Characterization of Ingredients Incorporated in the Traditional Mixed-Salad of the Capuchin Monks
Abstract
:1. Introduction
2. Results and Discussion
2.1. Macro- and Micromorphological Characterization
2.2. Phytchemical Investigations
2.3. Health Properties
3. Materials and Methods
3.1. Plant Material and Growth Conditions
3.2. Scanning Electron Microscopy (SEM) Analysis
3.3. Mixed Salad Traditional Recipe
3.4. Chemicals
3.5. Sample Processing and Extract Preparation
3.6. Phytochemical Screening
3.6.1. Total Phenols
3.6.2. Flavonoids
3.6.3. Vanillin Index
3.6.4. Proanthocyanidins
3.6.5. Chlorophyll Determination
3.6.6. Carbohydrates
3.7. Evaluation of Health Properties: Antioxidant and Anti-Inflammatory Activity
3.7.1. Antioxidant Activity
Trolox Equivalent Antioxidant Capacity (TEAC) Assay
Ferric Reducing Antioxidant Power (FRAP) Assay
Oxygen Radical Absorbance Capacity (ORAC) Assay
Iron-Chelating Activity
3.7.2. Anti-Inflammatory Activity
Bovine Serum Albumin (BSA) Denaturation Assay
Protease Inhibition Assay
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Łuczaj, Ł. Ethnobotanical Review of Wild Edible Plants of Slovakia. Acta Soc. Bot. Pol. 2012, 81, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Savo, V.; Salomone, F.; Mattoni, E.; Tofani, D.; Caneva, G. Traditional Salads and Soups with Wild Plants as a Source of Antioxidants: A Comparative Chemical Analysis of Five Species Growing in Central Italy. Evid.-Based Complement. Altern. Med. 2019, 2019, 6782472. [Google Scholar] [CrossRef] [Green Version]
- Guarrera, P.M.; Savo, V. Wild Food Plants Used in Traditional Vegetable Mixtures in Italy. J. Ethnopharmacol. 2016, 185, 202–234. [Google Scholar] [CrossRef]
- Motti, R.; Bonanomi, G.; Lanzotti, V.; Sacchi, R. The Contribution of Wild Edible Plants to the Mediterranean Diet: An Ethnobotanical Case Study Along the Coast of Campania (Southern Italy). Econ. Bot. 2020, 74, 249–272. [Google Scholar] [CrossRef]
- Pieroni, A.; Nebel, S.; Quave, C.; Münz, H.; Heinrich, M. Ethnopharmacology of Liakra: Traditional Weedy Vegetables of the Arbëreshë of the Vulture Area in Southern Italy. J. Ethnopharmacol. 2002, 81, 165–185. [Google Scholar] [CrossRef]
- Leonti, M. The Future Is Written: Impact of Scripts on the Cognition, Selection, Knowledge and Transmission of Medicinal Plant Use and Its Implications for Ethnobotany and Ethnopharmacology. J. Ethnopharmacol. 2011, 134, 542–555. [Google Scholar] [CrossRef]
- Watson, S. On Hospitals. Welfare, Law, and Christianity in Western Europe; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Mądra-Gackowska, K.; Gackowski, M.; Główczewska-Siedlecka, E. Medications of Medieval Monastery Medicine Assessment of Nutritional Status of Geriatric Patients View Project. J. Educ. Health Sport 2018, 8, 1667–1674. [Google Scholar] [CrossRef]
- Jotischky, A. A Hermit’s Cookbook. Monks, Food and Fasting in the Middle Ages; Continuum International Publishing Group: London, UK, 2011. [Google Scholar]
- Ubrizsy, A. Funzione Degli Orti Botanici Nella Scuola Medica Dal XVI al XVIII Secolo in Italia. In La Formazione del Medico in età Moderna (secc. XVIXVIII), Atti della XXXVIII Tornata di Studi Storici dell’Arte Medica e della Scienza, Fermo, Italy, 20–22 maggio 2010; Sani, R., Zurlini, F., Eds.; EUM: Macerata, Italy, 2012; p. 37. [Google Scholar]
- Åsen, P.A. Plants of possible monastic origin, growing in the past or present, at medieval monastery grounds in Norway, Plants and Culture: Seeds of the cultural heritage of Europe. In Plants and Culture: Seeds of the Cultural Heritage of Europe; Morel, J.P., Mercuri, A.M., Eds.; Edipuglia: Bari, Italy, 2009; pp. 227–238. [Google Scholar]
- Giannetti, L. Italian Renaissance Food-Fashioning or the Triumph of Greens. Calif. Ital. Stud. 2010, 1, 1–16. [Google Scholar] [CrossRef]
- Cesarini, G. II convento dei Cappuccini di Viterbo: Un esempio di organico sviluppo territoriale. Bibl. Società 2008, 61, 24–26. [Google Scholar]
- Kiser, L.J. The Garden of St. Francis: Plants, Landscape, and Economy in Thirteenth-Century Italy. Environ. Hist. 2003, 8, 229–245. [Google Scholar] [CrossRef]
- Cassiano da Langasco. Un Pizzico di Misticanza. La Presa di Tabacco; Sorriso Francescano: Genova, Italy, 1990. [Google Scholar]
- Pereira, C.G.; Custódio, L.; Rodrigues, M.J.; Neng, N.R.; Nogueira, J.M.F.; Carlier, J.; Costa, M.C.; Varela, J.; Barreira, L. Avaliação Do Potencial Antioxidante e Perfil Fitoquímico Do Plantago coronopus. Braz. J. Biol. 2017, 77, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Romano, A. The Medicinal Potential of Plants from the Genus Plantago (Plantaginaceae). Ind. Crops Prod. 2016, 83, 213–226. [Google Scholar] [CrossRef]
