Addition of Medicinal Plants Increases Antioxidant Activity, Color, and Anthocyanin Stability of Black Chokeberry (Aronia melanocarpa) Functional Beverages
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Herb Addition on Chemical Composition, Color Intensity, and Antioxidant Activity of Black Chokeberry Nectar
2.2. Changes in Chemical Composition, Color Intensity, and Antioxidant Activity of Black Chokeberry Nectar, without and with Herbs, during Pasteurization and Storage
2.2.1. Changes in Anthocyanin Content and Polyphenol Constituents of Black Chokeberry Nectar, without and with Herbs, during Pasteurization and Storage
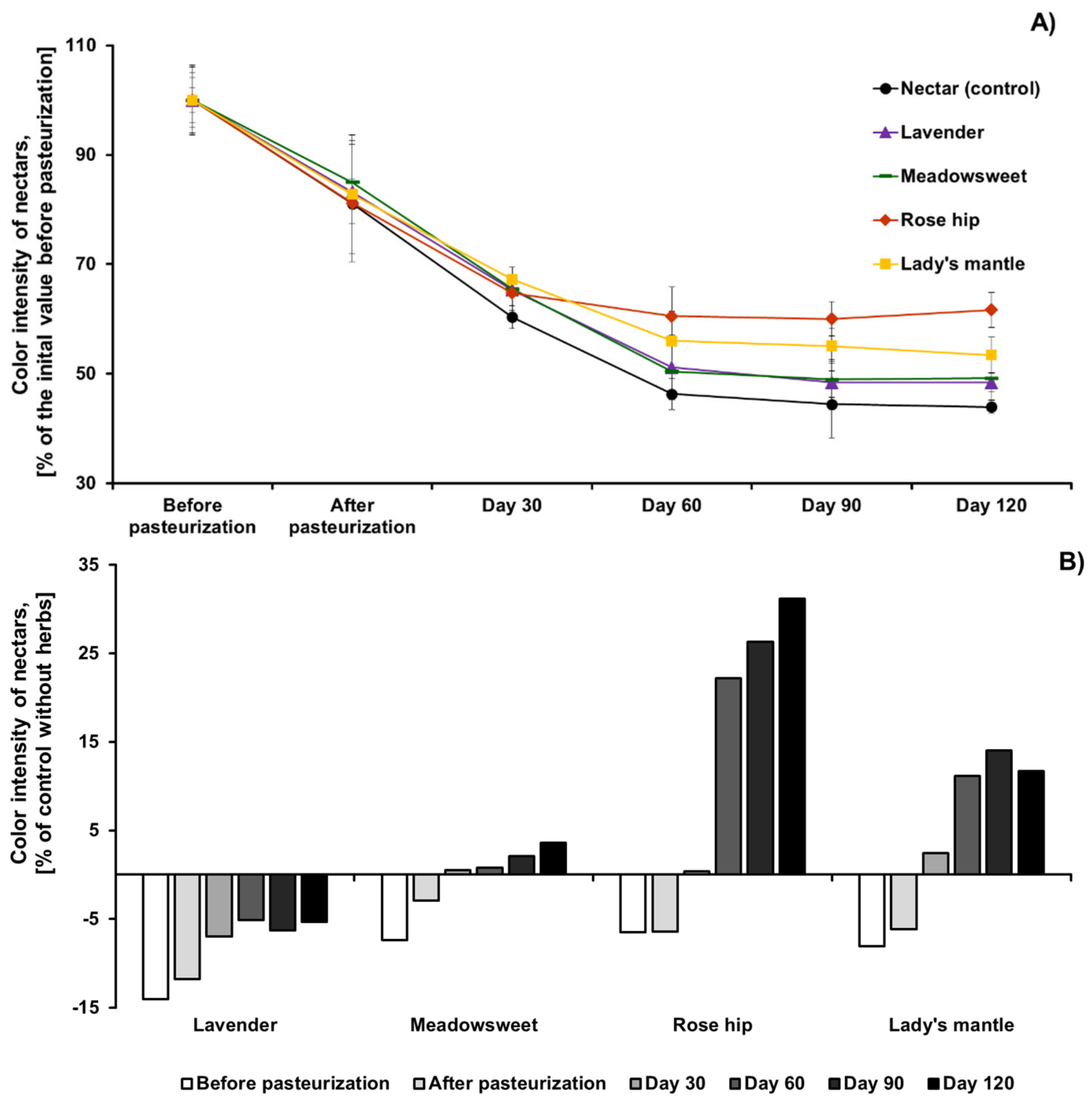
2.2.2. Changes in Color Intensity of Black Chokeberry Nectar, without and with Herbs, during Pasteurization and Storage
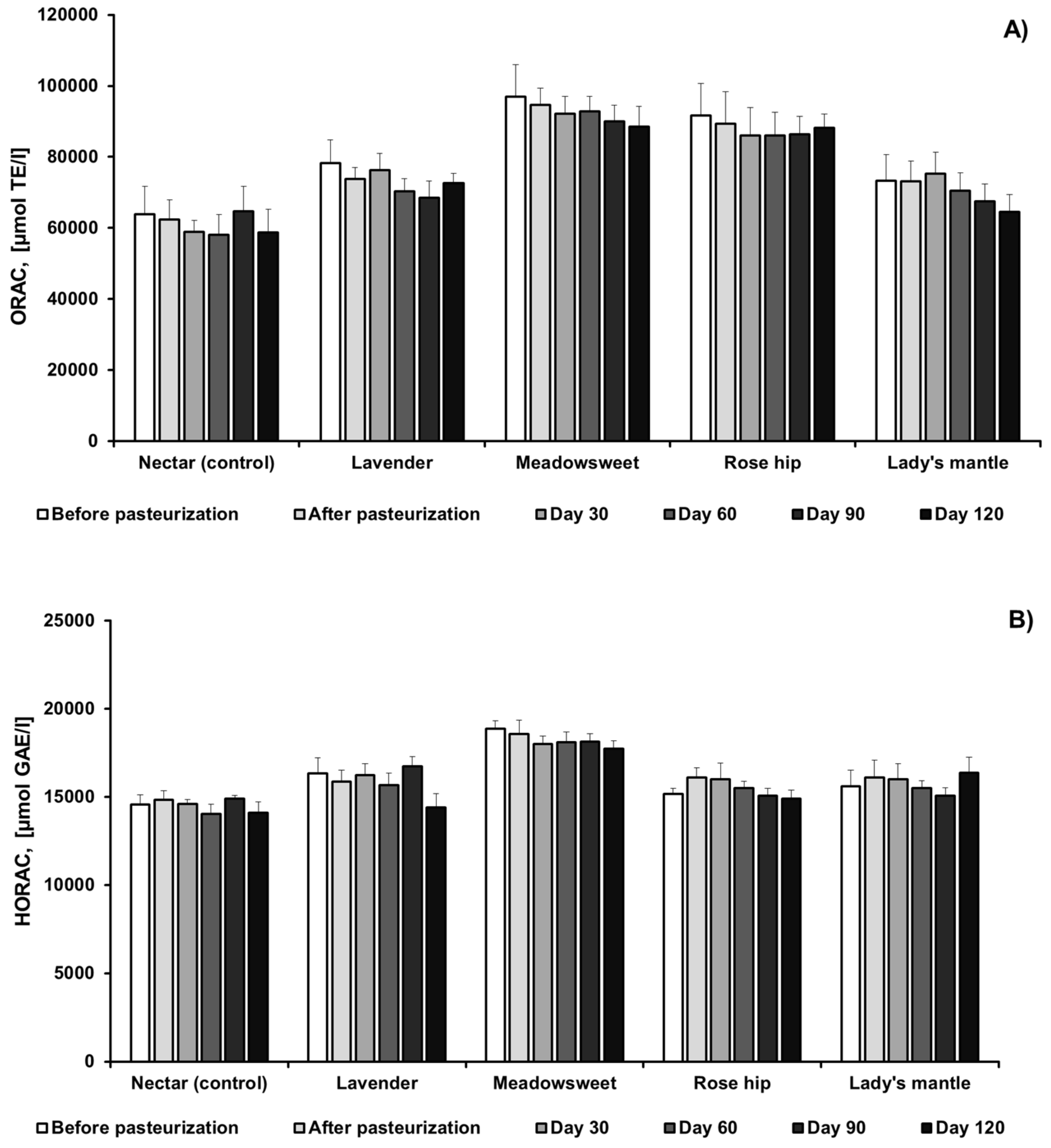
2.2.3. Changes in Antioxidant Activity of Black Chokeberry Nectar, without and with Herbs, during Pasteurization and Storage
3. Materials and Methods
3.1. Chemicals
3.2. Plant Materials
3.3. Preparation of Beverages
3.4. Pasteurization and Storage of Black Chokeberry Nectars with or without Herb Addition
3.5. Color Evaluation
3.6. Total Polyphenol Content Analysis
3.7. HPLC Determination of Anthocyanins
3.8. HPLC Analysis of Phenolic Compounds
3.9. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.10. Hydroxyl Radical Averting Capacity (HORAC) Assay
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sidor, A.; Gramza-Michałowska, A. Black chokeberry Aronia melanocarpa L. a qualitative composition, phenolic profile and antioxidant potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denev, P.; Kratchanov, C.; Ciz, M.; Lojek, A.; Kratchanova, M. Bioavailability and antioxidant activity of black chokeberry (Aronia melanocarpa) polyphenols: In vitro and in vivo evidences and possible mechanisms of action. A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 471–489. [Google Scholar] [CrossRef]
