Abstract
Population growth, food shortages, climate change and water scarcity are some of the frightening challenges being confronted in today’s world. Water deficit or drought stress has been considered a severe limitation for the productivity of rice, a widely popular nutritive cereal crop and the staple food of a large portion of the population. A key stage in crop growth is seed emergence, which is mostly constrained by abiotic elements such as high temperatures, soil crusting and low water potential, which are responsible for poor stand establishment. Seed priming is a pre-sowing treatment of seeds that primes them to a physiological state that allows them to emerge more proficiently. The purpose of this study was to investigate the potential of leaf extracts from local and exotic moringa landraces as seed priming agents in rice cultivated under water deficit (75% field capacity) and control conditions (100% field capacity). Rice seeds were placed in an aerated solution of moringa leaf extract (MLE) at 3% from three obtained landraces (Faisalabad, Multan and an exotic landrace of India). The results obtained from the experimentation show that the water deficit regime adversely affected the studied indicators including emergence and growth attributes as well as physiological parameters. Among the priming agents, MLE from the Faisalabad landrace significantly improved the speed and spread of emergence of rice seedlings (time to start emergence at 23%, emergence index at 75%, mean emergence time at 3.58% and final emergence percentage at 46%). All the priming agents enhanced the growth, photosynthetic pigments, gas exchange parameters and antioxidant activities, particularly under the water deficit regime, but the maximum improvement was recorded by the MLE from the Faisalabad landrace. Therefore, the MLE of the Faisalabad landrace can be productively used to boost the seedling establishment and growth of rice grown under normal and water deficit conditions.
1. Introduction
The human population is rapidly increasing, and food production is augmenting. There is massive pressure on all the available natural resources to feed the 9.7 billion people on earth by 2050. In addition, water scarcity and the changing climate are also leading challenges being faced by agricultural scientists. At present, the most distressing hazard for agricultural productivity is drought stress [1]. Among the cereals in the world, rice stands in third position, with a production of 495.74 million tones and cultivation on 167 million hectares, after maize and wheat [2]. Globally, three billion people consume rice as a staple food because it provides 20% of energy in the diet, whereas 19% and 5% energy supplies are from wheat and maize, respectively [3,4]. Field crop species are frequently exposed to disparaging environmental circumstances, especially abiotic stresses, which limit the yield and productivity of field crops [5]. Among these stresses, drought or water deficit is a major stress responsible for the low productivity of rice crops worldwide [6,7]. Drought stress also induces a set of biochemical, physiological and morpho-anatomical changes in plants to improve plant water use efficiency by minimizing the loss of water through reduced transpiration. In Asia, drought stress is one of the major limiting factors affecting rice productivity, with 23 M ha subject to drought conditions [8]. Plants are exposed to drought conditions when the loss of water through transpiration is higher than the supply of water to the root zone [9]. The level of mutilation produced by drought is normally capricious because it is influenced by a number of elements, particularly evapotranspiration, rainfall patterns and the water holding capacity of the soil.
Kim et al. [10] stated that water deficit conditions significantly influenced the morphology and growth of plant roots, the main component for nutrient and water absorption from the rhizosphere. It also causes a reduction in photosynthetic pigments and the photosynthetic rate, reduces the intake of CO2 and relative water contents and damages the processes of cell elongation and division [11]. Darwish et al. [12] reported that the WRKY transcription factor group plays a significant role in stress signaling pathways. Expression analysis revealed that TaWRKY32 was mainly expressed when plants were subjected to stress conditions. According to Nawaz et al. [13], drought stress at the reproductive stage (grain filling stage) is very dangerous because it adversely influences the partitioning of assimilates from source to sink. Water deficit conditions also lower the duration and speed of grain filling, which ultimately decreases the economical yield. Denaxa et al. [14] reported that reactive oxygen species, produced under drought stress, are detoxified by the important roles of enzymatic antioxidants, specifically catalase, superoxide dismutase and peroxidase, and non-enzymatic antioxidants. Farooq et al. [15] stated that there are various mechanisms including activation of antioxidant defensive systems, reduction in stomatal conductance and accumulation of compatible solutes that support crop plants in surviving under water deficit circumstances. Drought stress or aerobic environments caused variation in some parameters such as fruit TSS and firmness, the contents of carotenoids, flower number, proteins and activities of the catalase, peroxidase and superoxide dismutase enzymes [16].
In the agricultural sector, proficient water administration drives the handling of all possible routes for water demand and supply management approaches [17,18,19]. In Pakistan, Qamar et al. [20] reported that the productivity of field crops was highly dependent upon the availability of inputs, particularly water, at the critical growth stage, as the crop yield was significantly reduced by water shortage. Application of conservation tillage, stress signaling elements, plant water extracts, osmo-protectants and synthetic and natural mulches and the growth of drought-tolerant varieties are various practices and approaches that can be used to mitigate the adverse impacts of drought stress [21,22,23]. Soil puddling is also considered favorable for rice but unfavorable for post-rice upland crops [24]. Mineral fertilization is responsible for increased crop growth of rice [25]. In recent years, it has been reported that plant water extracts have a significant impact in enhancing the productivity of field crops [26]. Cheng and Cheng [27] reported that various crop water extracts have been identified in different plant species that improve the development, growth and productivity of field crops, particularly when cultivated under unfavorable circumstances, by manipulating physiological and biochemical processes including stomatal conductance, signal transduction, phytohormone metabolism, absorption of water/nutrients, antioxidant defensive systems and photosynthesis. Among most of the naturally available plant growth stimulants, leaf extracts of the moringa tree are at the top of the scientific community’s interests, being a natural source of many antioxidants, mineral elements, vitamins and growth-promoting substances [26,28,29]. Moringa oleifera is also considered as a potential allelopathic crop due to the presence of several allelochemicals [30,31].
Seed priming can be particularly beneficial to resource-poor farmers working in low-input agricultural systems where yield potential is limited by intrinsically stressed agronomic environments [32]. Usage of moringa leaf extracts via seed priming has been proven to enhance the emergence and establishment of seedlings and improve crop development and growth, leading to increased yields in unfavorable and normal environmental circumstances [26,33,34]. The ability of rice plants to cope with drought is supported by several parameters, including the structure of the root organ for water absorption [35]. Sarwar et al. [36] also stated that farmers can enhance crop productivity with the addition of organic supplements. Hence, the current experiment was designed to investigate the growth-promoting potential of leaf extracts obtained from exotic and local landraces of moringa when applied as seed priming agents to rice grown both under water deficit and normal conditions. It is also necessary to highlight that, prior to this study, no experiment thus far has explored the comparative seed priming potential of moringa leaf extracts (MLEs) from exotic and local landraces for rice crops.
2. Materials and Methods
2.1. Experimental Particulars
This experiment commenced during the rice cultivation season of 2018 and was set to explore the seed priming potential of leaf extracts obtained from exotic and local moringa landraces on emergence, physiological and gas exchange attributes, and enzymatic activities of rice seedlings (Oryza sativa L. Cul. Basmati Pak) grown under normal and water stress environments. The experiment was run at a greenhouse of the Department of Agronomy, Ghazi University, Dera Ghazi Khan, Pakistan. The trial was conducted according to a completely randomized design (CRD).