- Bello, O.M.; Fasinu, P.S.; Bello, O.E.; Ogbesejana, A.B.; Adetunji, C.O.; Dada, A.O.; Ibitoye, O.S.; Aloko, S.; Oguntoye, O.S. Wild Vegetable Rumex acetosa Linn.: Its Ethnobotany, Pharmacology and Phytochemistry—A Review. S. Afr. J. Bot. 2019, 125, 149–160. [Google Scholar] [CrossRef]
- Korpelainen, H.; Pietiläinen, M. Sorrel (Rumex acetosa L.): Not Only a Weed but a Promising Vegetable and Medicinal Plant. Bot. Rev. 2020, 86, 234–246. [Google Scholar] [CrossRef]
- Hussain, M.; Raza, S.M.; Janbaz, K.H. Pharmacologically Mechanistic Basis for the Traditional Uses of Rumex acetosa in Gut Motility Disorders and Emesis. Bangladesh J. Pharmacol. 2015, 10, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Vasas, A.; Orbán-Gyapai, O.; Hohmann, J. The Genus Rumex: Review of Traditional Uses, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2015, 175, 198–228. [Google Scholar] [CrossRef]
- Cornara, L.; la Rocca, A.; Terrizzano, L.; Dente, F.; Mariotti, M.G. Ethnobotanical and Phytomedical Knowledge in the North-Western Ligurian Alps. J. Ethnopharmacol. 2014, 155, 463–484. [Google Scholar] [CrossRef]
- Storl, W.D. A Curious History of Vegetables: Aphrodisiacal and Healing Properties, Folk Tales, Garden Tips, and Recipes; North Atlantic Books: Berkeley, CA, USA, 2016. [Google Scholar]
- Janda, K.; Gutowska, I.; Geszke-Moritz, M.; Jakubczyk, K. The Common Cichory (Cichorium intybus L.) as a Source of Extracts with Health-Promoting Properties—A Review. Molecules 2021, 26, 1814. [Google Scholar] [CrossRef]
- Bahmani, M.; Rafieian-Kopaei, M.; Saki, K. Chicory: A Review on Ethnobotanical Effects of Cichorium intybus L. Microbiologi View Project Melatonin and Human Mitochondrial Diseases View Project. J. Chem. Pharm. Sci. 2015, 8, 672–682. [Google Scholar]
- Street, R.A.; Sidana, J.; Prinsloo, G. Cichorium intybus: Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. Evid.-Based Complement. Altern. Med. 2013, 2013, 579319. [Google Scholar] [CrossRef] [Green Version]
- Cornara, L.; la Rocca, A.; Marsili, S.; Mariotti, M.G. Traditional Uses of Plants in the Eastern Riviera (Liguria, Italy). J. Ethnopharmacol. 2009, 125, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Manfrinato, C.; Canella, M.; Ardenghi, N.M.; Guzzon, F. Traditional Use of Tarragon/Pèrschtròmm (Artemisia dracunculus L., Asteraceae) in the Linguistic Island of Sappada/Plodn (European Alps, Northern Italy). Ethnobot. Res. Appl. 2019, 18, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Obolskiy, D.; Pischel, I.; Feistel, B.; Glotov, N.; Heinrich, M. Artemisia dracunculus L. (Tarragon): A Critical Review of Its Traditional Use, Chemical Composition, Pharmacology, and Safety. J. Agric. Food Chem. 2011, 59, 11367–11384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekiert, H.; Świątkowska, J.; Knut, E.; Klin, P.; Rzepiela, A.; Tomczyk, M.; Szopa, A. Artemisia dracunculus (Tarragon): A Review of Its Traditional Uses, Phytochemistry and Pharmacology. Front. Pharmacol. 2021, 12, 653993. [Google Scholar] [CrossRef]
- Wang, J.; Fernández, A.E.; Tiano, S.; Huang, J.; Floyd, E.; Poulev, A.; Ribnicky, D.; Pasinetti, G.M. An Extract of Artemisia dracunculus L. Promotes Psychological Resilience in a Mouse Model of Depression. Oxidative Med. Cell. Longev. 2018, 2018, 7418681. [Google Scholar] [CrossRef] [Green Version]
- D’Ambrosio, A. II Potere Degli Erbaggi Salutiferi. Influenze Leonardiane e Mensa Fitoterapica Nei Secoli XVI-XVII. L’Idomeneo 2019, 28, 165–190. [Google Scholar] [CrossRef]
- Cornara, L.; Smeriglio, A.; Frigerio, J.; Labra, M.; di Gristina, E.; Denaro, M.; Mora, E.; Trombetta, D. The Problem of Misidentification between Edible and Poisonous Wild Plants: Reports from the Mediterranean Area. Food Chem. Toxicol. 2018, 119, 112–121. [Google Scholar] [CrossRef]
- Colombo, M.L.; Assisi, F.; della Puppa, T.; Moro, P.; Sesana, F.M.; Bissoli, M.; Borghini, R.; Perego, S.; Galasso, G.; Banfi, E.; et al. Most Commonly Plant Exposures and Intoxications from Outdoor Toxic Plants. J. Pharm. Sci. Res. 2010, 2, 417. [Google Scholar]
- Chelsea Physic Garden. The Herb Almanac: A Seasonal Guide to Medicinal Plants; Aster: Hachette, UK, 2021; p. 160. [Google Scholar]