- Denev, P.; Číž, M.; Kratchanova, M.; Blazheva, D. Black chokeberry (Aronia melanocarpa) polyphenols reveal different antioxidant, antimicrobial and neutrophil-modulating activities. Food Chem. 2019, 284, 108–117. [Google Scholar] [CrossRef]
- Bushmeleva, K.; Vyshtakalyuk, A.; Terenzhev, D.; Belov, T.; Parfenov, A.; Sharonova, N.; Nikitin, E.; Zobov, V. Radical scavenging actions and immunomodulatory activity of Aronia melanocarpa propylene glycol extracts. Plants 2021, 10, 2458. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Wiloch, M.; Zawada, K.; Cyplik, W.; Kujawski, W. Evaluation of antioxidant and anti-inflammatory activity of anthocyanin-rich water-soluble aronia dry extracts. Molecules 2020, 25, 4055. [Google Scholar] [CrossRef]
- Daskalova, E.; Delchev, S.; Topolov, M.; Dimitrova, S.; Uzunova, Y.; Valcheva-Kuzmanova, S.; Kratchanova, M.; Vladimirova-Kitova, L.; Denev, P. Aronia melanocarpa (Michx.) Elliot fruit juice reveals neuroprotective effect and improves cognitive and locomotor functions of aged rats. Food Chem. Toxicol. 2019, 132, 110674. [Google Scholar] [CrossRef] [PubMed]
- Daskalova, E.; Delchev, S.; Vladimirova-Kitova, L.; Kitov, S.; Denev, P. Black chokeberry (Aronia melanocarpa) functional beverages increase HDL-cholesterol levels in aging rats. Foods 2021, 10, 1641. [Google Scholar] [CrossRef]
- Gajic, D.; Saksida, T.; Koprivica, I.; Vujicic, M.; Despotovic, S.; Savikin, K.; Jankovi, T.; Stojanovic, I. Chokeberry (Aronia melanocarpa) fruit extract modulates immune response in vivo and in vitro. J. Funct. Foods 2020, 66, 2–9. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Belcheva, A. Current knowledge of Aronia melanocarpa as a medicinal plant. Folia Med. 2006, 48, 11–17. [Google Scholar]
- Dorneanu, R.; Cioanca, O.; Chifiriuc, O.; Albu, E.; Tuchiluş, C.; Mircea, C.; Salamon, I.; Hăncianu, M. Synergic benefits of Aronia melanocarpa anthocyanin-rich extracts and antibiotics used for urinary tract infections. Farmacia 2017, 65, 778–783. [Google Scholar]
