Waste from Persea schiedeana Fruits as Potential Alternative for Biodiesel Production
Abstract
:1. Introduction
2. Results
2.1. Oil Extraction by Maceration
2.2. Chemical Properties of the Oil
2.3. Biodiesel Properties
2.3.1. Fatty Acid Profile
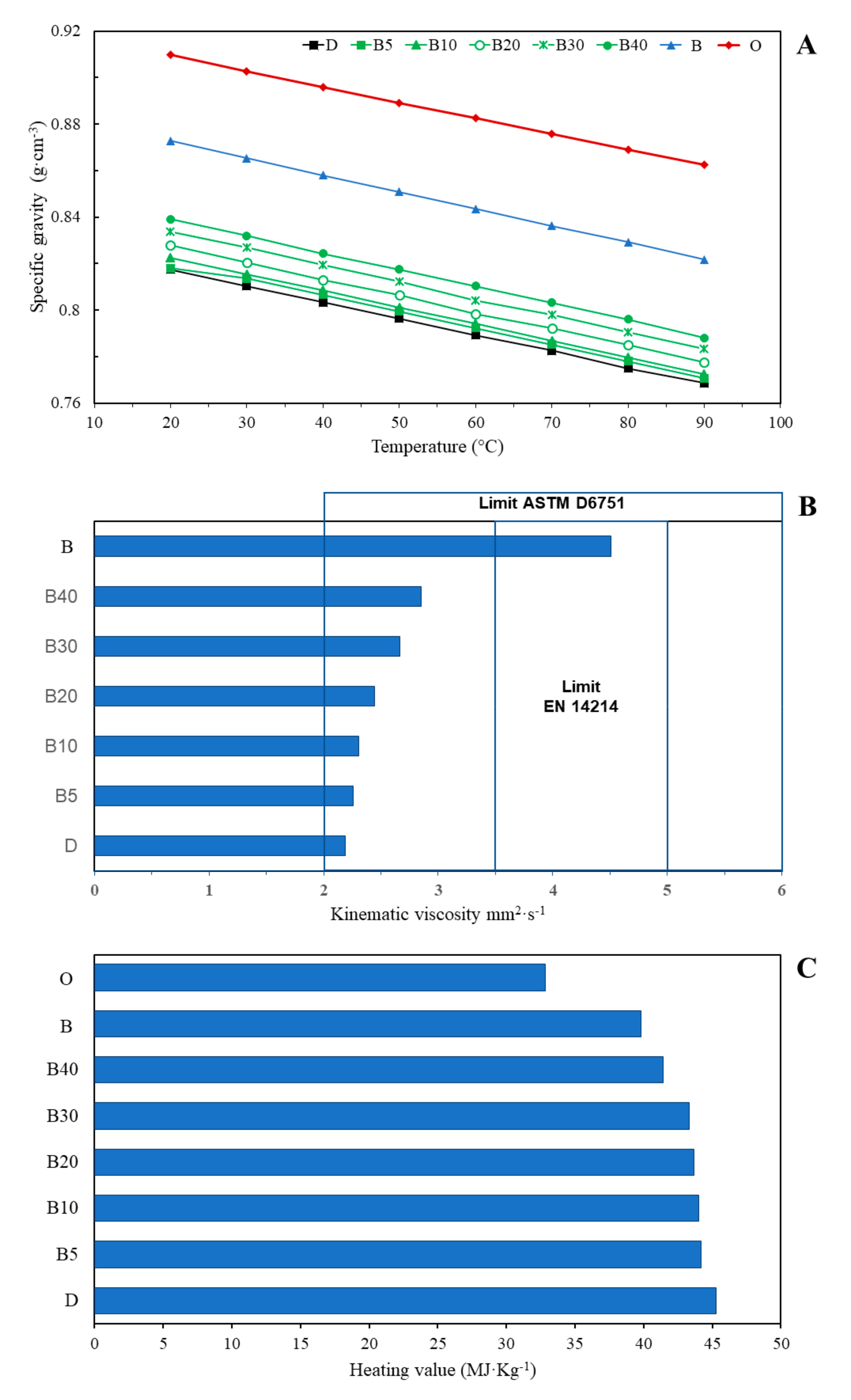
2.3.2. Mechanical Properties and Calorific Value of Oil and Biodiesel
3. Discussion
3.1. Biodiesel Chemical Characterization
3.2. Biodiesel Physical Characterization
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Oil Extraction
4.4. Oil Analysis
4.4.1. Chemical Properties of the Oil
4.4.2. Fatty Acid Profile
4.4.3. Physical Properties of Oil and Biodiesel
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Castillo, J.G.; Tinoco, J.A.; Famiani, F. Distribution of Persea schiedeana in Mexico and Potential for the Production of Fruits with High-quality Oil. HortScience 2017, 52, 661–666. [Google Scholar] [CrossRef] [Green Version]
- Boza, E.J.; Tondo, C.L.; Ledesma, N.; Campbell, R.J.; Bost, J.; Schnell, R.J.; Gutiérrez, O.A. Genetic differentiation, races and interracial admixture in avocado (Persea americana Mill.), and Persea spp. evaluated using SSR markers. Genet. Resour. Crop. Evol. 2018, 65, 1195–1215. [Google Scholar] [CrossRef]
- Bost, J. Persea schiedeana: A High Oil “Cinderella Species” Fruit with Potential for Tropical Agroforestry Systems. Sustainability 2013, 6, 99–111. [Google Scholar] [CrossRef] [Green Version]
- López-Yerena, A.; Guerra-Ramírez, D.; Jácome-Rincón, J.; Espinosa-Solares, T.; Reyes-Trejo, B.; Famiani, F.; Cruz-Castillo, J. Initial evaluation of fruit of accessions of Persea schiedeana Nees for nutritional value, quality and oil extraction. Food Chem. 2018, 245, 879–884. [Google Scholar] [CrossRef]
- Castañeda-Vildózola, A.; Ángel-Coronel, D.; Oscar, A.; Cruz-Castillo, J.G.; Váldez-Carrasco, J. Persea schiedeana (Lauraceae), a new host of Heilipus lauri Boheman (Coleoptera: Curculionidae) in Veracruz, Mexico. Neotrop. Entomol. 2009, 38, 871–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coronel, O.A.D.A.; Cruz-Castillo, J.G.; De La Cruz-Medina, J.; Famiani, F. Ripening and Physiological Changes in the Fruit of Persea schiedeana Nees during the Postharvest Period. HortScience 2010, 45, 172–175. [Google Scholar] [CrossRef] [Green Version]
- Simsek, S. Effects of biodiesel obtained from Canola, sefflower oils and waste oils on the engine performance and exhaust emissions. Fuel 2020, 265, 117026. [Google Scholar] [CrossRef]