- Rana Disel, N.; Yilmaz, M.; Kekec, Z.; Karanlik, M. Poisoned after Dinner: Dolma with Datura Stramonium. Turk. J. Emerg. Med. 2015, 15, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Gilotta, I.; Brvar, M. Accidental Poisoning with Veratrum album Mistaken for Wild Garlic (Allium ursinum). Clin. Toxicol. 2010, 48, 949–952. [Google Scholar] [CrossRef]
- Huxtable, R.J. The Harmful Potential of Herbal and Other Plant Products. Drug Saf. 1990, 5, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Smekens, M.J.; van Tienderen, P.H. Genetic Variation and Plasticity of Plantago coronopus under Saline Conditions. Acta Oecologica 2001, 22, 187–200. [Google Scholar] [CrossRef]
- Gemeinholzer, B.; Bachmann, K. Examining Morphological and Molecular Diagnostic Character States of Cichorium intybus L. (Asteraceae) and C. spinosum L. Plant Syst. Evol. 2005, 253, 105–123. [Google Scholar] [CrossRef]
- Ianovici, N. Histoanatomical Studies on Some Halophytes from Romania-Plantago coronopus. Biomonitoring in Urban Environment View Project COST Action FA 1203-Sustainable Management of Ambrosia artemisiifolia in Europe (SMARTER); 2013–2017 View Project; West University of Timişoara: Timișoara, Romania, 2010. [Google Scholar]
- Andrzejewska-Golec, E. Hair morphology in Plantago sect. Coronopus (Plantaginaceae). Plant Syst. Evol. 1992, 179, 107–113. [Google Scholar] [CrossRef]
- Yasmin, Y.G.; Khan, K.M.A.; Shaheen, S.N. Micromorphological Investigation of Foliar Anatomy of Fagopyrum Mill., and Rumex L. of Polygonaceae. Pak. J. Bot. 2010, 42, 47–57. [Google Scholar]
- El-Alfy, E.T.; El-Askary, E.H.; El-Tawab, E.S.; MKamel, M.S. A Pharmacognostical Study of Cichorium intybus L. Herb Growing in Egypt. Bull. Fac. Pharm. Cairo Univ. 2009, 47, 77–99. [Google Scholar]
- Krak, K.; Mráz, P. Trichomes in the Tribe Lactuceae (Asteraceae)—Taxonomic Implications. Biologia 2008, 63, 616–630. [Google Scholar] [CrossRef]
- Ivashchenko, I.V.; Rakhmetov, D.B.; Ivanenko, G.F. Anatomical Features of Leaf Blade of Artemisia dracunculus L. (Asteraceae) during Introduction in Zhytomyr Polissya. Mod. Phytomorphol. 2015, 8, 123–130. [Google Scholar] [CrossRef]
- Yilmaz, C.; Gökmen, V. Chlorophyll. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 37–41. [Google Scholar]
- Janković, T.; Zdunić, G.; Beara, I.; Balog, K.; Pljevljakušić, D.; Stešević, D.; Šavikin, K. Comparative Study of Some Polyphenols in Plantago Species. Biochem. Syst. Ecol. 2012, 42, 69–74. [Google Scholar] [CrossRef]
- Chukwumah, Y.; Walker, L.; Vogler, B.; Verghese, M. Changes in the Phytochemical Composition and Profile of Raw, Boiled, and Roasted Peanuts. J. Agric. Food Chem. 2007, 55, 9266–9273. [Google Scholar] [CrossRef]
- White, B.L.; Howard, L.R.; Prior, R.L. Impact of Different Stages of Juice Processing on the Anthocyanin, Flavonol, and Procyanidin Contents of Cranberries. J. Agric. Food Chem. 2011, 59, 4692–4698. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.; Kammerer, D.R.; Carle, R. Impact of Blanching on Polyphenol Stability and Antioxidant Capacity of Innovative Coriander (Coriandrum sativum L.) Pastes. Food Chem. 2013, 140, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Tian, C.; Luo, J.; Zhou, R.; Sun, X.; Ma, J. Influence of Technical Processing Units on Polyphenols and Antioxidant Capacity of Carrot (Daucus carrot L.) Juice. Food Chem. 2013, 141, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.; Carle, R.; Kammerer, D.R. Effects of Blanching on Polyphenol Stability of Innovative Paste-like Parsley (Petroselinum crispum (Mill.) Nym Ex A. W. Hill) and Marjoram (Origanum majorana L.) Products. Food Chem. 2013, 138, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Sablani, S.S.; Andrews, P.K.; Davies, N.M.; Walters, T.; Saez, H.; Syamaladevi, R.M.; Mohekar, P.R. Effect of Thermal Treatments on Phytochemicals in Conventionally and Organically Grown Berries. J. Sci. Food Agric. 2010, 90, 769–778. [Google Scholar] [CrossRef]
- Bernaert, N.; de Loose, M.; van Bockstaele, E.; van Droogenbroeck, B. Antioxidant Changes during Domestic Food Processing of the White Shaft and Green Leaves of Leek (Allium ampeloprasum Var. Porrum). J. Sci. Food Agric. 2014, 94, 1168–1174. [Google Scholar] [CrossRef]
- Ku, C.S.; Mun, S.P.; Jang, J.P. Effects of Water Extraction Temperatures on the Yield, Molecular Weight, and Antioxidant Activity of Proanthocyanidins Extracted from Pinus Radiata Bark. For. Prod. J. 2011, 61, 321–325. [Google Scholar] [CrossRef]
- CREA. Linee Guida per una sana Alimentazione 2018. Available online: https://www.crea.gov.it/documents/59764/0/LINEE-GUIDA+DEFINITIVO.pdf/28670db4-154c-0ecc-d187-1ee9db3b1c65?t=1576850671654 (accessed on 16 January 2022).