- Spinardi, A.; Cola, G.; Gardana, C.; Mignani, I. Variation of anthocyanin content and profile throughout fruit development and ripening of highbush blueberry cultivars grown at two different altitudes. Front. Plant Sci. 2019, 19, 3–5. [Google Scholar] [CrossRef]
- Fan, R.; Sun, Q.; Zeng, J.; Zhang, X. Contribution of anthocyanin pathways to fruit flesh coloration in pitayas. Plant Biol. 2020, 20, 361. [Google Scholar] [CrossRef]
- Ponder, A.; Hallmann, E.; Kwolek, M.; Srednicka-Tober, D.; Kazimierczak, R. Genetic differentiation in anthocyanin content among berry fruits. Mol. Biol. 2021, 43, 4. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.H.; Connolly, B.A.; Levine, L.H.; Richards, J.T.; Shine, S.M.; Spencer, L.E. Anthocyanins, total phenolics, ORAC and moisture content of wild and cultivated dark-fruited Aronia species. Sci. Hortic. 2017, 224, 332–342. [Google Scholar] [CrossRef]
- Sadilova, E.; Stintzing, F.C.; Carle, R. Thermal degradation of acylated and nonacylated anthocyanins. J. Food Sci. 2006, 71, 504–512. [Google Scholar] [CrossRef]
- Rhim, J.W. Kinetics of thermal degradation of anthocyanin pigment solutions driven from red flower cabbage. Food Sci. Biotechnol. 2002, 11, 361–364. [Google Scholar]
- Brownmiller, C.; Howard, L.R.; Prior, R.L. Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed blueberry. J. Food Sci. 2008, 73, 72–79. [Google Scholar] [CrossRef]
- García-Viguera, C.; Zafrilla, P. Changes in anthocyanins during food processing: Influence on color. In Chemistry and Physiology of Selected Food Colorants; Ames, J.M., Hofmann, T., Eds.; American Chemical Society: Washington, DC, USA, 2001; Volume 4, pp. 56–65. [Google Scholar]
- Kasparaviciene, G.; Briedis, V. Stability and antioxidant activity of black currant and black aronia berry juices. Medicina 2003, 39 (Suppl. 2), 65–69. (In Lithuanian) [Google Scholar]