- Reyes-Trejo, B.; Guerra-Ramírez, D.; Zuleta-Prada, H.; Cuevas-Sánchez, J.A.; Reyes, L.; Reyes-Chumacero, A.; Rodríguez-Salazar, J.A. Annona diversifolia seed oil as a promising non-edible feedstock for biodiesel production. Ind. Crop. Prod. 2014, 52, 400–404. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels securing the planet’s future energy needs. Energy Convers. Manag. 2009, 50, 2239–2249. [Google Scholar] [CrossRef]
- Lai, J.-Q.; Hu, Z.-L.; Wang, P.-W.; Yang, Z. Enzymatic production of microalgal biodiesel in ionic liquid [BMIm][PF6]. Fuel 2011, 95, 329–333. [Google Scholar] [CrossRef]
- Mazutti, M.A.; Voll, F.A.; Cardozo-Filho, L.; Corazza, M.L.; Lanza, M.; Priamo, W.L.; Oliveira, J.V. Thermophysical properties of biodiesel and related systems: (Liquid+liquid) equilibrium data for soybean biodiesel. J. Chem. Thermodyn. 2013, 58, 83–94. [Google Scholar] [CrossRef]
- Fasina, O.; Colley, Z. Viscosity and Specific Heat of Vegetable Oils as a Function of Temperature: 35 °C to 180 °C. Int. J. Food Prop. 2008, 11, 738–746. [Google Scholar] [CrossRef]
- Diamante, L.M.; Lan, T. Absolute Viscosities of Vegetable Oils at Different Temperatures and Shear Rate Range of 64.5 to 4835 s−1. J. Food Process. 2014, 2014, 234583. [Google Scholar] [CrossRef] [Green Version]
- Guarieiro, L.L.N.; Pereira, P.A.D.P.; Torres, E.A.; da Rocha, G.O.; de Andrade, J.B. Carbonyl compounds emitted by a diesel engine fuelled with diesel and biodiesel–diesel blends: Sampling optimization and emissions profile. Atmos. Environ. 2008, 42, 8211–8218. [Google Scholar] [CrossRef] [Green Version]
- Kannah, R.Y.; Merrylin, J.; Devi, T.P.; Kavitha, S.; Sivashanmugham, P.; Kumar, G.; Banu, J.R. Food waste valorization: Biofuels and value added product recovery. Bioresour. Technol. Rep. 2020, 11, 100524. [Google Scholar] [CrossRef]
- Bobadilla, M.C.; Lorza, R.L.; García, R.E.; Gómez, F.S.; González, E.P.V. An Improvement in Biodiesel Production from Waste Cooking Oil by Applying Thought Multi-Response Surface Methodology Using Desirability Functions. Energies 2017, 10, 130. [Google Scholar] [CrossRef] [Green Version]
- Rachimoellah, H.M.; Resti, D.A.; Zibbeni, A.; Susila, I.W. Production of biodiesel through transesterification of avocado (Persea gratissima) seed oil using base catalyst. J. Tek. Mesin 2009, 11, 85–90. [Google Scholar]
- Hiwot, T. Determination of oil and biodiesel content, physicochemical properties of the oil extracted from avocado seed (Persea Americana) grown in Wonago and Dilla (gedeo zone), southern Ethiopia. Chem. Int. 2017, 3, 311–319. [Google Scholar]