- Puccinelli, M.; Pezzarossa, B.; Pintimalli, L.; Malorgio, F. Selenium Biofortification of Three Wild Species, Rumex acetosa L., Plantago coronopus L., and Portulaca oleracea L., Grown as Microgreens. Agronomy 2021, 11, 1155. [Google Scholar] [CrossRef]
- Jan, G.; Kahan, M.; Ahmad, M.; Iqbal, Z.; Afzal, A.; Afzal, M.; Shah, G.M.; Majid, A.; Fiaz, M.; Zafar, M.; et al. Nutritional analysis, micronutrients and chlorophyll contents of Cichorium intybus L. J. Med. Plants Res. 2011, 5, 2452–2456. [Google Scholar]
- Liopa-Tsakalidi, A.; Chalikiopoulos, D.; Papasavvas, A. Effect of chitin on growth and chlorophyll content of two medicinal plants. J. Med. Plants Res. 2010, 4, 499–508. [Google Scholar]
- Nartnampong, A.K.W.; Porasuphatana, S. Blanching process increases health promoting phytochemicals in green leafy Thai vegetables. Int. Food Res. J. 2016, 23, 2426–2435. [Google Scholar]
- Xiao, H.W.; Pan, Z.; Deng, L.-Z.; El-Mashad, H.M.; Yang, X.-H.; Mujumdar, A.S.; Gao, Z.-J.; Zhang, Q. Recent developments and trends in thermal blanching—A comprehensive review. IPA 2017, 4, 101–127. [Google Scholar] [CrossRef]
- Smeriglio, A.; de Francesco, C.; Denaro, M.; Trombetta, D. Prickly Pear Betalain-Rich Extracts as New Promising Strategy for Intestinal Inflammation: Plant Complex vs. Main Isolated Bioactive Compounds. Front. Pharmacol. 2021, 12, 2067. [Google Scholar] [CrossRef]
- Lin, B.; Johnson, B.J.; Rubin, R.A.; Malanoski, A.P.; Ligler, F.S. Iron Chelation by Cranberry Juice and Its Impact on Escherichia coli Growth. BioFactors 2011, 37, 121–130. [Google Scholar] [CrossRef]
- Yun, S.; Chu, D.; He, X.; Zhang, W.; Feng, C. Protective Effects of Grape Seed Proanthocyanidins against Iron Overload-Induced Renal Oxidative Damage in Rats. J. Trace Elem. Med. Biol. 2020, 57, 126407. [Google Scholar] [CrossRef]
- Maffei Facino, R.; Carini, M.; Aldini, G.; Bombardelli, E.; Morazzoni, P.; Morelli, R. Free radicals scavenging action and anti-enzyme activities of procyanidines from Vitis vinifera. A mechanism for their capillary protective action. Arzneimittelforschung 1994, 44, 592–601. [Google Scholar]
- Singh, P.; Bajpai, V.; Khandelwal, N.; Varshney, S.; Gaikwad, A.N.; Srivastava, M.; Singh, B.; Kumar, B. Determination of Bioactive Compounds of Artemisia Spp. Plant Extracts by LC–MS/MS Technique and Their in-Vitro Anti-Adipogenic Activity Screening. J. Pharm. Biomed. Anal. 2021, 193, 113707. [Google Scholar] [CrossRef]
- Bergantin, C.; Maietti, A.; Cavazzini, A.; Pasti, L.; Tedeschi, P.; Brandolini, V.; Marchetti, N. Bioaccessibility and HPLC-MS/MS Chemical Characterization of Phenolic Antioxidants in Red Chicory (Cichorium intybus). J. Funct. Foods 2017, 33, 94–102. [Google Scholar] [CrossRef]