- Asen, S.; Stewart, R.N.; Norris, K.H. Co-pigmentation of anthocyanins in plant tissues and its effect on color. Phytochemistry 1972, 11, 1139–1144. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-Garcia, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klisurova, D.; Petrova, I.; Ognyanov, M.; Georgiev, Y.; Kratchanova, M.; Denev, P. Co-pigmentation of black chokeberry (Aronia melanocarpa) anthocyanins with phenolic co-pigments and herbal extracts. Food Chem. 2019, 279, 162–170. [Google Scholar] [CrossRef]
- Wilska-Jeszka, J.; Korzuchowska, A. Anthocyanins and chlorogenic acid copigmentation—Influence on the colour of strawberry and chokeberry juices. Z. Lebensm. Unters. Forsch. 1996, 203, 38–42. [Google Scholar] [CrossRef]
- Skąpska, S.; Marszałek, K.; Woźniak, L.; Zawada, K.; Wawer, I. Aronia dietary drinks fortified with selected herbal extracts preserved by thermal pasteurization and high pressure carbon dioxide. LWT-Food Sci. Technol. 2017, 85, 423–426. [Google Scholar] [CrossRef]
- Sidor, A.; Drożdżyńska, A.; Brzozowska, A.; Szwengiel, A.; Gramza-Michałowska, A. The effect of plant additives on the stability of polyphenols in cloudy and clarified juices from black chokeberry (Aronia melanocarpa). Antioxidants 2020, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Kratchanova, M.; Denev, P.; Kratchanov, C. Rosehip extract synergistically increase antioxidant activity of fruit and herb extracts. Bulg. Chem. Commun. 2014, 46A, 59–64. [Google Scholar]
- Gonzalez-Manzano, S.; Dueñas, M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T.; Santos-Buelga, C. Studies on the copigmentation between anthocyanins and flavan-3-ols and their influence in the colour expression of red wine. Food Chem. 2009, 114, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Directive 2012/12/EU of the European parliament and of the Council amending Council Directive 2001/112/EC relating to fruit juices and certain similar products intended for human consumption. Off. J. Eur. Union 2012, L115/1–L115/11.
