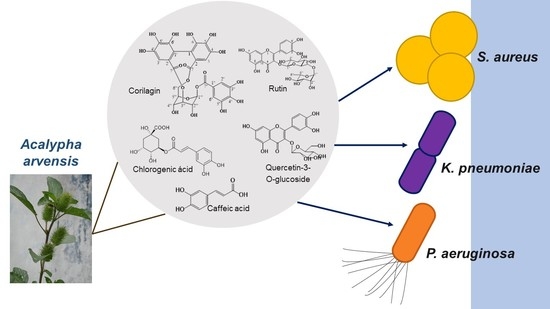
Ellagitannin, Phenols, and Flavonoids as Antibacterials from Acalypha arvensis (Euphorbiaceae)
Abstract
:1. Introduction
2. Results
2.1. Antibacterial Effect of A. arvensis’s Extracts
2.2. Antibacterial Activity of the AaEtOH Fractions (AaR1, AaR2, AaR3, AaR4, and AaR5)
2.3. HPLC Analysis of Extract and Fractions
2.4. Isolation and Structural Elucidation of Compounds 1–3
3. Discussion
4. Materials and Methods
4.1. Equipment and Reagents
4.2. Plant Material
4.3. Extracts
4.4. Isolation and Identification of Compounds (1–8)
4.5. Antibacterial Activity
4.5.1. Strains Used
4.5.2. In Vitro Evaluation of the Organic Extracts Using a Plate Dilution Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OMS. Datos Recientes Revelan los Altos Niveles de Resistencia a los Antibióticos en Todo el Mundo. Available online: https://www.who.int/es/news-room/detail/29-01-2018-high-levels-of-antibiotic-resistance-found-worldwide-new-data-shows (accessed on 2 December 2021).
- Giono-Cerezo, S.; Santos-Preciado, J.I.; Rayo Morfín-Otero, M.D.; Torres-López, F.J.; Alcántar-Curiel, M.D. Resistencia antimicrobiana. Importancia y esfuerzos por contenerla. Gac. Med. Mex. 2020, 156, 172–180. [Google Scholar] [CrossRef]
- Fauci, A.S. Emerging and reemerging infectious diseases: The perpetual challenge. Acad. Med. 2005, 80, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Watkins, K. Emerging infectious diseases: A review. Curr. Emerg. Hosp. Med. Rep. 2018, 6, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cordero, S.; Kirwan, R.; Noguera-Julian, A.; Cardellach, F.; Fortuny, C.; Morén, C. A Mitocentric View of the Main Bacterial and Parasitic Infectious Diseases in the Pediatric Population. Int. J. Mol. Sci. 2021, 22, 3272. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Sharma, A.; George, A.J.; Anvitha, D.; Kumar, P.; Dwivedi, V.P.; Chandra, N.S. Antibacterial activity of medicinal plants against ESKAPE: An update. Heliyon 2021, 7, e06310. [Google Scholar] [CrossRef]
- Velázquez Meza, M.E. Surgimiento y diseminación de Staphylococcus aureus meticilinorresistente. Salud Pública México 2005, 47, 381–387. [Google Scholar] [CrossRef] [PubMed]
- León-Buitimea, A.; Garza-Cárdenas, C.R.; Garza-Cervantes, J.A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. The Demand for New Antibiotics: Antimicrobial Peptides, Nanoparticles, and Combinatorial Therapies as Future Strategies in Antibacterial Agent Design. Front. Microbiol. 2020, 11, 1669. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar]
- Hemeg, H.A.; Moussa, I.M.; Ibrahim, S.; Dawoud, T.M.; Alhaji, J.H.; Mubarak, A.S.; Kabli, S.A.; Alsubki, R.A.; Tawfik, A.M.; Marouf, S.A. Antimicrobial effect of different herbal plant extracts against different microbial population. Saudi J. Biol. Sci. 2020, 27, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [Green Version]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241. [Google Scholar] [CrossRef] [Green Version]
- Standley, P.C.; Steyermark, J.A. Flora of Guatemala; Chicago Natural History Museum: Chicago, IL, USA, 1949; Volume 24, pp. 25–170. [Google Scholar]
- Méndez Gómez, E.; López Noverola, U.; López Naranjo, J.I.; Salaya Domínguez, J.M.; Díaz González, J.A. Catálogo de Plantas Medicinales de Uso Actual en el Estado de Tabasco, 1st ed.; Fundación Produce Tabasco-UJAT: Tabasco, Mexico, 2004; p. 39. [Google Scholar]
- Duke, J.A. Duke’s Handbook of Medicinal Plants of Latin America, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 4–5. [Google Scholar]
- Argueta, A.L.; Cano, L.; Asselein, L.; Rodarte, M.E. Atlas de las Plantas de la Medicina Tradicional Mexicana, 1st ed.; Instituto Nacional Indigenista: Ciudad de México, Mexico, 1994; p. 236. [Google Scholar]
- Vibrans, H. Acalypha arvensis Poepp. & Endl. (Hierba del Gusano). Available online: http://www.conabio.gob.mx/malezasdemexico/euphorbiaceae/acalypha-arvensis/fichas/ficha.htm (accessed on 2 December 2021).