- Ferrare, K.; Bidel, L.P.R.; Awwad, A.; Poucheret, P.; Cazals, G.; Lazennec, F.; Azay-Milhau, J.; Tournier, M.; Lajoix, A.-D.; Tousch, D. Increase in Insulin Sensitivity by the Association of Chicoric Acid and Chlorogenic Acid Contained in a Natural Chicoric Acid Extract (NCRAE) of Chicory (Cichorium intybus L.) for an Antidiabetic Effect. J. Ethnopharmacol. 2018, 215, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Poorter, H.; Fiorani, F.; Stitt, M.; Schurr, U.; Finck, A.; Gibon, Y.; Usadel, B.; Munns, R.; Atkin, O.K.; Tardieu, F.; et al. The Art of Growing Plants for Experimental Purposes: A Practical Guide for the Plant Biologist. Funct. Plant Biol. 2012, 39, 821–838. [Google Scholar] [CrossRef] [Green Version]
- Lenka, S.K. Cultivation of Crops under Shade-Net Greenhouse. In Protected Cultivation and Smart Agriculture; New Delhi Publishers: New Delhi, India, 2020. [Google Scholar]
- Chieco, C.; Rotondi, A.; Morrone, L.; Rapparini, F.; Baraldi, R. An Ethanol-Based Fixation Method for Anatomical and Micro-Morphological Characterization of Leaves of Various Tree Species. Biotech. Histochem. 2013, 88, 109–119. [Google Scholar] [CrossRef]
- Boverio, Z. De Sacris Ritibus iuxta Romanam Regulam vsui Fratrum Minorum S. Francisci, qui vulgo Capuccini nuncupantur accommodatis; Typis Scorigianis: Napoli, Italy, 1626. [Google Scholar]
- Massonio, S. Archidipno Overo Dell’insalata e Dell’uso di Es; Marcantonio Brogiollo: Venezia, Italy, 1990. [Google Scholar]
- Cucinotta, F.; Pieroni, A. “If You Want to Get Married, You Have to Collect Virdura”: The Vanishing Custom of Gathering and Cooking Wild Food Plants on Vulcano, Aeolian Islands, Sicily. Food Cult. Soc. 2018, 21, 539–567. [Google Scholar] [CrossRef]
- Sicari, V.; Loizzo, M.R.; Sanches Silva, A.; Romeo, R.; Spampinato, G.; Tundis, R.; Leporini, M.; Musarella, C.M. The Effect of Blanching on Phytochemical Content and Bioactivity of Hypochaeris and Hyoseris Species (Asteraceae), Vegetables Traditionally Used in Southern Italy. Foods 2021, 10, 32. [Google Scholar] [CrossRef]
- Bazzicalupo, M.; Cornara, L.; Burlando, B.; Cascini, A.; Denaro, M.; Smeriglio, A.; Trombetta, D. Carpobrotus edulis (L.) N.E.Br. Extract as a Skin Preserving Agent: From Traditional Medicine to Scientific Validation. J. Integr. Med. 2021, 19, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Denaro, M.; Trombetta, D.; Ragusa, S.; Circosta, C. New Insights on Euphorbia dendroides L. (Euphorbiaceae): Polyphenol Profile and Biological Properties of Hydroalcoholic Extracts from Aerial Parts. Plants 2021, 10, 1621. [Google Scholar] [CrossRef]