- Denev, P.; Kratchanova, M.; Petrova, I.; Klisurova, D.; Georgiev, Y.; Ognyanov, M.; Yanakieva, I. Black chokeberry (Aronia melanocarpa (Michx.) Elliot) fruits and functional drinks differ significantly in their chemical composition and antioxidant activity. J. Chem. 2018, 2018, 9574587. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, J.; Shivhare, U.S.; Raghavan, G.S.V. Thermal degradation kinetics of anthocyanin and visual colour of plum puree. Eur. Food Res. Technol. 2004, 218, 525–528. [Google Scholar] [CrossRef]
- Adams, J.B. Thermal degradation of anthocyanins with particular reference to the 3-glycosides of cyanidin. I. In acidified aqueous solution at 100 °C. J. Sci. Food Agric. 1973, 24, 747–762. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Ki, K.-N. Stability of the anthocyanin pigment extracted from aronia (Aronia melancocarpa). Korean J. Food Sci. Technol. 2013, 45, 416–421. [Google Scholar] [CrossRef] [Green Version]
- Wilkes, K.; Howard, L.R.; Brownmiller, C.; Prior, R.L. Changes in chokeberry (Aronia melanocarpa L.) polyphenols during juice processing and storage. J. Agric. Food Chem. 2014, 62, 4018–4025. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-M.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-mediated synthesis of silver nanoparticles: Their characteristic properties and therapeutic applications. Nanoscale Res. Lett. 2016, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.B.; López-de-Dicastillo Bergamo, C.A.; Payet, R.M.; Liu, X.; Konczak, I. Influence of copigment derived from Tasmannia pepper leaf on Davidson’s plum anthocyanins. J. Food Sci. 2011, 76, C447–C453. [Google Scholar] [CrossRef]
- Glories, Y. La couleur des vins rouges. 2e partie: Mesure, origine et interprétation. J. Int. Sci. Vigne du Vin. 1984, 18, 253–271. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-San José, M.L. Polyphenols and colour variability of red wines made from grapes harvested at different ripeness grade. Food Chem. 2006, 96, 197–208. [Google Scholar] [CrossRef]
- Bimpilas, A.; Panagopoulou, M.; Tsimogiannis, D.; Oreopoulou, V. Anthocyanin copigmentation and color of wine: The effect of naturally obtained hydroxycinnamic acids as cofactors. Food Chem. 2016, 197, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Remini, H.; Mertz, C.; Belbahi, A.; Achir, N.; Dornier, M.; Madani, K. Degradation kinetic modelling of ascorbic acid and colour intensity in pasteurized blood orange juice during storage. Food Chem. 2015, 173, 665–673. [Google Scholar] [CrossRef] [PubMed]
- National Academy of Sciences, Institute of Medicine. Panel on dietary antioxidants and related compounds: Vitamin C, vitamin E, selenium, and α-carotene and other carotenoids: Overview, antioxidant definition, and relationship to chronic disease. In Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000; pp. 35–57. [Google Scholar]
- Číž, M.; Čížová, H.; Denev, P.; Kratchanova, M.; Slavov, A.; Lojek, A. Different methods for control and comparison of the antioxidant properties of vegetables. Food Control 2010, 21, 518–523. [Google Scholar] [CrossRef]
- Furtado, P.; Figueiredo, P.; Chaves das Neves, H.; Pina, F. Photochemical and thermal degradation of anthocyanidins. J. Photochem. Photobiol. A Chem. 1993, 75, 113–118. [Google Scholar] [CrossRef]
- Seeram, N.P.; Bourquin, L.D.; Nair, M.G. Degradation products of cyanidin glycosides from tart cherries and their bioactivities. J. Agric. Food Chem. 2001, 49, 4924–4929. [Google Scholar] [CrossRef] [PubMed]
- Roidoung, S.; Dolan, K.; Siddiq, M. Gallic acid as a protective antioxidant against anthocyanin degradation and color loss in vitamin-C fortified cranberry juice. Food Chem. 2016, 210, 422–427. [Google Scholar] [CrossRef]