- Dagde, K. Extraction of vegetable oil from avocado seeds for production of biodiesel. J. Appl. Sci. Environ. Manag. 2019, 23, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Campos-Hernández, N.; Cruz-Castillo, J.G.; Hernández-Montes, A.; Rubio-Hernández, D. Persea schiedeana oil of over-mature fruit harvested from wild trees. Universidad Ciencia 2011, 27, 179–189. [Google Scholar]
- NMX-F-052-SCFI-2008. Aceites y Grasas. Aceite de Aguacate. Especificaciones. Fats and Oils Avocado Especifications. 2008. Available online: https://aniame.com/mx/wp-content/uploads/Normatividad/CTNNIAGS/NMX-F-052-SCFI-2008.pdf (accessed on 1 November 2018).
- Cruz-Castillo, J.; Del Angel-Coronel, O.; De La Cruz-Medina, J.; Joaquín-Martínez, M. Características morfológicas y bioquímicas de frutos de chinene (Persea schiedeana Nees.). Rev. Chapingo Ser. Hortic. 2007, 13, 141–147. [Google Scholar] [CrossRef]
- López-Yerena, A.; Ninot, A.; Lozano-Castellón, J.; Escribano-Ferrer, E.; Romero-Aroca, A.J.; Belaj, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Conservation of Native Wild Ivory-White Olives from the MEDES Islands Natural Reserve to Maintain Virgin Olive Oil Diversity. Antioxidants 2020, 9, 1009. [Google Scholar] [CrossRef]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Zuleta, E.C.; Baena, L.; Rios, L.A.; Calderon, J.A. The oxidative stability of biodiesel and its impact on the deterioration of metallic and polymeric materials: A review. J. Braz. Chem. Soc. 2012, 23, 2159–2175. [Google Scholar] [CrossRef] [Green Version]
- Ogunkunle, O.; Ahmed, N.A. Response surface analysis for optimisation of reaction parameters of biodiesel production from alcoholysis of Parinari polyandra seed oil. Int. J. Sustain. Energy 2018, 38, 630–648. [Google Scholar] [CrossRef]
- Knothe, G. Avocado and olive oil methyl esters. Biomass Bioenergy 2013, 58, 143–148. [Google Scholar] [CrossRef]
- De Sousa, L.S.; de Moura, C.V.R.; de Moura, E.M. Action of natural antioxidants on the oxidative stability of soy biodiesel during storage. Fuel 2021, 288, 119632. [Google Scholar] [CrossRef]
- Knothe, G. Some aspects of biodiesel oxidative stability. Fuel Process. Technol. 2007, 88, 669–677. [Google Scholar] [CrossRef]
- Pinzi, S.; Garcia, I.L.; Giménez, F.J.L.; De Castro, M.D.L.; Dorado, G.; Dorado, M.P. The Ideal Vegetable Oil-based Biodiesel Composition: A Review of Social, Economical and Technical Implications. Energy Fuels 2009, 23, 2325–2341. [Google Scholar] [CrossRef]