- Cáceres, A.; Cano, O.; Samayoa, B.; Aguilar, L. Plants used in Guatemala for the treatment of gastrointestinal disorders. 1. Screening of 84 plants against enterobacteria. J. Ethnopharmacol. 1990, 30, 55–73. [Google Scholar] [CrossRef]
- Cáceres, A.; Alvarez, A.V.; Ovando, A.E.; Samayoa, B.E. Plants used in Guatemala for the treatment of respiratory diseases. 1. Screening of 68 plants against gram-positive bacteria. J. Ethnopharmacol. 1991, 31, 193–208. [Google Scholar] [CrossRef]
- Jiménez, M.; Cruz, S.M.; Cáceres, A. Determinación de la actividad biocida de cinco especies del genero Acalypha (A. guatemalensis, A. arvensis, A. polystaquia, A. hispida y A. pseudoalopecuroides). Rev. Científica (IIQB USAC) 2005, 3, 35–38. [Google Scholar]
- Yamada, H.; Nagao, K.; Dokei, K.; Kasai, Y.; Michihata, N. Total synthesis of (−)-Corilagin. J. Am. Chem. Soc. 2008, 130, 7566–7567. [Google Scholar] [CrossRef] [PubMed]
- Kochumadhavan, A.; Mangal, P.; Kumar, L.S.; Meenakshi, B.M.; Venkanna, B.U.; Muguli, G. Corilagin: First time isolation from the whole plant of Phyllanthus maderaspatensis L. Pharmacogn. Commun. 2019, 9, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Sudjaroen, Y.; Hull, W.E.; Erben, G.; Würtele, G.; Changbumrung, S.; Ulrich, C.M.; Owen, R.W. Isolation and characterization of ellagitannins as the major polyphenolic components of Longan (Dimocarpus longan Lour) seeds. Phytochemistry 2012, 77, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Hu, S.H.; Wang, J.T.; Chen, K.S.; Chia, Y.C. Hypoglycemic effect of extract of Hericium erinaceus. J. Sci. Food Agric. 2005, 85, 641–646. [Google Scholar] [CrossRef]
- Walters, K.R.; Pan, Q.; Serianni, A.S.; Duman, J.G. Cryoprotectant biosynthesis and the selective accumulation of threitol in the freeze-tolerant Alaskan beetle, Upis ceramboides. J. Biol. Chem. 2009, 284, 16822–16831. [Google Scholar] [CrossRef] [Green Version]
- Szymczyk, M. Unexpected course of Wittig reaction when using cinnamyl aldehyde as a substrate. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 264–266. [Google Scholar] [CrossRef]
- Pawlak, J.L.; Berchtold, G.A. Total synthesis of (−)-chorismic acid and (−)-shikimic acid. J. Org. Chem. 1987, 52, 1765–1771. [Google Scholar] [CrossRef]
- Seebaluck, R.; Gurib-Fakim, A.; Mahomoodally, F. Medicinal plants from the genus Acalypha (Euphorbiaceae)—A review of their ethnopharmacology and phytochemistry. J. Ethnopharmacol. 2015, 159, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, A.; Girón, L.M.; Alvarado, S.R.; Torres, M.F. Screening of antimicrobial activity of plants popularly used in Guatemala for the treatment of dermatomucosal diseases. J. Ethnopharmacol. 1987, 20, 223–237. [Google Scholar] [CrossRef]
- Lentz, D.L.; Clark, A.M.; Hufford, C.D.; Meurer-Grimes, B.; Passreiter, C.M.; Cordero, J.; Ibrahimi, O.; Okunade, A.L. Antimicrobial properties of Honduran medicinal plants. J. Ethnopharmacol. 1998, 63, 253–263. [Google Scholar] [CrossRef]
- Nino, J.; Mosquera, O.M.; Corea, Y.M. Antibacterial and antifungal activities of crude plant extracts from Colombian biodiversity. Rev. Biol. Trop. 2012, 60, 1535–1542. [Google Scholar] [CrossRef] [Green Version]