2.2. Treatment Plan, Drought Imposition, Extract Preparation and Application
The following treatment plan was followed for studying the mentioned objective:
Factor A—Drought levels:
- Control conditions (CC, plants irrigated continuously at 100% field capacity);
- Drought stress (DS, plants irrigated continuously at 75% field capacity).
Factor B—Priming treatments:
- Control (no priming);
- Hydro priming (priming with water);
- Priming with a water extract from white seeded moringa leaves (local landrace, Faisalabad origin), FM;
- Priming with a water extract from black seeded moringa leaves (local landrace, DG Khan origin), DM;
- Priming with a water extract from PKM1 moringa leaves (exotic landrace, established at Faisalabad), EM.
Two levels of water treatment (control conditions and drought stress) were induced by maintaining the soil moisture contents at 100% and 75% for the control conditions and drought stress, respectively. Plants grown under control conditions were irrigated at field capacity (FC) and considered as controls. Drought stress was imposed by maintaining the soil moisture contents at 75% as compared to FC. These soil moisture contents were maintained up to harvesting of rice seedlings starting from sowing. In order to determine the water loss by transpiration, pots were weighed on a daily basis, and soil moisture contents were sustained by recompensing this water loss through the addition of tap water up to the initial weight of the respective pot. The gravimetric method described by Nachabe [37] was followed to determine FC.
Fresh leaves were collected from a well-established moringa plantation at the respective sites which were healthy, mature and disease free. After rinsing under tap water, leaves were placed in a deep freezer overnight. A locally designed and assembled machine was used for juice extraction from overnight frozen leaves [34]. A 3% solution (100 mL) of moringa leaf extract (3 mL) was prepared by adding distilled water (97 mL) after sieving the extract.
Regarding seed priming treatments, seeds were placed in an aerated solution of moringa leaf extracts at 3%, for eight hours. Ten seeds were sown in each earthen pot (45 and 30 cm in height and diameter, respectively), filled with growth media (sand, clay, silt and compost in the same proportions). Overall, there were 80 pots in total: 40 for CC, and 40 for DS, as each priming treatment had four replications. Thinning was performed after fifteen days of emergence to maintain the four seedlings in each pot for further experimentation.
2.3. Emergence and Vigor Evaluation of Seedlings
ISTA protocols [38] were followed to record data of seedling vigor and emergence parameters. On a daily basis, emerged seedlings were counted until a persistent count was achieved. Emergence of the first seedling was considered as the time to start emergence (TSE). The formula proposed by Ellis and Roberts [39] was adopted to measure the mean emergence time:
where D is the number of days counted from the beginning of emergence, and n is the number of seeds which emerged on the respective day.
Mean emergence time (MET) = Ʃ Dn/Ʃ n
To determine the emergence index, the formula proposed by the Association of Official Seed Analysis [40] was followed:
Emergence index (EI) = (number of emerged seedling(s)/day of first count) + - - - + (number of emerged seedlings/idem of final count)
The final emergence percentage was calculated according to following formula:
Final emergence percentage (FEP) = number of emerged seedlings/number of seeds sown × 100
2.4. Measurement of Growth Attributes
Seedlings were harvested 30 days after emergence from each experimental unit to record the data of seedling growth. A meter rod was used to measure the lengths of shoots and roots. An electronic weighing balance was used to record the data of the fresh and dry biomasses of rice seedlings. After recording the fresh biomass, to record the dry biomass, seedlings were placed in the oven at 70 °C until a constant weight was achieved.
2.5. Estimation of Physiological Attributes
At the harvesting time (30 days after emergence), samples were collected from the flag leaf of mother tillers from each experimental unit to record the data regarding chlorophyll pigments (chlorophyll a and b and carotenoid contents). A spectrophotometer was used to analyze the chlorophyll pigments of the leaf samples of rice seedlings. In accordance with Arnon [41], absorbance of filtrates at 663, 645 and 480 nm for chlorophyll a and b and carotenoids, respectively, was observed, and the following formulas were used to measure the chlorophyll pigment concentrations in the leaf samples:
where
Chlorophyll a content = [12.7 (OD 663) − 2.69 (OD 645)] × V/1000 × W
Chlorophyll b content = [12.7 (OD 645) − 4.68 (OD 663)] × V/1000 × W
Carotenoids = [(OD 480) + 0.114 (OD 663) − 0.638 (OD 645)] × V/1000 × W
- V = volume of extract in mL;
- W = weight of sample (fresh leaf) in grams (g).
By summation of the chlorophyll a content and chlorophyll b content, the total chlorophyll content was recorded:
Total chlorophyll content = chlorophyll a content + chlorophyll b content
2.6. Measurement of Gas Exchange Attributes
An infrared gas analyzer (IRGA LI-6400; a portable device) was used for the estimation of the photosynthesis rate (A; µmol CO2 m−2 s−1), stomatal conductance (gs; mmol m−2 s−1) and respiration rate (E; mmol H2O m−2 s−1). The flag leaf of mother tillers from each experimental unit was considered suitable to record the data of gas exchange attributes. Data of gas exchange attributes were recorded for two hours between 10:00 and 12:00 a.m. by using the flag leaf of rice seedlings according to procedures described by Long [42].
2.7. Determination of Enzymatic Activities
Samples from the flag leaf of mother tillers of each experimental unit were collected at the harvesting time to estimate the enzymatic activities. The superoxide dismutase activity was determined according to the method described by Giannopolitis and Ries [43]. The procedure proposed by Chance and Maehly [44] was followed to estimate the activity of catalase. With a slight modification, the protocol suggested by Nakano and Asada [45] for ascorbate peroxidase was followed accordingly to observe the ascorbate peroxidase activity. The method proposed by Velikova [46] was used to measure the concentration of hydrogen peroxide.
2.8. Statistical Analysis
Collected data of emergence, physiological and gas exchange attributes, growth and enzymatic activities were analyzed and evaluated statistically by using the statistical package “Statistic 8.1”, employing Fisher’s analysis of variance (ANOVA) technique under a completely randomized design. Microsoft Excel was used for calculation and graphical presentation. The statistical model includes the replications (4), drought levels (2), foliar treatments (5) of MLEs and the interaction between drought levels and foliar treatments of MLEs. Different letters (a, b, c, etc.) were used to portray the significant differences among treatments’ effects via LSD at a 5% probability level [47].
3. Results
The effect of the moringa leaf extracts as a priming agent as well as the water regimes on rice seedlings was studied through various indicators such as emergence and growth attributes, photosynthetic pigments, gas exchange parameters and antioxidant activities. Significant levels of seedling emergence and growth, photosynthetic pigments, gas exchange parameters and antioxidant activities, as influenced by priming agents and water regimes, are presented in Table 1.

Table 1.
Analysis of variance for emergence, growth, photosynthetic pigment, gas exchange and enzymatic attributes of rice seedlings cultivated under normal and water deficit regimes in response to priming treatments of moringa leaf extracts (n = 4).