- Knothe, G. Analyzing biodiesel: Standards and other methods. J. Am. Oil Chem. Soc. 2006, 83, 823–833. [Google Scholar] [CrossRef]
- Martínez, M.C.J.; Castillo, J.G.C.; Medina, J.D.L.C.; del Ángel Coronel, Ó. Distribución ecogeográfica y características del fruto de persea schiedeana nees. en los Tuxtlas, Veracruz, México. Rev. Fitotec. Mex. 2007, 30, 403–410. [Google Scholar]
- Shahidi, F. Quality Assurance of Fats and Oils. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; Wiley: Hoboken, NJ, USA, 2005; Volume 6. [Google Scholar]
- Hoffmann, J.-F.; Vaitilingom, G.; Henry, J.-F.; Chirtoc, M.; Olives, R.; Goetz, V.; Py, X. Temperature dependence of thermophysical and rheological properties of seven vegetable oils in view of their use as heat transfer fluids in concentrated solar plants. Sol. Energy Mater. Sol. Cells 2018, 178, 129–138. [Google Scholar] [CrossRef]
- Cen, E.N. Automotive fuels—fatty acid methyl esters (FAME) for diesel engines—Requirement methods. EN 2008, 14214, 2008. [Google Scholar]
- Anawe, P.; Adewale, F.J. Data on physico-chemical, performance, combustion and emission characteristics of Persea Americana Biodiesel and its blends on direct-injection, compression-ignition engines. Data Brief 2018, 21, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- ASTM. Standard Specification for Biodiesel Fuel (B100) Blend Stock for Distillate Fuels; Annual book of ASTM standards 2008; ASTM: West Conshohocken, PA, USA, 2008. [Google Scholar]
- Paul, A.A.; Adewale, F.J. Data on optimization of production parameters on Persea Americana (Avocado) plant oil biodiesel yield and quality. Data Brief 2018, 20, 855–863. [Google Scholar] [CrossRef]
- Knothe, G. Biodiesel and renewable diesel: A comparison. Prog. Energy Combust. Sci. 2010, 36, 364–373. [Google Scholar] [CrossRef]
- Levine, F.; Kayea, R.V., III; Wexler, R.; Sadvary, D.J.; Melick, C.; La Scala, J. Heats of combustion of fatty acids and fatty acid esters. J. Am. Oil Chem. Soc. 2014, 91, 235–249. [Google Scholar] [CrossRef]
- Mansourpoor, M.; Shariati, A. Optimization of biodiesel production from sunflower oil using response surface methodology. J. Chem. Eng. Process Technol. 2012, 3, 141. [Google Scholar] [CrossRef] [Green Version]