- Moreira, J.; Klein-Júnior, L.C.; Filho, V.C.; Buzzi, F.C. Anti-hyperalgesic activity of corilagin, a tannin isolated from Phyllanthus niruri L. (Euphorbiaceae). J. Ethnopharmacol. 2013, 146, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Duhnam, N.W.; Miya, T.S. A note on a simple apparatus for detecting neurobiological deficit in rats and mice. J. Am. Pharm. Assoc. 1957, 46, 208–209. [Google Scholar]
- Chen, Y.; Chen, C. Corilagin prevents tert-butyl hydroperoxide-induced oxidative stress injury in cultured N9 murine microglia cells. Neurochem. Int. 2011, 59, 290–296. [Google Scholar] [CrossRef]
- Kinoshita, S.; Inoue, Y.; Nakama, S.; Ichiba, T.; Aniya, Y. Antioxidant and hepatoprotective actions of medicinal herb, Terminalia catappa L. from Okinawa Island and its tannin corilagin. Phytomedicine 2007, 14, 755–762. [Google Scholar] [CrossRef]
- Hau, D.K.; Zhu, G.; Leung, A.K.; Wong, R.S.; Cheng, G.Y.; Lai, P.B.; Tong, S.; Lau, F.; Chan, K.; Wong, W.; et al. In vivo anti-tumor activity of corilagin on Hep3B hepatocellular carcinoma. Phytomedicine 2010, 18, 11–15. [Google Scholar] [CrossRef]
- Burapadaja, S.; Bunchoo, A. Antimicrobial Activity of Tannins from Terminalia citrina. Planta Med. 1995, 61, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, H.; Kayser, O.; Latt’e, K.P.; Ferreira, D. Evaluation of the antimicrobial potency of tannins and related compounds using the microdilution broth method. Planta Med. 1999, 65, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Boakye, Y.D.; Agyare, C.; Hensel, A. Anti-infective properties and time-kill kinetics of Phyllanthus muellerianus and its major constituent, geraniin. Med. Chem. Curr. Res. 2016, 6, 95–104. [Google Scholar] [CrossRef]
- Farha, A.K.; Yang, Q.-Q.; Kim, G.; Li, H.B.; Zhu, F.; Liu, H.Y.; Gan, R.Y.; Corke, H. Tannins as an alternative to antibiotics. Food Biosci. 2020, 38, 100751–100770. [Google Scholar] [CrossRef]
- Miao, M.; Xiang, L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020, 87, 71–88. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T.J. Antimicrobial Potential of Caffeic Acid against Staphylococcus aureus Clinical Strains. Biomed. Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parra, A.L.; Yhebra, R.S.; Sardiñas, I.G.; Buela, L.I. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine 2001, 8, 395–400. [Google Scholar]
- Thilakchand, K.R.; Mathai, R.T.; Simon, P.; Ravi, R.T.; Baliga-Rao, M.P.; Baliga, M.S. Hepatoprotective properties of the Indian gooseberry (Emblica officinalis Gaertn): A review. Food Funct. 2013, 4, 1431–1441. [Google Scholar] [CrossRef]
- Rozing, G. Micropillar array columns for advancing nanoflow HPLC. Microchem. J. 2021, 170, 106629. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Jiménez-Ferrer, E.; Román-Ramos, R.; Zamilpa, A.; González-Cortazar, M.; León-Rivera, I.; Vargas-Villa, G.; Herrera-Ruiz, M. A mixture of quercetin 4′-O-rhamnoside and isoquercitrin from Tilia americana var. mexicana and its biotransformation products with antidepressant activity in mice. J. Ethnopharmacol. 2021, 267, 113619. [Google Scholar] [CrossRef]