3.1. Emergence Attributes
The time to start emergence, mean emergence time and emergence index of rice seedlings were significantly influenced by the priming agents and water regimes, but the interaction was statistically non-significant (Table 1). A lower time to start emergence (5.15 days) and mean emergence time (10.74 days) of seedlings were recorded under well-watered conditions as compared to the water deficit regime (Table 2). The water deficit regime increased the time to start emergence (5.65 days) and mean emergence time (10.91). In the case of priming agents, the maximum reduction in the time to start emergence was observed for FM priming, which was statistically at par with DM and EM priming. FM priming also reduced the mean emergence time of rice seedlings (10.60 days). FM priming and the normal regime of water treatment produced the maximum emergence index (6.97). Water deficit conditions also reduced the emergence index (5.13) of seedlings. The final emergence percentage was statistically improved by all priming agents, but the maximum outcome was recorded for FM priming (98.75%) (Table 2). The water regimes did not significantly influence the final emergence percentage.

Table 2.
Impact of moringa leaf extracts from various landraces as priming agents on emergence attributes of rice seedlings cultivated under control and water deficit regimes.
3.2. Growth Attributes
Growth attributes including the fresh and dry biomasses and the shoot and root lengths of rice seedlings were significantly affected by the priming agents and water treatments (Table 1), but the interaction was only significant regarding the dry biomass of rice seedlings. All the priming agents enhanced the fresh and dry biomasses and the shoot and root lengths of rice seedlings, but the maximum improvement was observed for FM priming (Table 3). The water deficit regime reduced the fresh and dry biomass as well as shoot length of seedlings, while root length was increased. The maximum dry biomass (14.43 g) was recorded for FM priming under the control water treatment, while the minimum (8.45 g) was recorded under the water deficit regimes (Table 3).

Table 3.
Impact of moringa leaf extracts from various landraces as priming agents on growth attributes of rice seedlings cultivated under control and water deficit regimes.
3.3. Photosynthetic Pigments
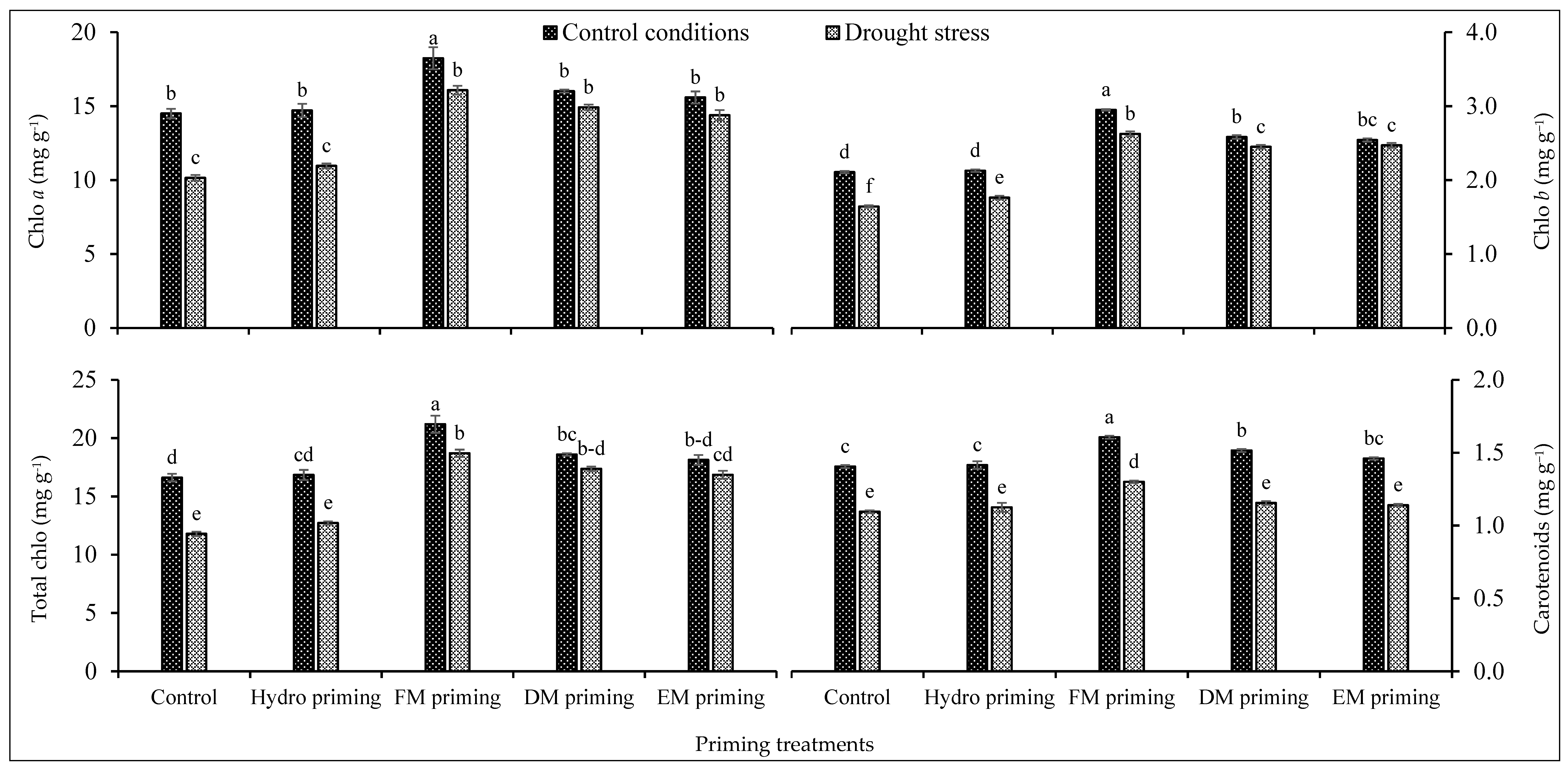
Chlorophyll a and b and total chlorophyll contents were significantly affected by the priming treatments as well as water regimes, and even the interaction of both factors was significant (Table 1), while the interaction was non-significant regarding carotenoids. FM priming was responsible for the maximum synthesis of chlorophyll contents including chlorophyll a and b, total chlorophyll and carotenoid contents (Figure 1). FM priming performed better under water deficit regimes as well. FM priming produced the maximum total chlorophyll contents under control conditions followed by the same treatment under water deficit regimes (Figure 1). DM and EM priming were statistically at par with each other regarding chlorophyll pigments. A lower concentration of chlorophyll pigments was observed in non-primed seeds as well as in hydro priming. Water regimes also influenced the concentration of photosynthetic pigments in rice seedlings. Water deficit regimes reduced the production of photosynthetic pigments, while the maximum concentration was recorded under normal water regimes (Figure 1).
Figure 1.
Impact of moringa leaf extracts as priming agents on photosynthetic pigments of rice seedlings cultivated under control and water deficit regimes. Bars sharing the same letter did not differ significantly at p = 0.05.