- ISO. Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity (ISO 660: 2009) 2009; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- International Organization dor Standardization. Animal and Vegetable Fats and Oils: Determination of Peroxide Value: Iodometric (Visual) Endpoint Determination; International Organization dor Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- ISO. Animal Vegetable Fats and Oils—Determination of Peroxide Value; ISO 3960; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- ISO. Animal and Vegetable Fats and Oils—Determination of Iodine Value; ISO 3961; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- Zhang, J.; Jiang, L. Acid-catalyzed esterification of Zanthoxylum bungeanum seed oil with high free fatty acids for biodiesel production. Bioresour. Technol. 2008, 99, 8995–8998. [Google Scholar] [CrossRef]
- Cuevas-Sanchez, J.A.; Ramirez, D.G.; Reyes, L.; Reyes-Chumacero, A.; Reyes-Trejo, B. Proximate composition, mineral nutrient and fatty acids of the seed of ilama, Annona diversifolia Saff. Sci. Res. Essays 2011, 6, 3089–3093. [Google Scholar]
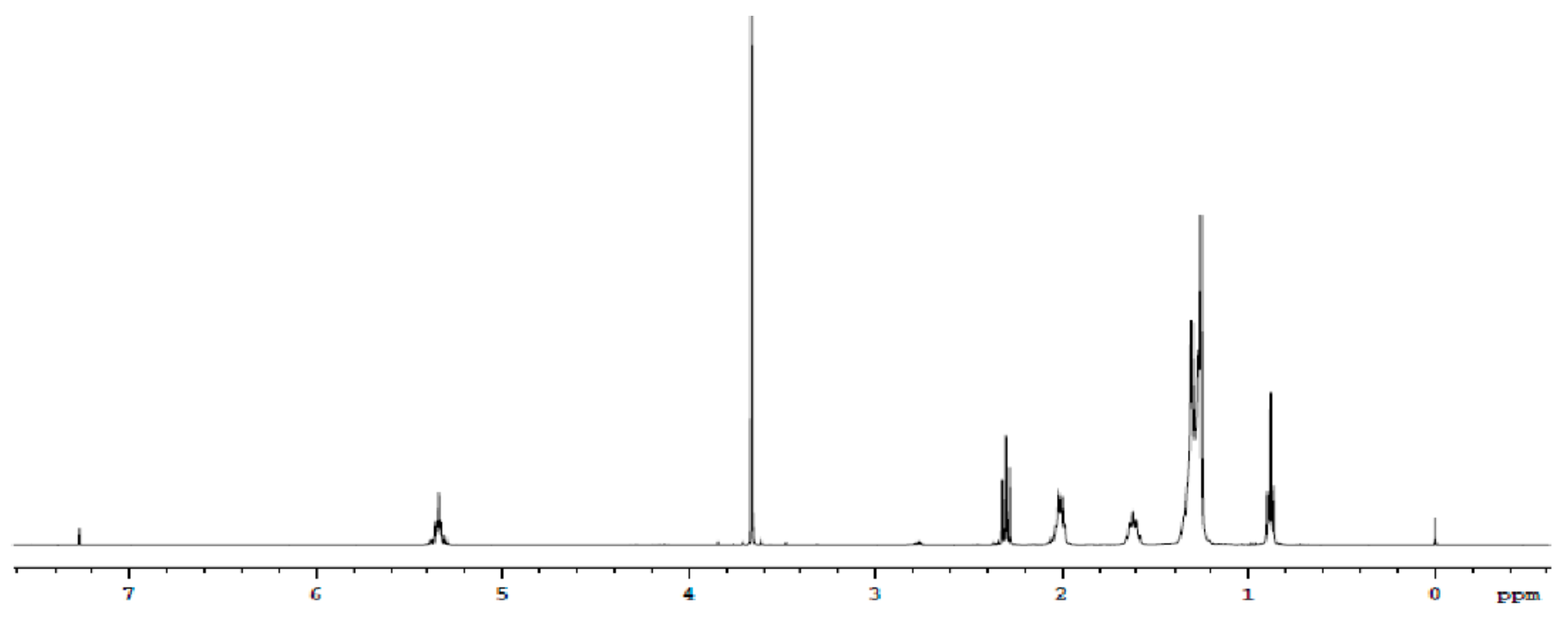
| FFA (%) | Iodine (g I2 100·g−1) | Peroxides (meq O2·kg−1) | Saponification (mg KOH·g−1) | Study |
|---|---|---|---|---|
| 8.36 ± 1.35 | 75.05 ± 1.09 | 3.99 ± 0.58 | 179.52 ± 2.85 | Current study |
| 0.16 | 84.13 | 9.67 | 197.13 | [20] |
| 1.5 | 85–90 | 10 | 177-198 | [21] |
| Fatty Acid | % |
|---|---|
| Hexadecanoic (palmitic; 16:0) | 25.75 |
| 9(Z)-Hexadecenoic (palmitoleic; 16:1) | 5.62 |
| Octadecanoic (stearic; 18:0) | 3.31 |
| 9(Z)-Octadecenoic (oleic; 18:1) | 53.12 |
| 9,12(Z,Z)-Octadecadienoic (linoleic; 18:2) | 4.84 |
| 9,12,15(Z,Z,Z)-Octadecatrienoic (linolenic; 18:3) | 0.57 |
| Docosanoic acid (behenic; 22:0) | 2.24 |
| Others | 4.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Yerena, A.; Guerra-Ramírez, D.; Reyes-Trejo, B.; Salgado-Escobar, I.; Cruz-Castillo, J.G. Waste from Persea schiedeana Fruits as Potential Alternative for Biodiesel Production. Plants 2022, 11, 252. https://doi.org/10.3390/plants11030252
López-Yerena A, Guerra-Ramírez D, Reyes-Trejo B, Salgado-Escobar I, Cruz-Castillo JG. Waste from Persea schiedeana Fruits as Potential Alternative for Biodiesel Production. Plants. 2022; 11(3):252. https://doi.org/10.3390/plants11030252
Chicago/Turabian StyleLópez-Yerena, Anallely, Diana Guerra-Ramírez, Benito Reyes-Trejo, Irma Salgado-Escobar, and Juan Guillermo Cruz-Castillo. 2022. "Waste from Persea schiedeana Fruits as Potential Alternative for Biodiesel Production" Plants 11, no. 3: 252. https://doi.org/10.3390/plants11030252