- Boudjelal, A.; Smeriglio, A.; Ginestra, G.; Denaro, M.; Trombetta, D. Phytochemical Profile, Safety Assessment and Wound Healing Activity of Artemisia absinthium L. Plants 2020, 9, 1744. [Google Scholar] [CrossRef]
- Baali, F.; Boumerfeg, S.; Boudjelal, A.; Denaro, M.; Ginestra, G.; Baghiani, A.; Righi, N.; Deghima, A.; Benbacha, F.; Smeriglio, A.; et al. Wound-Healing Activity of Algerian Lavandula stoechas and Mentha pulegium Extracts: From Traditional Use to Scientific Validation. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2021, 1–13. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; di Gristina, E.; Mastracci, L.; Grillo, F.; Cornara, L.; Trombetta, D. Pharmacognostic Approach to Evaluate the Micromorphological, Phytochemical and Biological Features of Citrus lumia Seeds. Food Chem. 2022, 375, 131855. [Google Scholar] [CrossRef]
- Muscarà, C.; Smeriglio, A.; Trombetta, D.; Mandalari, G.; la Camera, E.; Grassi, G.; Circosta, C. Phytochemical characterization and biological properties of two standardized extracts from a non-psychotropic Cannabis sativa L. cannabidiol (CBD)-chemotype. Phytother. Res. 2021, 35, 5269–5281. [Google Scholar] [CrossRef]
- Smeriglio, A.; Bonasera, S.; Germanò, M.P.; D’Angelo, V.; Barreca, D.; Denaro, M.; Monforte, M.T.; Galati, E.M.; Trombetta, D. Opuntia ficus-indica (L.) Mill. Fruit as Source of Betalains with Antioxidant, Cytoprotective, and Anti-angiogenic Properties. Phytother. Res. 2019, 33, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Muscarà, C.; Smeriglio, A.; Trombetta, D.; Mandalari, G.; la Camera, E.; Occhiuto, C.; Grassi, G.; Circosta, C. Antioxidant and antimicrobial activity of two standardized extracts from a new Chinese accession of non-psychotropic Cannabis sativa L. Phytother. Res. 2021, 35, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Denaro, M.; Smeriglio, A.; Trombetta, D. Antioxidant and Anti-Inflammatory Activity of Citrus Flavanones Mix and Its Stability after In Vitro Simulated Digestion. Antioxidants 2021, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Denaro, M.; D’Angelo, V.; Germanò, M.P.; Trombetta, D. Antioxidant, Anti-Inflammatory and Anti-Angiogenic Properties of Citrus lumia Juice. Front. Pharmacol. 2020, 11, 2033. [Google Scholar] [CrossRef] [PubMed]

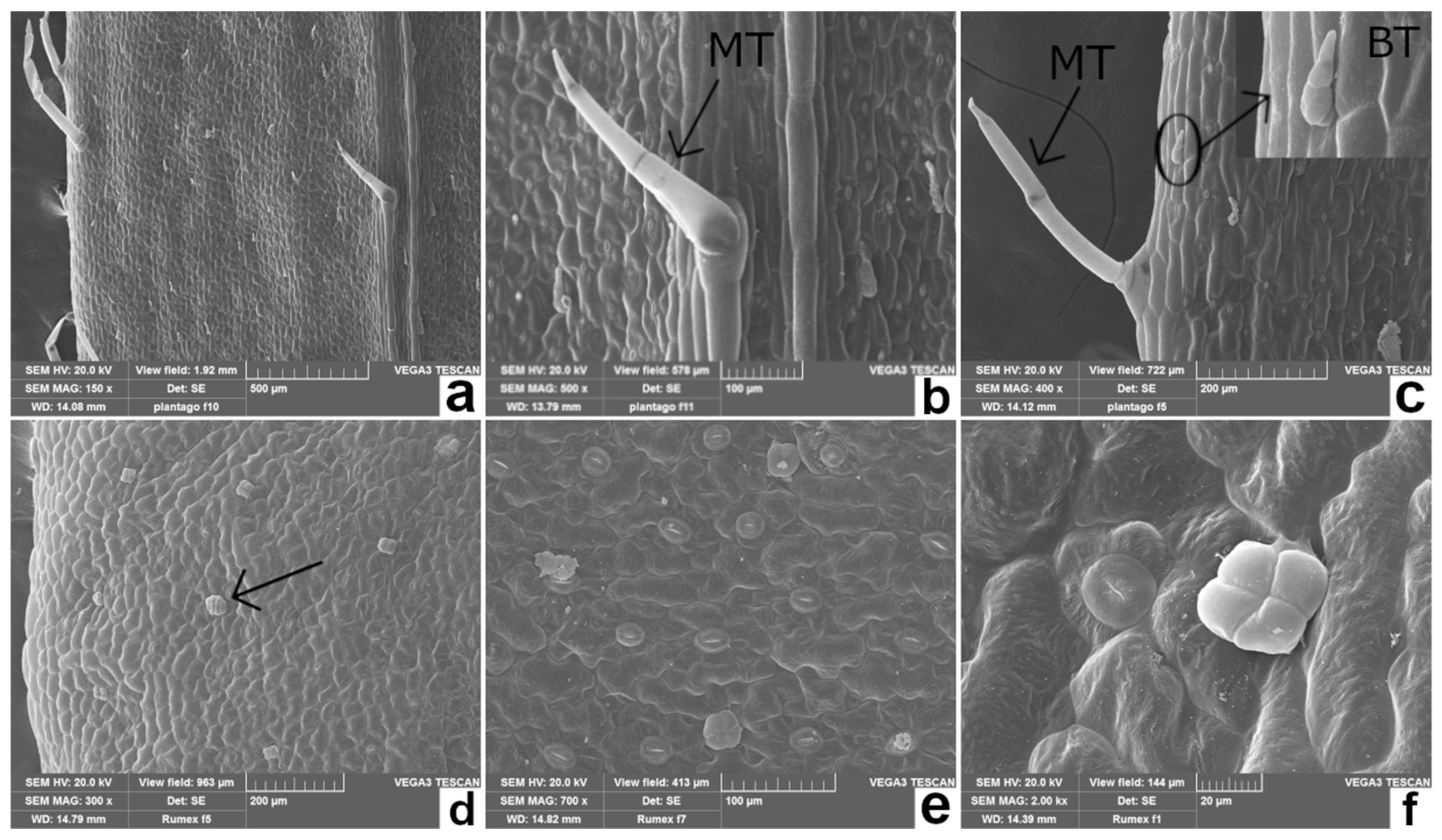

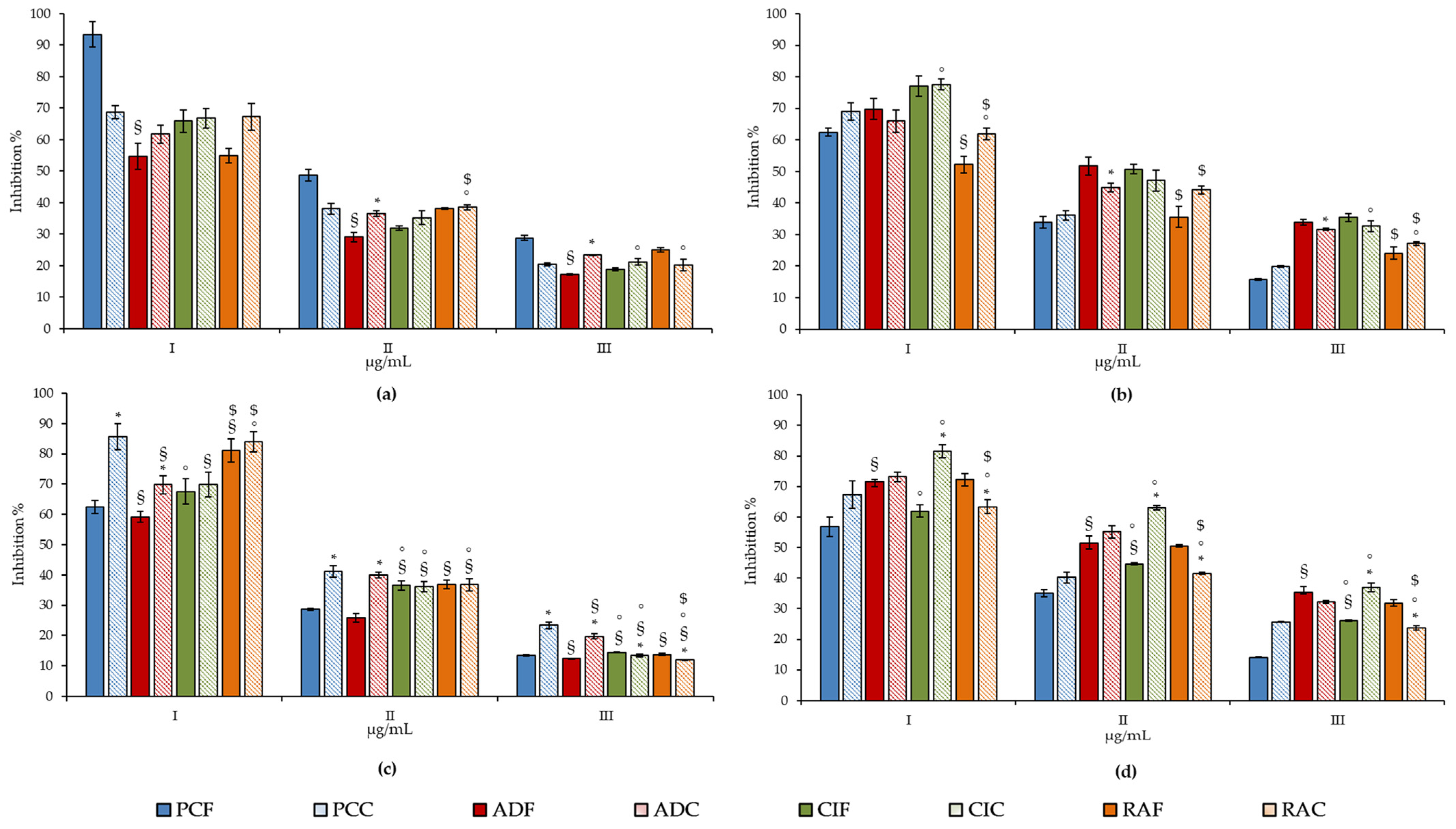
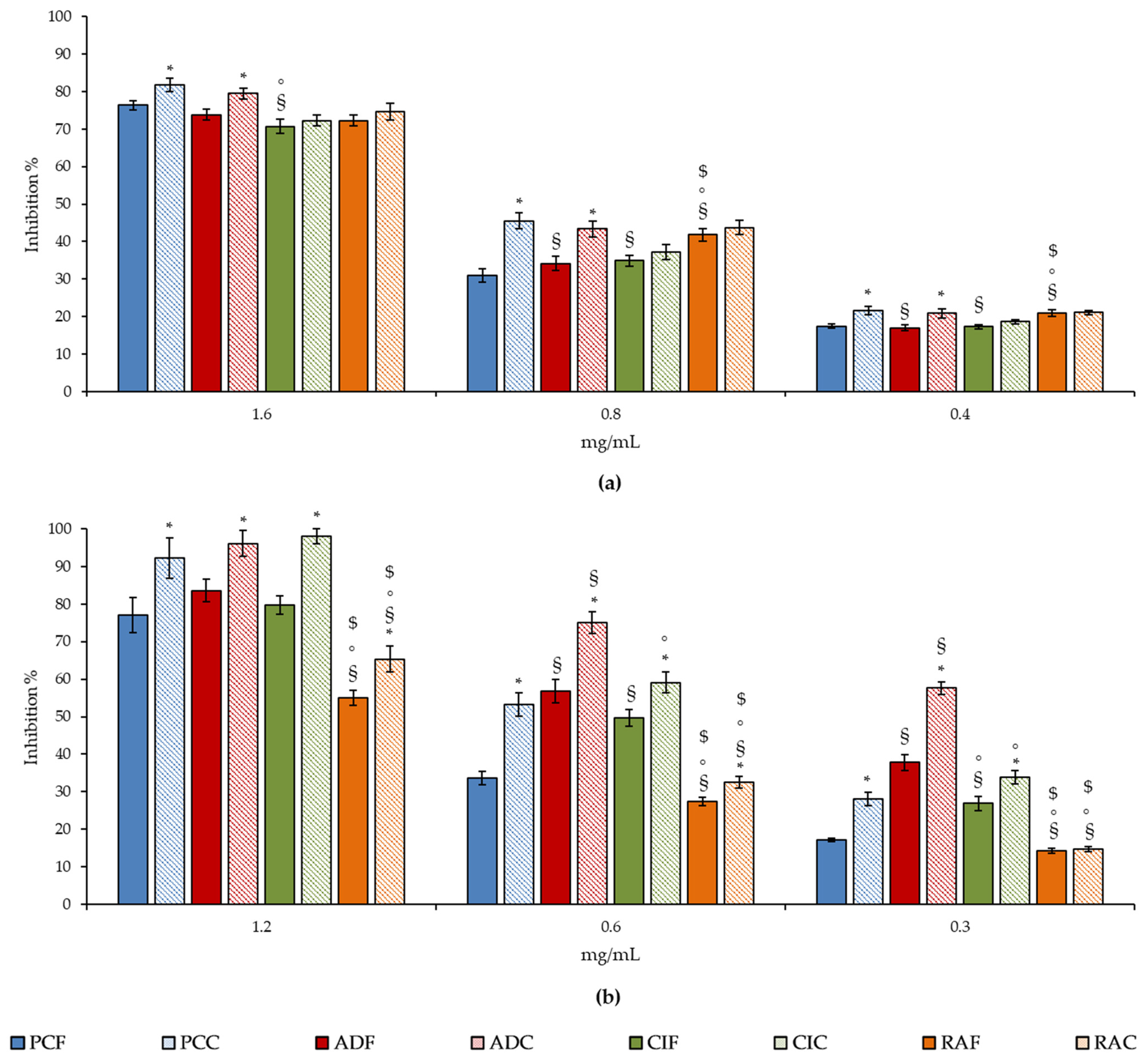
| Species | Leaf Macromorphological Features | Leaf Micromorphological Features |
|---|---|---|