3.4. Gas Exchange Attributes
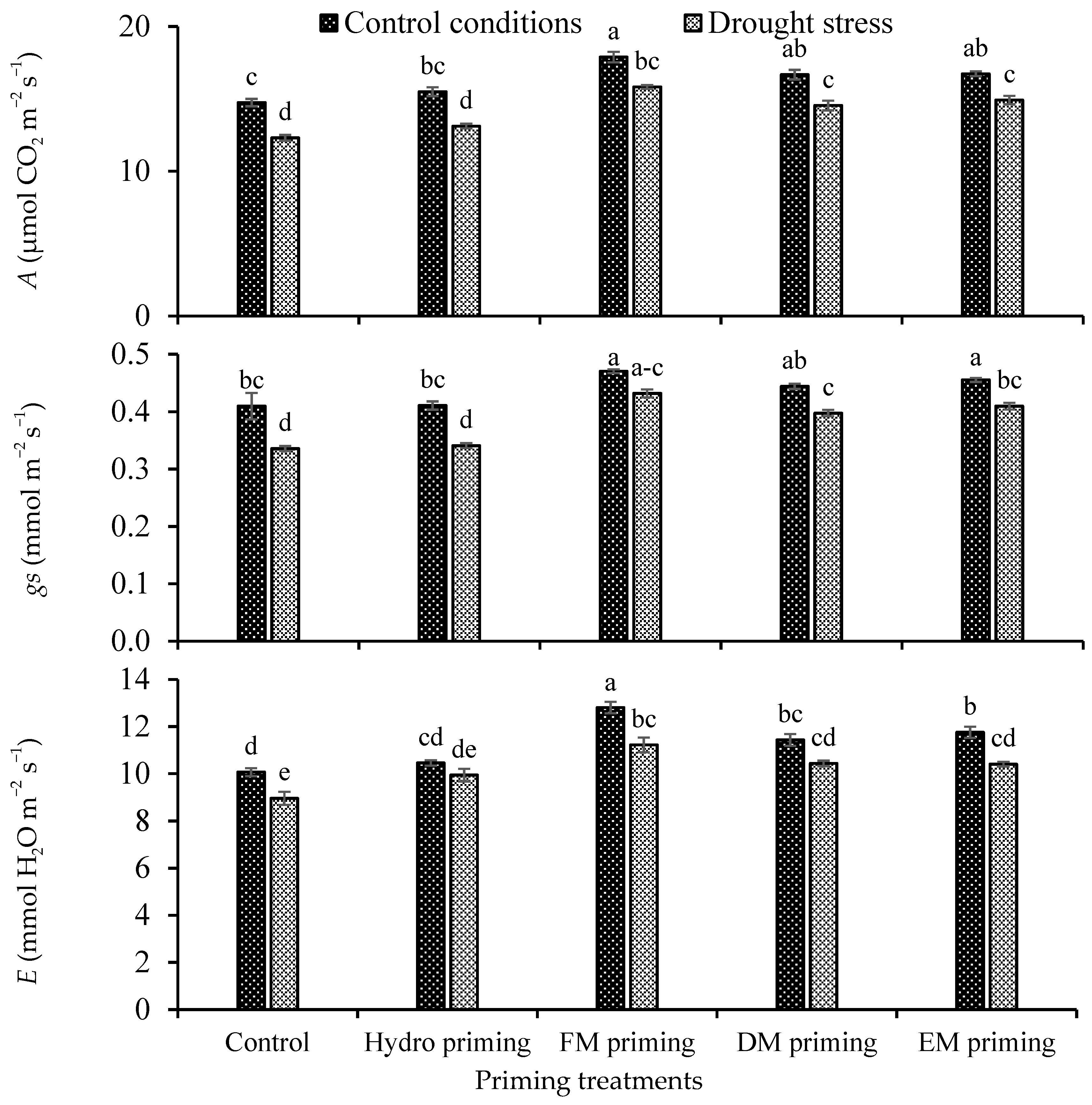
The priming agents and water regimes significantly influenced the gas exchange attributes, i.e., photosynthesis rate (A), stomatal conductance to water (gs) and respiration rate (E), of rice seedlings, while their interaction was non-significant (Table 1). A better photosynthesis rate was observed under control conditions as compared to drought stress. In the case of priming, all the priming agents showed an improvement in the photosynthesis rate in normal control conditions as well as under water deficit regimes, but the maximum response was recorded by FM priming, followed by DM and EM priming, in that order (Figure 2). The minimum photosynthesis rate was observed in control and hydro priming treatments. FM priming also improved the stomatal conductance under the water deficit regime, which was statistically similar under the normal condition. The water deficit regime reduced the respiration rate as compared to the normal condition, while FM priming improved the respiration rate either in normal or deficit conditions (Figure 2). The minimum respiration rate was found under non-primed and hydro priming treatments, which were statistically at par with each other.
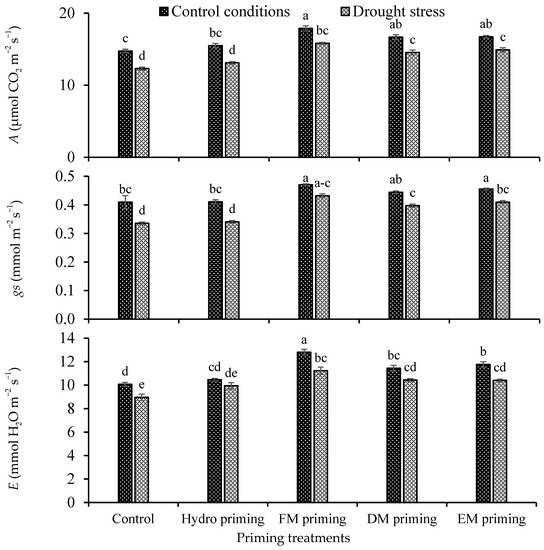
Figure 2.
Impact of moringa leaf extracts as priming agents on gas exchange attributes of rice seedlings cultivated under control and water deficit regimes. Bars sharing the same letter did not differ significantly at p = 0.05.