- Rabie, M.A.; Soliman, A.Z.; Diaconeasa, Z.S.; Constantin, B. Effect of pasteurization and shelf life on the physicochemical properties of physalis (Physalis peruviana L.) Juice. J. Food Process. Preserv. 2015, 39, 1051–1060. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescence probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Denev, P.; Ciz, M.; Ambrozova, G.; Lojek, A.; Yanakieva, I.; Kratchanova, M. Solid-phase extraction of berris’ anthocyanins and evaluation of their antioxidative properties. Food Chem. 2010, 123, 1055–1061. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D.J. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef] [PubMed]



| Chokeberry | Chokeberry with Lavender | Chokeberry with Meadowsweet | Chokeberry with Rosehip | Chokeberry with Lady’s Mantle | |
|---|---|---|---|---|---|
| Anthocyanin content, mg/L | 700.4 c ± 16.0 | 587.0 a ± 12.5 | 642.9 b ± 13.5 | 571.9 a ± 19.7 | 565.2 a ± 21.1 |
| Total polyphenol content, mg/L | 3020.0 a ± 65.4 | 3395.7 b ± 59.4 | 4177.2 d ± 120.3 | 4281.4 d± 88.7 | 3851.5 c ± 53.3 |
| ORAC 1, µmol TE/L | 63,878 a ± 190 | 73,198 b ± 1750 | 96,973 d ± 1488 | 91,713 d ± 3322 | 78,227 c ± 2319 |
| HORAC 2, µmol GAE 3/L | 14,556 a ± 32 | 16,336 c ± 54 | 18,860 d ± 745 | 15,158 b ± 873 | 15,612 b ± 203 |
| CI 4 | 42.8 b ± 1.0 | 36.8 a ± 2.3 | 39.6 a ± 2.4 | 40.0 a ± 1.6 | 39.3 a ± 1.6 |
| Color hue | 9.0 ab ± 0.8 | 9.8 abc ± 0.6 | 8.7 a ± 0.4 | 11.7 d ± 1.0 | 10.7 cd ± 0.6 |
| Chokeberry | Chokeberry with Lavender | Chokeberry with Meadowsweet | Chokeberry with Rosehip | Chokeberry with Lady’s Mantle | |
|---|---|---|---|---|---|
| Gallic acid | - | - | 34.9 a ± 3.4 | 57.2 b ± 4.8 | 32.4 a ± 4.1 |
| Neochlorogenic acid | 322.1 b ± 18.5 | 242.1 a ± 12.4 | 273.3 a ± 16.5 | 254.3 a ± 21.0 | 240.2 a ± 20.3 |
| Chlorogenic acid | 269.1 b ± 11.2 | 181.6 a ± 10.8 | 280.6 b ± 19.6 | 273.8 b ± 28.1 | 283.1 b ± 21.2 |
| Caffeic acid | - | 19.9 a ± 2.6 | - | - | - |
| Epicatechin | 42.4 a ± 3.1 | 50.7 ab ± 4.8 | 115.3 c ± 9.6 | 53.9 b ± 4.1 | 53.7 b ± 2.3 |
| p-Coumaric acid | - | 17.4 b ± 1.2 | 16.9 b ± 2.1 | 11.7 a ± 0.9 | 20.5 b ± 0.8 |
| Ferulic acid | - | 22.1 a ± 0.8 | 58.3 c ± 3.1 | 45.2 b ± 2.1 | 47.2 b ± 4.0 |
| Rutin | 178.0 a ± 8.1 | 124.7 a ± 9.6 | 188.4 b ± 9.2 | 172.8 b ± 5.1 | 579.8 c ± 14.6 |
| Ellagic acid | - | - | 34.2 a ± 1.2 | 49.5 b ± 2.9 | 65.9 c ± 4.1 |
| Quercetin-3-glucoside | 116.0 a ± 7.2 | 132.7 ab ± 5.6 | 176.2 c ± 9.9 | 145.5 b ± 13.1 | 118.8 a ± 10.5 |
| Rosmarinic acid | - | 29.2 a ± 0.2 | - | - | - |
| Quercetin | 14.5 b ± 0.8 | 6.2 a ± 0.2 | 12.3 b ± 0.6 | 8.7 a ± 0.9 | 8.4 a ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teneva, D.; Pencheva, D.; Petrova, A.; Ognyanov, M.; Georgiev, Y.; Denev, P. Addition of Medicinal Plants Increases Antioxidant Activity, Color, and Anthocyanin Stability of Black Chokeberry (Aronia melanocarpa) Functional Beverages. Plants 2022, 11, 243. https://doi.org/10.3390/plants11030243
Teneva D, Pencheva D, Petrova A, Ognyanov M, Georgiev Y, Denev P. Addition of Medicinal Plants Increases Antioxidant Activity, Color, and Anthocyanin Stability of Black Chokeberry (Aronia melanocarpa) Functional Beverages. Plants. 2022; 11(3):243. https://doi.org/10.3390/plants11030243
Chicago/Turabian StyleTeneva, Desislava, Daniela Pencheva, Ani Petrova, Manol Ognyanov, Yordan Georgiev, and Petko Denev. 2022. "Addition of Medicinal Plants Increases Antioxidant Activity, Color, and Anthocyanin Stability of Black Chokeberry (Aronia melanocarpa) Functional Beverages" Plants 11, no. 3: 243. https://doi.org/10.3390/plants11030243
APA StyleTeneva, D., Pencheva, D., Petrova, A., Ognyanov, M., Georgiev, Y., & Denev, P. (2022). Addition of Medicinal Plants Increases Antioxidant Activity, Color, and Anthocyanin Stability of Black Chokeberry (Aronia melanocarpa) Functional Beverages. Plants, 11(3), 243. https://doi.org/10.3390/plants11030243







