, 542–552. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Title, P.O.; Bemmels, J.B. ENVIREM: An Expanded Set of Bioclimatic and Topographic Variables Increases Flexibility and Improves Performance of Ecological Niche Modeling. Ecography 2018, 41, 291–307. [Google Scholar] [CrossRef]
- Jarvis, A.; Reuter, H.I.; Nelson, A.; Guevara, E. Hole-Filled SRTM for the Globe Version 4. Available from the CGIAR-CSI SRTM 90 m Database. 2008, 15, p. 5. Available online: http://srtm.csi.cgiar.org (accessed on 8 December 2021).
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling. R Package Version 1.1-4. Cran 2017. Available online: http://CRAN.R-project.org/package=dismo (accessed on 8 December 2021).
- Marchi, M.; Castellanos-Acuña, D.; Hamann, A.; Wang, T.; Ray, D.; Menzel, A. ClimateEU, Scale-Free Climate Normals, Historical Time Series, and Future Projections for Europe. Sci. Data 2020, 7, 428. [Google Scholar] [CrossRef] [PubMed]
- Hamann, A.; Wang, T.; Spittlehouse, D.L.; Murdock, T.Q. A Comprehensive, High-Resolution Database of Historical and Projected Climate Surfaces for Western North America. Bull. Am. Meteorol. Soc. 2013, 94, 1307–1309. [Google Scholar] [CrossRef]
- Wang, T.; Hamann, A.; Spittlehouse, D.L.; Murdock, T.Q. ClimateWNA-High-Resolution Spatial Climate Data for Western North America. J. Appl. Meteorol. Climatol. 2012, 51, 16–29. [Google Scholar] [CrossRef]
- Hengl, T.; de Jesus, J.M.; Heuvelink, G.B.M.; Gonzalez, M.R.; Kilibarda, M.; Blagotić, A.; Shangguan, W.; Wright, M.N.; Geng, X.; Bauer-Marschallinger, B.; et al. SoilGrids250m: Global Gridded Soil Information Based on Machine Learning. PLoS ONE 2017, 12, e0169748. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Liu, X. Global Land Projection Based on Plant Functional Types with a 1-km Resolution under Socio-Climatic Scenarios. Sci. Data 2022, 9, 1–18. [Google Scholar]
- Hijmans, R.J. Package Raster: Geographic Data Analysis and Modeling with Raster Data: R package, Version 3.1-5 2020. 3.1. CRAN Repos. R Core Team: Vienna, Austria, 2020. Available online: http://CRAN.R-project.org/package=raster (accessed on 8 December 2021).
- Evans, J.S. SpatialEco R Package Version 1.2-0; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Cao, Y.; Wang, F.; Tseng, T.H.; Carver, S.; Chen, X.; Zhao, J.; Yu, L.; Li, F.; Zhao, Z.; Yang, R. Identifying Ecosystem Service Value and Potential Loss of Wilderness Areas in China to Support Post-2020 Global Biodiversity Conservation. Sci. Total Environ. 2022, 846, 157348. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where Is Positional Uncertainty a Problem for Species Distribution Modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Exavier, R.; Zeilhofer, P. OpenLand: Software for Quantitative Analysis and Visualization of Land Use and Cover Change. R J. 2020, 12, 359. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The Art of Modelling Range-Shifting Species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Breiner, F.T.; Nobis, M.P.; Bergamini, A.; Guisan, A. Optimizing Ensembles of Small Models for Predicting the Distribution of Species with Few Occurrences. Methods Ecol. Evol. 2018, 9, 802–808. [Google Scholar] [CrossRef]
- Breiner, F.T.; Guisan, A.; Nobis, M.P.; Bergamini, A. Including Environmental Niche Information to Improve IUCN Red List Assessments. Divers. Distrib. 2017, 23, 484–495. [Google Scholar] [CrossRef]
- Breiner, F.T.; Guisan, A.; Bergamini, A.; Nobis, M.P. Overcoming Limitations of Modelling Rare Species by Using Ensembles of Small Models. Methods Ecol. Evol. 2015, 6, 1210–1218. [Google Scholar] [CrossRef]