3.5. Antioxidant Activities
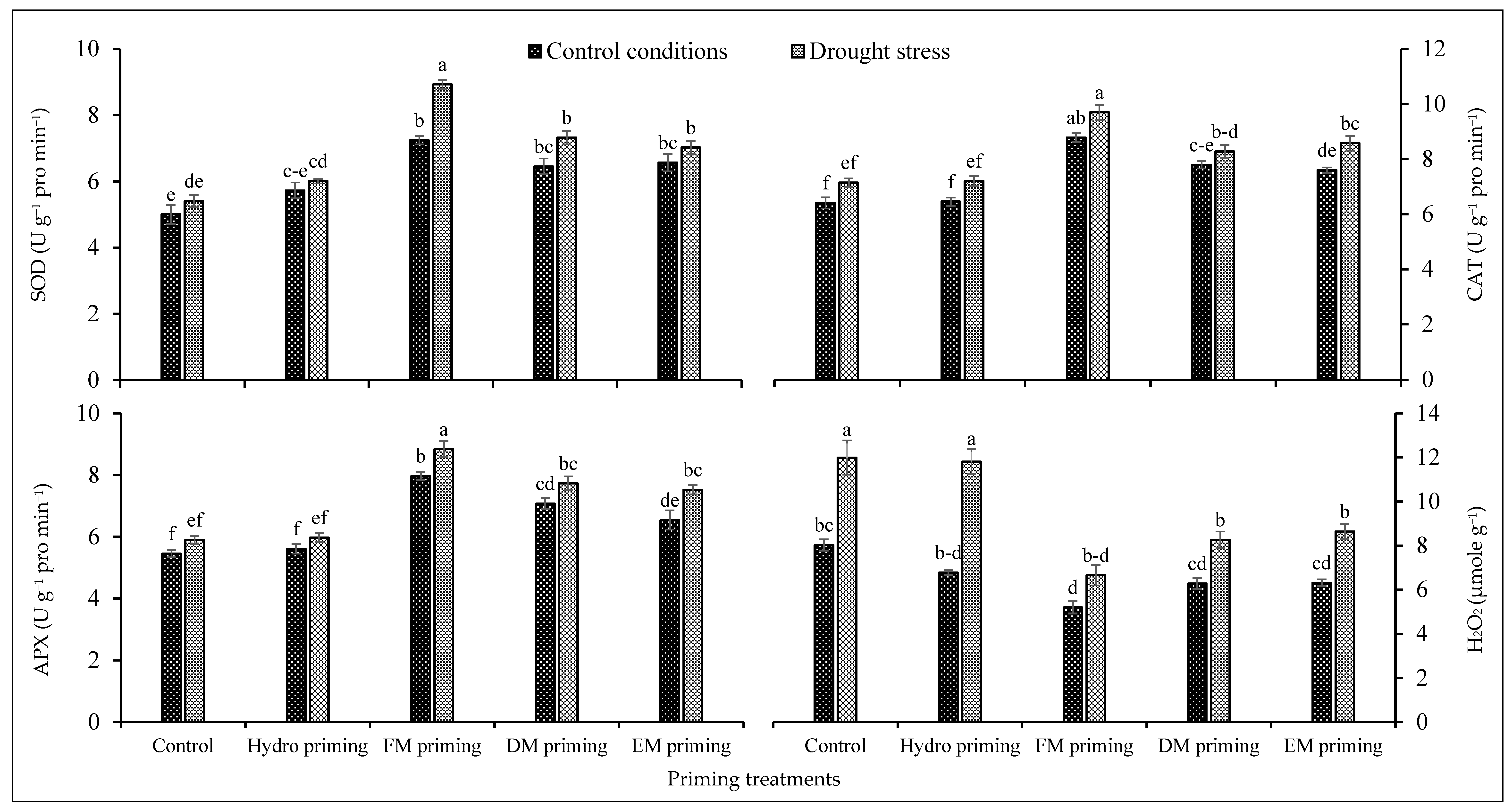
The activities of superoxide dismutase, catalase, ascorbate peroxidase and H2O2 were significantly affected by the water regimes and priming agents (Table 1). The water deficit regimes improved the antioxidant activities of rice seedlings, while low antioxidant activities were observed under normal conditions (Figure 3). Drought stress significantly enhanced the activities of superoxide dismutase, catalase, ascorbate peroxidase and H2O2 comparatively. All priming agents, particularly FM priming, improved the activities of superoxide dismutase, catalase and ascorbate peroxidase under normal and water deficit regimes, but the maximum improvement was recorded by FM priming. On the other hand, the H2O2 concentration was increased by water deficit regimes, but the priming agents reduced the concentration of H2O2 either under normal or water deficit regimes; the maximum reduction was noted by FM priming under both water regimes (Figure 3). DM and EM priming performed statistically similarly under the normal and deficit regimes. The maximum production of H2O2 was recorded in non-primed and hydro priming treatments under water deficit regimes (Figure 3).
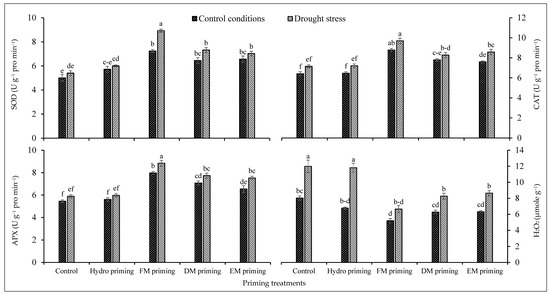
Figure 3.
Impact of moringa leaf extracts as priming agents on antioxidant activities of rice seedlings cultivated under control and water deficit regimes. Bars sharing the same letter did not differ significantly at p = 0.05.
4. Discussion
In this study, it was observed that water regimes and priming agents significantly influenced the emergence and growth attributes, photosynthetic pigments, gas exchange parameters and antioxidant activities. Under drought stress, plants showed a reduction in the concentrations of photosynthetic pigments and rate of gas exchange attributes, but with enhanced enzymatic activities, in the present experimentation. The priming agents appeared to mitigate the adverse impacts of water deficit by improving the emergence and growth attributes of rice seedlings, as also shown in wheat seeds [26]. The improved emergence attributes (time to start emergence, mean emergence time, emergence index and final emergence percentage) of rice seedlings with respect to the controls may be associated with greater and more rapid intake of water by the seeds (imbibition process) and the triggering of enzymatic activities linked with rupturing of the seed coat due to the seed priming agent [30,48]. The improved speed and spread of emergence indicate the considerable potential of MLEs for agricultural use as priming agents to improve drought tolerance and thereby increase crop productivity in environmentally stressed regions. Our scientific outcomes are also supported by previous studies demonstrating that plant growth and vigor were improved by application of MLEs as priming agents in maize [33]. Our findings are also supported by Khan et al. [34], who found that a leaf extract from a moringa landrace from the Faisalabad region had more growth-enhancing potential as compared to the leaf extracts of other available landraces regarding seedling growth and vigor. Farooq and Koul [49] observed variation regarding various bioactive compounds in the seed and leaf extracts of various cultivars of moringa. They concluded that seed and leaf extracts of the Jaffin variety contain strong antioxidant and antibacterial activities with greater total flavonoid and phenolic contents as compared to other landraces. Therefore, higher plant growth-enhancing activity was present in the extract of the Jaffin cultivar. Seed priming enhances the rate of metabolism, which results in an increase in the speed of germination and emergence [50].
Growth parameters including the fresh and dry biomasses and the shoot and root lengths of rice seedlings were adversely affected by the water deficit regime in the present experimentation. On the other hand, application of MLEs as priming agents enhanced the growth of rice seedlings under normal conditions, but improvement was also observed under the water deficit regime. Not only are moringa leaves good reservoirs of mineral nutrients, i.e., K, Ca, Mg and vitamin C, but they also contain zeatin, a type of cytokinin, which speeds up the cell division process [28]. These observations suggest that the mechanism of improved growth by MLE application is based on the improvement in emergence attributes of seedlings and the subsequent increase in the plant growth components. Thus, the application of MLEs as priming agents may enhance the performance of rice seedlings. Most of the mineral nutrients of MLEs during seed priming seemed to be partitioned to the embryo of the seed, which boosted the emergence of seedlings and ultimately the growth and development of plants. Seed priming can be particularly beneficial to resource-poor farmers working in low-input agricultural systems where the yield potential is limited by intrinsically stressed agronomic environments [32]. The findings of the current study are also in line with the outcomes of Khan et al. [29,34], who found that extracts prepared from the leaves of landraces in Faisalabad exhibited a greater biostimulant potential that might be due to the availability of bioactive compounds, antioxidants, mineral nutrients and plant growth-promoting substances. Moreover, Basra et al. [33] reported that seed priming of maize with MLEs improved plant growth and economic yield. Similarly, Abdalla [51] noted that a foliar spray of MLEs enhanced the plant length, fresh (68.1%) and dry biomasses (51.5%), photosynthetic pigments, photosynthetic rate, stomatal conductance, total soluble protein, ascorbic acid content and phytohormones in rocket plants.