| Plantago coronopus L. (PC) Buck’s-horn plantain (Figure 1a) | Pubescent, toothed at the tip, slightly fleshy; narrow and pinnately lobed, arranged in a dense ascending rosette at the apex of a short stem [41]. | Figure 2a–c Epidermal cells: rectangular with almost straight cell walls. Stomata apparatus: diacytic-type. Trichomes: two non-glandular types: bottle-like and larger, long stalked, multicellular trichomes [42]. Rarely, secretory trichomes could be observed [41]. |
| Rumex acetosa L. (RA) Common sorrel (Figure 1b) | Large, ovate, hairless, fleshy; the lobes of basal leaves are pointed, and the petiole elongated; the stem leaves are almost stalkless [18]. | Figure 2d–f Epidermal cells: irregularly shaped, with slightly undulating cell walls. Stomata apparatus: anisocytic and paracytic types. Trichomes: non-glandular trichomes lacking; glandular trichomes peltate, normally showing four-celled secretory heads [43]. |
| Cichorium intybus L. (CI) Chicory (Figure 1c) | Hairy, arranged in an ascending rosette; oblong lanceolate, pinnate shape; basal leaves oblanceolate, toothed, with short petiole; cauline leaves smaller and sessile [24]. | Figure 3a–c Epidermal cells: undulating cell walls. Stomata apparatus: anomocytic-type [44]. Trichomes: mutiseriate glandular trichomes on abaxial surface; multiseriate non-glandular trichomes with non-projecting cell apices on both surfaces [45]. |
| Artemisia dracunculus L. (AD) Wild tarragon (Figure 1d) | Sessile, arranged alternately along the stem, with a sharp tip and entire leaf margins; lower leaves tripartite at the apex, the middle and upper leaves are lanceolate [30]. | Figure 3d–f Epidermal cells: highly undulating cell walls. Stomata apparatus: anomocytic-type. Trichomes: stellate non-glandular trichomes and biseriate glandular trichomes with a subcuticular space filled with secondary compounds [46]. |
| Plant Extracts | Total Phenols | Flavonoids | Vanillin Index | Proanthocyanidins | Total Chlorophyll | Carbohydrates |
|---|---|---|---|---|---|---|
| PCF | 1704.00 ± 93.22 a,d | 8594.25 ± 43.49 a,d | 446.96 ± 35.67 a,d | 1.27 ± 0.01 a,d | 0.93 ± 0.02 a | 0.60 ± 0.02 a,d |
| RAF | 644.71 ± 25.28 b,e | 2364.09 ± 7.01 b,e | 53.74 ± 3.88 b | 6.00 ± 0.25 b,e | 1.04 ± 0.03 b,e | 0.39 ± 0.01 b,e |
| CIF | 1025.83 ± 95.63 f | 3613.59 ± 68.05 c,f | 10.75 ± 0.67 c,f | 3.73 ± 0.17 c,f | 1.14 ± 0.02 f | 0.54 ± 0.02 c,f |
| ADF | 1170.29 ± 101.90 | 1567.33 ± 95.21 g | 134.36 ± 5.24 g | 0.07 ± 0.00 g | 1.13 ± 0.01 | 0.48 ± 0.01 |
| PCC | 0.88 ± 686.37 a | 0.83 ± 1002.87 a | 557.38 ± 10.34 a | 16.33 ± 0.58 a | 0.94 ± 0.02 a | 1.92 ± 0.03 a |
| RAC | 1202.31 ± 50.21 b | 2921.38 ± 83.13 b | 60.65 ± 3.94 b | 2.83 ± 0.05 b | 0.84 ± 0.01 b | 0.14 ± 0.00 b |