- Broennimann, O.; di Cola, V.; Petitpierre, B.; Breiner, F.; Scherrer, D.; D’Amen, M.; Randin, C.; Engler, R.; Hordijk, W.; Mod, H.; et al. Ecospat: Spatial Ecology Miscellaneous Methods. Package “Ecospat”. R Package Version 2018, 30, 2; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Liu, C.; White, M.; Newell, G. Selecting Thresholds for the Prediction of Species Occurrence with Presence-Only Data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Hirzel, A.H.; le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the Ability of Habitat Suitability Models to Predict Species Presences. Ecol. Model. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A Review of Methods for the Assessment of Prediction Errors in Conservation Presence/Absence Models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Sofaer, H.R.; Hoeting, J.A.; Jarnevich, C.S. The Area under the Precision-Recall Curve as a Performance Metric for Rare Binary Events. Methods Ecol. Evol. 2019, 10, 565–577. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Measuring and Comparing the Accuracy of Species Distribution Models with Presence-Absence Data. Ecography 2011, 34, 232–243. [Google Scholar] [CrossRef]
- Raes, N.; ter Steege, H. A Null-Model for Significance Testing of Presence-Only Species Distribution Models. Ecography 2007, 30, 727–736. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting Thresholds of Occurrence in the Prediction of Species Distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the Selection of Thresholds for Predicting Species Occurrence with Presence-Only Data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Aruajo, M.B. BIOMOD–A Platform for ensemble forecasting for species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Vittoz, P.; Engler, R. Seed Dispersal Distances: A Typology Based on Dispersal Modes and Plant Traits. Bot. Helv. 2007, 117, 109–124. [Google Scholar] [CrossRef]
- Brown, J.L.; Carnaval, A.C. A Tale of Two Niches: Methods, Concepts, and Evolution. Front. Biogeogr. 2019, 11. [Google Scholar] [CrossRef]
- Mannocci, L.; Roberts, J.J.; Halpin, P.N.; Authier, M.; Boisseau, O.; Bradai, M.N.; Canãdas, A.; Chicote, C.; David, L.; Di-Méglio, N.; et al. Assessing Cetacean Surveys throughout the Mediterranean Sea: A Gap Analysis in Environmental Space. Sci. Rep. 2018, 8, 3126. [Google Scholar] [CrossRef]
- Bouchet, P.J.; Miller, D.L.; Roberts, J.J.; Mannocci, L.; Harris, C.M.; Thomas, L. Dsmextra: Extrapolation Assessment Tools for Density Surface Models. Methods Ecol. Evol. 2020, 11, 1464–1469. [Google Scholar] [CrossRef]
- Dauby, G.; Stévart, T.; Droissart, V.; Cosiaux, A.; Deblauwe, V.; Simo-Droissart, M.; Sosef, M.S.M.; Lowry, P.P.; Schatz, G.E.; Gereau, R.E.; et al. ConR: An R Package to Assist Large-Scale Multispecies Preliminary Conservation Assessments Using Distribution Data. Ecol. Evol. 2017, 7, 11292–11303. [Google Scholar] [CrossRef]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kougioumoutzis, K.; Trigas, P.; Tsakiri, M.; Kokkoris, I.P.; Koumoutsou, E.; Dimopoulos, P.; Tzanoudakis, D.; Iatrou, G.; Panitsa, M. Climate and Land-Cover Change Impacts and Extinction Risk Assessment of Rare and Threatened Endemic Taxa of Chelmos-Vouraikos National Park (Peloponnese, Greece). Plants 2022, 11, 3548. https://doi.org/10.3390/plants11243548
Kougioumoutzis K, Trigas P, Tsakiri M, Kokkoris IP, Koumoutsou E, Dimopoulos P, Tzanoudakis D, Iatrou G, Panitsa M. Climate and Land-Cover Change Impacts and Extinction Risk Assessment of Rare and Threatened Endemic Taxa of Chelmos-Vouraikos National Park (Peloponnese, Greece). Plants. 2022; 11(24):3548. https://doi.org/10.3390/plants11243548
Chicago/Turabian StyleKougioumoutzis, Konstantinos, Panayiotis Trigas, Maria Tsakiri, Ioannis P. Kokkoris, Eleni Koumoutsou, Panayotis Dimopoulos, Dimitris Tzanoudakis, Gregoris Iatrou, and Maria Panitsa. 2022. "Climate and Land-Cover Change Impacts and Extinction Risk Assessment of Rare and Threatened Endemic Taxa of Chelmos-Vouraikos National Park (Peloponnese, Greece)" Plants 11, no. 24: 3548. https://doi.org/10.3390/plants11243548
APA StyleKougioumoutzis, K., Trigas, P., Tsakiri, M., Kokkoris, I. P., Koumoutsou, E., Dimopoulos, P., Tzanoudakis, D., Iatrou, G., & Panitsa, M. (2022). Climate and Land-Cover Change Impacts and Extinction Risk Assessment of Rare and Threatened Endemic Taxa of Chelmos-Vouraikos National Park (Peloponnese, Greece). Plants, 11(24), 3548. https://doi.org/10.3390/plants11243548