In the current study, MLE treatment significantly improved the chlorophyll pigments and gas exchange attributes under water shortage. Production of chlorophyll pigments improves efficiency and utilization of inputs. Water use efficiency is also improved by higher production of chlorophyll pigments. As the concentration of chlorophyll pigments increases, gas exchange attributes are also improved. Photosynthetic and respiration activities are enhanced, which are responsible for the stay-green period. MLEs are rich in cytokinins which delay leaf senescence, resulting in a high leaf area and a greater amount of chlorophyll pigments [52]. It has been previously reported that exogenous application of MLEs improved the chlorophyll pigments [28], gas exchange attributes, antioxidant activities, growth and yield of field crops cultivated under normal and stressful circumstances [53,54]. Similar findings were also noted in rocket plants, where a foliar spray of MLEs enhanced the plant length, fresh and dry masses, chlorophyll contents, photosynthetic rate, stomatal conductance, ascorbic acid, total soluble proteins and phytohormones [51]. Moringa leaves are an excellent source of zeatin (5 to 200 µg/g of fresh leaves), antioxidants and tocopherols which enhance plant tolerance against environmental stresses [55,56]. The findings of the current experiment are also in line with a previous study in which MLE use increased the chlorophyll contents in abiotically stressed wheat. In addition, exogenous use of MLEs improved the wheat chlorophyll pigments [13]. Improved plant growth with fresh MLEs can be attributed to the presence of various secondary metabolites such as phenols and ascorbates [33]. These findings are also supported by Khan et al. [34], who found that a moringa leaf extract from a landrace of Faisalabad origin showed a higher biostimulant potential that might be due to the presence of a high concentration of plant growth-promoting compounds, mineral nutrients and antioxidants. Use of MLEs from all landraces significantly enhanced the morphological, physiological and yield traits both under ambient and stressed conditions as compared to the controls [29]. The application of minerals, either alone or in combination with growth promoters, improved the growth attributes of field crops [57].
In biochemical attributes, MLE application showed positive synergy with other bio-chemical stimulants. In the present findings, the improvement in biochemical attributes may be due to the presence of allelochemicals and secondary compounds such as ascorbic acid, total phenolics [28] and zeatin [58]. Drought stress adversely reduced the chlorophyll and carotenoid contents, and their reduction was directly associated with the intensity of stress. In the current study, drought stress decreased the photosynthetic pigments, which is in line with previous findings in which water shortage lowered the chlorophyll and carotenoid contents of rice [59]. Drought stress decreased the level of chlorophyll contents, while under ambient conditions, more chlorophyll pigments were observed as compared to the drought stress condition [60]. Zhang et al. [61] also reported that drought stress reduced the photosynthetic rate, water contents and transpiration rate and enhanced stomatal resistance. Thus, the chlorophyll content and photosynthetic rate can be used to check the intensity of drought stress [62]. The application of mineral elements and organic compounds is considered a helpful practice in maintaining crop productivity with improved soil fertility to achieve the maximum plant growth and economical yield under stressful conditions [63,64].
Antioxidants play an inevitable role in increasing plant tolerance against abiotic stress by improving the plant defense system. In the current study, exogenous use of MLEs increased the antioxidant activities (SOD, CAT and APX), which protect the plants from oxidative damage. The activities of antioxidants are higher under stressful conditions as compared to normal and favorable conditions, which protects cells and organelles from oxidative damage [65]. Therefore, to increase plant tolerance, there is a need to improve antioxidant activity to overcome oxidative stress. SOD acts as the basis of defense in response to oxidative stress [66]. SOD expression plays a vital role against drought stress by scavenging the H2O2 concentration [67]. Hanafy [68] found that under drought, the activity of the enzymatic antioxidants glutathione reductase (GR), SOD and APX was enhanced in soybean and canola [69]. Moreover, in common bean, MLE treatment, both foliar spray and seed priming, significantly increased enzymatic antioxidant activities such as those of GR, SOD and APX [70]. Moringa leaf extract (MLE) is a natural seed priming agent and crop growth enhancer. The farmer community can use MLE at 3% as a seed priming agent to enhance the growth and productivity of field crops cultivated under normal and/or unfavorable conditions.
5. Conclusions
The results obtained from the experimentation show that the water deficit regime adversely affected the studied indicators including emergence and growth attributes as well as physiological parameters. Among the priming agents, the MLE from the Faisalabad landrace significantly improved the speed and spread of emergence of rice seedlings. Therefore, the MLE of the Faisalabad landrace can be productively used to boost the stand establishment of seedlings and growth of rice grown under normal and water deficit conditions. Further research is required to support MLEs’ impact as seed priming agents on the final yield, even under field conditions.
Author Contributions
Conceptualization, S.K., S.B., D.I. and N.R.; methodology, S.K., Z.H. and M.N.; software, M.N., A.A.A.-G. and H.D.; validation, S.K., D.I. and S.B.; formal analysis, S.K. and N.R.; writing—original draft preparation, S.K., D.I., N.R., Z.H., M.N., M.S.E. and J.D.; writing—review and editing, S.K., S.B., N.R., A.A.A.-G., M.S.E., H.D. and J.D.; project administration, S.K. and S.B.; funding acquisition, S.K., A.A.A.-G., M.S.E., H.D. and J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Higher Education Commission (HEC), Pakistan, under the Startup Research Grant Program (SRGP: No:21-2333/SRGP/R&D/HEC/2019) for the project entitled, “Exploring the growth enhancing potential of leaf extracts from local and exotic moringa landraces for rice” (financial support). The corresponding author, Shahbaz Khan, received funding for this research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the outcomes of the current experimentation are available from the corresponding author (S.K.) upon reasonable request.
Acknowledgments
The authors are thankful to the Department of Agronomy, Ghazi University, Dera Ghazi Khan, Pakistan, for providing the greenhouse and laboratory facilities for conducting the experimentation. The authors also extend their appreciation to the Researchers Supporting Project number (RSP2022R483), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research progress and perspective on drought stress in legumes: A review. Int. J. Mole. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef] [Green Version]
- Statista. 2019. Available online: https://www.statista.com/statistics/263977/world-grain-production-by-type/ (accessed on 25 October 2021).
- Alexandratos, N.; Jelle, B. World Agriculture towards 2030/2050: The 2012 Revision. FAO Agricultural Development Economics Division. Food and Agriculture Organization of the United Nations. 2012. Available online: www.fao.org/economic/esa (accessed on 12 September 2021).