- Alegría-Herrera, E.; Herrera-Ruiz, M.; Román-Ramos, R.; Zamilpa, A.; Santillán-Urquiza, M.A.; Aguilar, M.I.; Aviles-Flores, M.; Fuentes-Mata, M.; Jiménez-Ferrer, E. Effect of Ocimum basilicum, Ocimum selloi, and rosmarinic acid on cerebral vascular damage in a chronic hypertension model. Biol. Pharm. Bull. 2019, 42, 201–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Hernández, C.; Rojo-Rubio, R.; Olmedo-Juárez, A.; Zamilpa, A.; Mendoza-de Gives, P.; Antonio-Romo, I.A.; Aguilar-Marcelino, L.; Arece-García, J.; Tapia-Maruri, D.; González-Cortazar, M. Galloyl derivatives from Caesalpinia coriaria exhibit in vitro ovicidal activity against cattle gastrointestinal parasitic nematodes. Exp. Parasitol. 2019, 200, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Navarro García, V.M.; Rojas, G.; Gerardo Zepeda, L.; Aviles, M.; Fuentes, M.; Herrera, A.; Jiménez, E. Antifungal and Antibacterial Activity of Four Selected Mexican Medicinal Plants. Pharm. Biol. 2006, 44, 297–300. [Google Scholar] [CrossRef]








| Extract (mg/mL) | |||||
|---|---|---|---|---|---|
| Bacteria | AaHex | AaAcOEt | AaEtOH | Control (+) | Control (−) |
| Sa | >2 | 2 | 2 | --- | * |
| SaRM1 | >2 | >2 | 2 | --- | * |
| SaRM2 | >2 | >2 | 2 | --- | * |
| Se | >2 | >2 | 2 | --- | * |
| Sh | >2 | >2 | >2 | --- | * |
| Ef | >2 | >2 | >2 | --- | * |
| Kp1 | >2 | >2 | 2 | --- | * |
| Kp2 | >2 | >2 | 2 | --- | * |
| Pa | >2 | 2 | 2 | --- | * |
| Ec1 | >2 | >2 | >2 | --- | * |
| Ec2 | >2 | >2 | >2 | --- | * |
| Ec3 | >2 | >2 | >2 | --- | * |
| Sd | >2 | >2 | >2 | --- | * |
| Fractions (mg/mL) | ||||||
|---|---|---|---|---|---|---|
| Bacteria | AaR2 | AaR3 | AaR4 | AaR5 | Control (+) | Control (−) |
| Sa | >2 | >2 | 1 | 0.5 | --- | * |
| SaRM1 | >2 | >2 | <0.5 | <0.5 | --- | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ble-González, E.A.; Gómez-Rivera, A.; Zamilpa, A.; López-Rodríguez, R.; Lobato-García, C.E.; Álvarez-Fitz, P.; Gutierrez-Roman, A.S.; Perez-García, M.D.; Bugarin, A.; González-Cortazar, M. Ellagitannin, Phenols, and Flavonoids as Antibacterials from Acalypha arvensis (Euphorbiaceae). Plants 2022, 11, 300. https://doi.org/10.3390/plants11030300
Ble-González EA, Gómez-Rivera A, Zamilpa A, López-Rodríguez R, Lobato-García CE, Álvarez-Fitz P, Gutierrez-Roman AS, Perez-García MD, Bugarin A, González-Cortazar M. Ellagitannin, Phenols, and Flavonoids as Antibacterials from Acalypha arvensis (Euphorbiaceae). Plants. 2022; 11(3):300. https://doi.org/10.3390/plants11030300
Chicago/Turabian StyleBle-González, Ever A., Abraham Gómez-Rivera, Alejandro Zamilpa, Ricardo López-Rodríguez, Carlos Ernesto Lobato-García, Patricia Álvarez-Fitz, Ana Silvia Gutierrez-Roman, Ma Dolores Perez-García, Alejandro Bugarin, and Manasés González-Cortazar. 2022. "Ellagitannin, Phenols, and Flavonoids as Antibacterials from Acalypha arvensis (Euphorbiaceae)" Plants 11, no. 3: 300. https://doi.org/10.3390/plants11030300
APA StyleBle-González, E. A., Gómez-Rivera, A., Zamilpa, A., López-Rodríguez, R., Lobato-García, C. E., Álvarez-Fitz, P., Gutierrez-Roman, A. S., Perez-García, M. D., Bugarin, A., & González-Cortazar, M. (2022). Ellagitannin, Phenols, and Flavonoids as Antibacterials from Acalypha arvensis (Euphorbiaceae). Plants, 11(3), 300. https://doi.org/10.3390/plants11030300