| CIC | 1476.96 ± 61.66 c | 6355.65 ± 93.30 c | 17.47 ± 0.49 c | 9.55 ± 0.12 c | 1.05 ± 0.03 | 0.21 ± 0.00 c |
| ADC | 1298.73 ± 84.36 | 8767.83 ± 430.86 | 685.52 ± 5.23 | 13.13 ± 0.24 | 1.09 ± 0.04 | 0.48 ± 0.01 |
| Plant Extracts | TEAC | FRAP | ORAC | ICA | BDA | APA |
|---|---|---|---|---|---|---|
| PCF | 230.20 (190.47–278.22) a,d | 244.38 (171.30–348.57) a,d | 2.41 (1.94–2.50) d,g | 22.66 (19.28–26.62) a,d | 970.22 (405.66–2330.12) | 701.55 (350.55–1430.22) |
| RAF | 1111.82 (831.15–1487.27) b,e | 1970.96 (1493.70–2600.70) b,e | 5.67 (4.60–6.98) b,e | 6.63 (5.79–7.60) c | 910.11 (762.33–1092.11) | 1141.22 (891.99–1480.21) b |
| CIF | 510.96 (419.14–622.89) f | 841.48 (709.86–997.51) c,f | 1.89 (1.50–2.38) f | 7.80 (6.53–9.33) | 1020.31 (862.05–1224.58) | 540.34 (462.22–640.12) |
| ADF | 331.66 (258.54–425.47) | 546.40 (444.0–672.0) | 1.36 (0.95–1.96) | 9.85 (8.10–11.97) | 980.08 (842.34–1162.66) | 441.22 (380.05–510.11) |
| PCC | 31.89 (27.06–37.59) a | 31.35 (26.51–37.09) a | 0.30 (0.25–0.37) a | 9.05 (7.49–10.95) | 760.25 (650.22–890.27) | 440.04 (190.10–992.90) |
| RAC | 377.70 (296.34–481.39) | 383.59 (322.90–455.69) | 1.94 (1.58–2.39) f | 6.52 (5.76–7.38) | 861.33 (731.22–1020.66) | 871.22 (730.22–044.10) b |
| CIC | 273.80 (228.12–328.63) | 396.67 (331.67–474.40) | 1.10 (0.89–1.36) | 7.64 (6.47–9.02) | 970.02 (812.49–1151.08) | 371.02 (142.04–960.07) |
| ADC | 278.10 (220.15–353.57) | 425.20 (343.0–527.0) | 1.22 (0.93–1.60) | 7.11 (6.06–8.35) | 812.42 (692.77–951.55) | 240.20 (212.33–298.99) c |
| Standard | 3.28 (2.44–3.89) h | 3.88 (1.62–5.78) h | 0.79 (0.39–1.65) i | 6.48 (5.22–7.68) l | 31.82 (26.58–38.10) h | 32.88 (25.22–39.13) h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornara, L.; Ambu, G.; Alberto, A.; Trombetta, D.; Smeriglio, A. Characterization of Ingredients Incorporated in the Traditional Mixed-Salad of the Capuchin Monks. Plants 2022, 11, 301. https://doi.org/10.3390/plants11030301
Cornara L, Ambu G, Alberto A, Trombetta D, Smeriglio A. Characterization of Ingredients Incorporated in the Traditional Mixed-Salad of the Capuchin Monks. Plants. 2022; 11(3):301. https://doi.org/10.3390/plants11030301
Chicago/Turabian StyleCornara, Laura, Gabriele Ambu, Alex Alberto, Domenico Trombetta, and Antonella Smeriglio. 2022. "Characterization of Ingredients Incorporated in the Traditional Mixed-Salad of the Capuchin Monks" Plants 11, no. 3: 301. https://doi.org/10.3390/plants11030301
APA StyleCornara, L., Ambu, G., Alberto, A., Trombetta, D., & Smeriglio, A. (2022). Characterization of Ingredients Incorporated in the Traditional Mixed-Salad of the Capuchin Monks. Plants, 11(3), 301. https://doi.org/10.3390/plants11030301