- Milani, P.; Carnahan, E.; Kapoor, S.; Ellison, C.; Manus, C.; Spohrer, R.; van den Berg, G.; Wolfson, J.; Kreis, K. Social marketing of a fortified staple food at scale: Generating demand for fortified rice in Brazil. J. Food Prod. Mark. 2017, 23, 955–978. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The Impact of Drought in Plant Metabolism: How to Exploit Tolerance Mechanisms to Increase Crop Production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Sikder, S.; Husna, A.; Sultana, S.; Akhter, S.; Alim, A.; Joardar, J.C. Influence of water stress on morphology, physiology and yield contributing characteristics of rice. SAARC J. Agric. 2020, 18, 61–71. [Google Scholar] [CrossRef]
- Basal, O.; Szabó, A. Physiology, yield and quality of soybean as affected by drought stress. Asian J. Agric. Biol. 2020, 8, 247–252. [Google Scholar] [CrossRef]
- Huke, R.E.; Huke, E.H. Rice Area by Type of Culture: South, Southeast, and East Asia; IRRI: Los Ban, CA, USA, 1997. [Google Scholar]
- Anjum, S.A.; Wang, L.C.; Farooq, M.; Hussain, M.; Xue, L.L.; Zou, C.M. Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J. Agron. Crop Sci. 2011, 197, 177–185. [Google Scholar] [CrossRef]
- Kim, Y.; Chung, Y.S.; Lee, E.; Tripathi, P.; Heo, S.; Kim, K.H. Root Response to Drought Stress in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2020, 21, 1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, M.; Malik, M.A.; Farooq, M.; Ashraf, M.Y.; Cheema, M.A. Improving drought tolerance by exogenous application of glycinebetaine and salicylic acid in sunflower. J. Agron. Crop Sci. 2008, 194, 193–199. [Google Scholar] [CrossRef]
- Darwish, E.; Rehman, S.U.; Mao, X.; Jing, R. A wheat stress induced WRKY transcription factor TaWRKY32 confers drought stress tolerance in Oryza sativa. Asian J. Agric. Biol. 2021. [Google Scholar] [CrossRef]
- Nawaz, A.; Farooq, M.; Alam, S.; Wahid, A. Stay green character at grain filling ensures resistance against terminal drought in wheat. Int. J. Agric. Biol. 2013, 15, 1272–1276. [Google Scholar]
- Denaxa, N.K.; Damvakaris, T.; Roussos, P.A. Antioxidant defense system in young olive plants against drought stress and mitigation of adverse effects through external application of alleviating products. Sci. Hortic. 2020, 259, 108812. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Dordrecht, The Netherland, 2009; pp. 153–188. [Google Scholar]
- Kazemi, S.; Zakerin, A.; Abdossi, V.; Moradi, P. Fruit yield and quality of the grafted tomatoes under different drought stress conditions. Asian J. Agric. Biol. 2021. [Google Scholar] [CrossRef]
- Rehman, A.; Hassan, F.; Qamar, R.; Rehman, A.U. Application of plant growth promoters on sugarcane (Saccharum officinarum L.) budchip under subtropical conditions. Asian J. Agric. Biol. 2021, 2, 202003202. [Google Scholar] [CrossRef]
- Al-Zboon, K.K.; Al-Tabbal, J.A.; Al-Kharabsheh, N.M.; Al-Mefleh, N.K. Natural volcanic tuff as a soil mulching: Effect on plant growth and soil chemistry under water stress. Appl. Water Sci. 2019, 9, 123. [Google Scholar] [CrossRef] [Green Version]
- Makawita, G.I.P.S.; Wickramasinghe, I.; Wijesekara, I. Using brown seaweed as a biofertilizer in the crop management industry and assessing the nutrient upliftment of crops. Asian J. Agric. Biol. 2021. [Google Scholar] [CrossRef]
- Qamar, R.; Anjum, I.; Rehman, A.U.; Safdar, M.E.; Javeed, H.M.R.; Rehman, A.; Ramzan, Y. Mitigating water stress on wheat through foliar application of silicon. Asian J. Agric. Biol. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Tabaxi, I.; Ζisi, C.; Karydogianni, S.; Folina, A.E.; Kakabouki, I.; Kalivas, A.; Bilalis, D. Effect of organic fertilization on quality and yield of oriental tobacco (Nicotiana tabacum L.) under Mediterranean conditions. Asian J. Agric. Biol. 2021. [Google Scholar] [CrossRef]
- Batool, S.; Khan, S.; Basra, S.M.A. Foliar application of moringa leaf extract improves the growth of moringa seedlings in wInt. S. Afri. J. Bot. 2020, 129, 347–353. [Google Scholar] [CrossRef]
- Gondal, M.R.; Saleem, M.Y.; Rizvi, S.A.; Riaz, A.; Naseem, W.; Muhammad, G.; Hayat, S.; Iqbal, M. Assessment of drought tolerance in various cotton genotypes under simulated osmotic settings. Asian J. Agric. Biol. 2021, 2, 202008437. [Google Scholar] [CrossRef]
- Hossain, M.A.; Rana, M.M.; Al-Rabbi, S.M.H.; Mitsui, T. Management of puddled soil through organic amendments for post-rice mungbean. Asian J. Agric. Biol. 2021. [Google Scholar] [CrossRef]
- Khasanah, R.A.N.; Rachmawati, D. Potency of silicon in reducing cadmium toxicity in Cempo Merah rice. Asian J. Agric. Biol. 2020, 4, 405–412. [Google Scholar] [CrossRef]
- Khan, S.; Basra, S.M.A.; Nawaz, M.; Hussain, I.; Foidl, N. Combined application of moringa leaf extract and chemical growth-promoters enhances. the plant growth and productivity of wheat crop (Triticum aestivum L.). S. Afr. J. Bot. 2020, 129, 74–81. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Foidl, N.; Makkar, H.P.; Becker, K. The potential of Moringa oleifera for agricultural and industrial uses. The miracle tree: The multiple attributes of Moringa. In What Development Potential For Moringa Product? CIRAD: Managua, Nicaragua, 2001; pp. 45–76. [Google Scholar]
- Khan, S.; Basit, A.; Hafeez, M.B.; Irshad, S.; Bashir, S.; Bashir, S.; Maqbool, M.M.; Saddiq, M.S.; Hasnain, Z.; Aljuaid, B.S.; et al. Moringa leaf extract improves biochemical attributes, yield and grain quality of rice (Oryza sativa L.) under drought stress. PLoS ONE 2021, 16, e0254452. [Google Scholar] [CrossRef]
- Khan, S.; Basra, S.M.A.; Afzal, I.; Nawaz, M.; Rehman, H.U. Growth promoting potential of fresh and stored Moringa oleifera leaf extracts in improving seedling vigor, growth and productivity of wheat crop. Environ. Sci. Poll. Res. 2017, 24, 27601–27612. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.F.; Basra, S.M.A.; Hafeez, M.B.; Khan, S.; Irshad, S.; Iqbal, S.; Saddiq, M.S.; Akram, M.Z. Inorganic fertilization improves quality and biomass of Moringa oleifera L. Agrofor. Syst. 2020, 94, 975–983. [Google Scholar] [CrossRef]
- Carrillo-Reche, J.; Vallejo-Marín, M.; Quilliam, R.S. Quantifying the potential of ‘on-farm’ seed priming to increase crop performance in developing countries. A meta-analysis. Agron. Sustain. Dev. 2018, 38, 64. [Google Scholar] [CrossRef] [Green Version]
- Basra, S.M.A.; Iftikhar, M.; Afzal, I. Potential of moringa (Moringa oleifera) leaf extract as priming agent for hybrid maize seeds. Int. J. Agric. Biol. 2011, 13, 1006–1010. [Google Scholar]
- Khan, S.; Basra, S.M.A.; Afzal, I.; Wahid, A. Screening of moringa landraces for leaf extract as biostimulant in wheat. Int. J. Agric. Biol. 2017, 19, 999–1006. [Google Scholar] [CrossRef]
- Salsinha, Y.C.F.; Maryani, I.D.; Purwestri, Y.A.; Rachmawati, D. Morphological and anatomical characteristics of Indonesian rice roots from East Nusa Tenggara contribute to drought tolerance. Asian J. Agric. Biol. 2021. [Google Scholar] [CrossRef]
- Sarwar, N.; Mubeen, K.; Wasaya, A.; Rehman, A.U.; Farooq, O.; Shehzad, M. Response of hybrid maize to multiple soil organic amendments under sufficient or deficient soil zinc situation. Asian J. Agric. Biol. 2020, 8, 38–43. [Google Scholar] [CrossRef]
- Nachabe, M.H. Refining the definition of field capacity in the literature. J. Irrig. Drain. Eng. 1998, 124, 230–232. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; ISTA: Secretariat, Switzerland, 2010. [Google Scholar]
- Ellis, R.A.; Roberts, E.H. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Association of Official Seed Analysis (AOSA). Rules for testing seeds. J. Seed Technol. 1990, 12, 1–112. [Google Scholar]
- Arnon, D.T. Copper enzyme in isolated chloroplasts polyphenols oxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, S.P.; Farage, P.K.; Garcia, R.L. Measurement of leaf and canopy photosynthetic CO2 exchange in the field. J. Exp. Bot. 1996, 47, 1629–1642. [Google Scholar] [CrossRef] [Green Version]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalase and peroxidase. Meth. Enzym. 1955, 2, 764–775. [Google Scholar]
- Nakano, Y.; Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts: Its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid raintreated bean plants. Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dicky, D.A. Principles And Procedures Of Statistics, A Biometrical Approach, 3rd ed.; McGraw Hill, Inc. Book Co.: New York, NY, USA, 1997; pp. 352–358. [Google Scholar]
- Jena, A.; Sing, R.K.; Sing, M.K. Mitigation measures for wheat production under heat stress condition. Int. J. Agric. Sci. Res. 2017, 7, 359–376. [Google Scholar]
- Farooq, B.; Koul, B. Comparative analysis of the antioxidant, antibacterial and plant growth promoting potential of five Indian varieties of Moringa oleifera L. S. Afri. J. Bot. 2020, 129, 47–55. [Google Scholar] [CrossRef]
- Ashraf, M.; Athar, H.R.; Harris, P.J.C.; Kwon, T.R. Some prospective strategies for improving crop salt tolerance. Adv. Agron. 2008, 97, 45–110. [Google Scholar]
- Abdalla, M.M. The potential of Moringa oleifera extract as a biostimulant in enhancing the growth, biochemical and hormonal contents in rocket (Eruca vesicaria subsp. sativa) plants. Int. J. Plant Physiol. Biochem. 2013, 5, 42–49. [Google Scholar]
- Rehman, H.; Basra, S.M.A. Growing Moringa oleifera as a Multipurpose Tree; Some Agro-Physiological and Industrial Perspectives. American Chronicle. 28 May 2010. Available online: http://www.americanchronicle.com/articles/view/159447 (accessed on 21 May 2020).
- Rashid, N.; Khan, S.; Wahid, A.; Basra, S.M.A.; Alwahibi, M.S.; Jacobsen, S.E. Impact of Natural and Synthetic Growth Enhancers on the Productivity and Yield of Quinoa (Chenopodium quinoa Willd.) Cultivated under Normal and Late Sown Circumstances. J. Agron. Crop Sci. 2021, 1–15. [Google Scholar] [CrossRef]
- Rashid, N.; Khan, S.; Wahid, A.; Ibrar, D.; Irshad, S.; Bakhsh, A.; Hasnain, Z.; Alkahtani, J.; Alwahibi, M.S.; Gawwad, M.R.A.; et al. Exogenous application of moringa leaf extract improves growth, biochemical attributes, and productivity of late-sown quinoa. PLoS ONE 2021, 16, e0259214. [Google Scholar] [CrossRef] [PubMed]
- Owusu, D. Phytochemical Composition of Ipomea Batatus and Moringa Oleifera Leaves and Crackers from Underutilized Flours. Master’s Thesis, Department of Biochem and Biotech, Faculty of Bio Science, College of Science, Kwame Nkrumah University of Science Technology, Kumasi, Ghana, 2008. [Google Scholar]
- Yasmeen, A.; Basra, S.M.A.; Farooq, M.; Rehman, H.; Hussain, N.; Athar, H.R. Exogenous application of moringa leaf extract modulates the antioxidant enzyme system to improve wheat performance under saline conditions. Plant Growth Regul. 2013, 69, 225–233. [Google Scholar] [CrossRef]
- Farooq, O.; Ali, M.; Sarwar, N.; Rehman, A.; Iqbal, M.M.; Naz, T.; Asghar, M.; Ehsan, F.; Nasir, M.; Hussain, Q.M. Foliar applied brassica water extract improves the seedling development of wheat and chickpea. Asian J. Agric. Biol. 2021. [Google Scholar] [CrossRef]
- Fuglie, L.J. The Miracle Tree: Moringa oleifera: Natural Nutrition for the Tropics; Church World Service: Dakar, Senegal, 1999; p. 172. [Google Scholar]
- Swapna, S.; Shylaraj, K.S. Screening for osmotic stress responses in rice varieties under drought condition. Rice Sci. 2017, 24, 253–263. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, X.; Lv, T.; Bai, M.; Wand, Z.; Niu, J. Effects of water stress on fluorescence parameters and photosynthetic characteristics of drip irrigation in rice. Water 2020, 12, 289. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.P.; Zhu, D.F.; Lin, X.Q.; Chen, H.Z. Effects of water stress on rice growth and yield at different growth stages. Agric. Res. Arid Areas. 2005, 2, 48–53. [Google Scholar]
- Sun, L.F. Rice Roots of Drought Stress on the Photosynthetic Fluorescence Characteristic Influence. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2013. [Google Scholar]
- Safdar, M.E.; Aslam, A.; Qamar, R.; Ali, A.; Javaid, M.M.; Hayyat, M.S.; Raza, A. Allelopathic effect of prickly chaff flower (Achyranthes aspera L.) used as a tool for managing noxious weeds. Asian J. Agric. Biol 2021, 10. [Google Scholar] [CrossRef]
- Zahid, N.; Ahmed, M.J.; Tahir, M.M.; Maqbool, M.; Shah, S.Z.A.; Hussain, S.J.; Khaliq, A.; Rehmani, M.I.A. Integrated effect of urea and poultry manure on growth, yield and postharvest quality of cucumber (Cucumis sativus L.). Asian J. Agric. Biol. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Planta. 2003, 119, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- McKersie, B.D.; Bowley, S.R.; Jones, K.S. Winter survival of transgenic alfalfa overexpressing superoxide dismutase. Plant Physiol. 1999, 119, 839–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanafy, R. Using Moringa olifera Leaf Extract as a bio-fertilizer for drought stress mitigation of Glycine max L. plants. Egypt. J. Bot. 2017, 57, 281–292. [Google Scholar] [CrossRef]
- Mirzaee, M.; Moieni, A.; Ghanati, F. Effects of drought stress on the lipid peroxidation and antioxidant enzymes activities in two canola (Brassica napus L.) cultivars. J. Agric. Sci. Technol. 2013, 15, 593–602. [Google Scholar]
- Zaki, S.S.; Rady, M.M. Moringa oleifera leaf extract improves growth, physiochemical attributes, antioxidant defense system and yields of salt-stressed Phaseolus vulgaris L. plants. Int. J. Chem. Technol. Res. 2015, 8, 120–134. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).