Identification and Functional Characterization of FLOWERING LOCUS T in Platycodon grandiflorus
Abstract
:1. Introduction
2. Results and Discussion
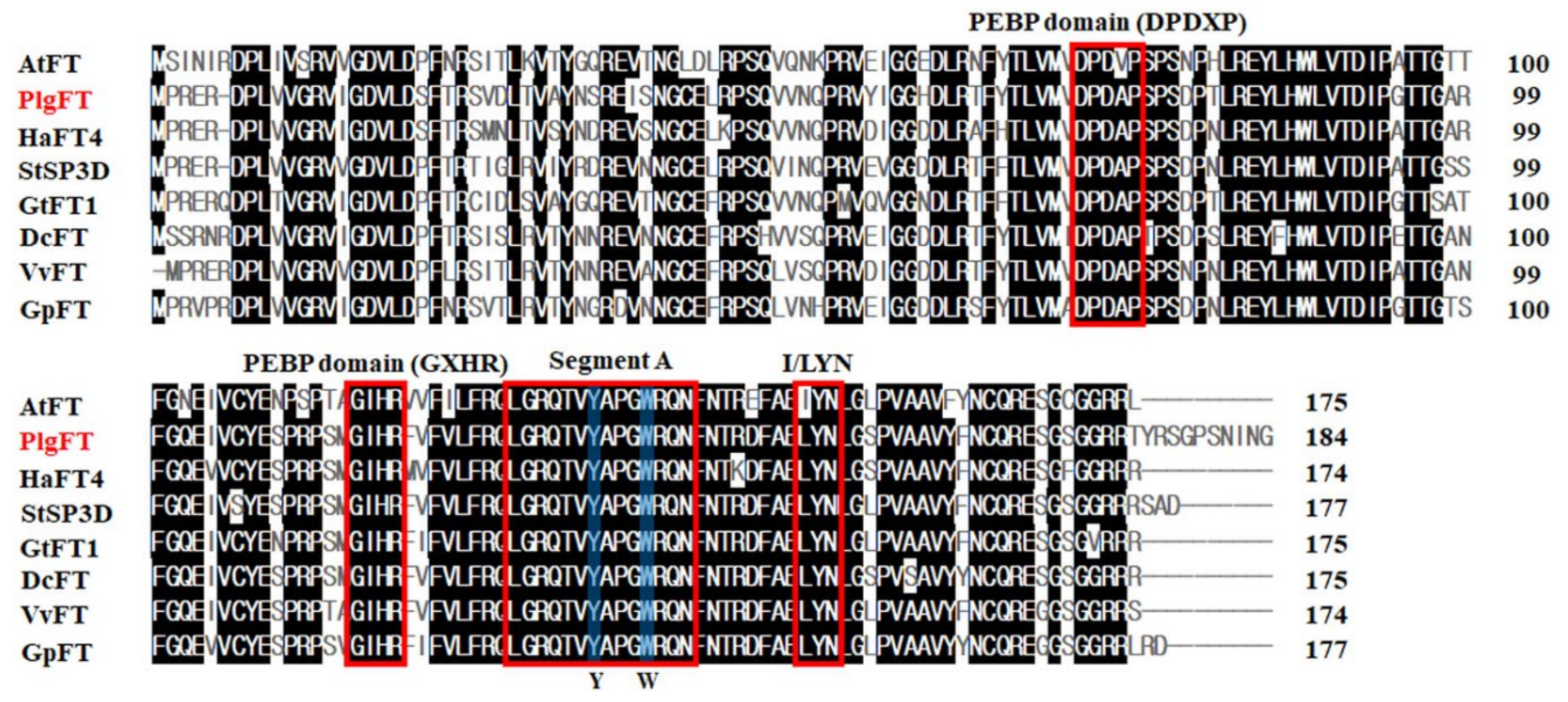
2.1. Identification of FT Gene in P. grandiflorus
2.2. Expression Pattern of PlgFT in Various Organs
2.3. The Diurnal Expression Pattern of PlgFT
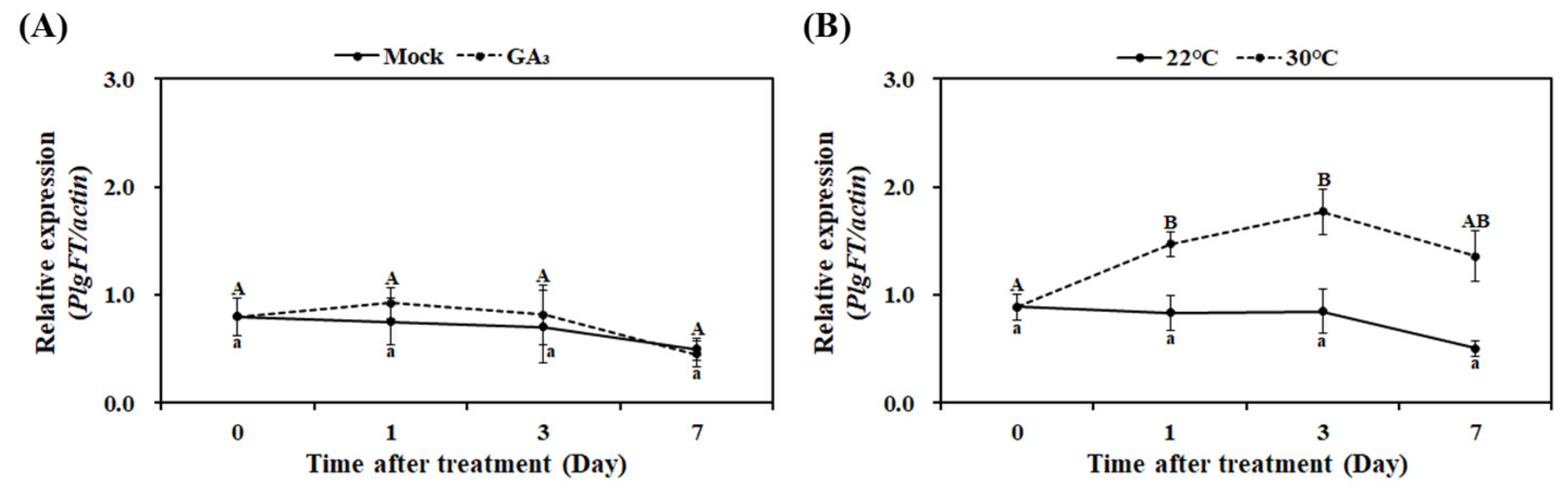
2.4. Effects of High Temperature and GA3 on PlgFT Expression
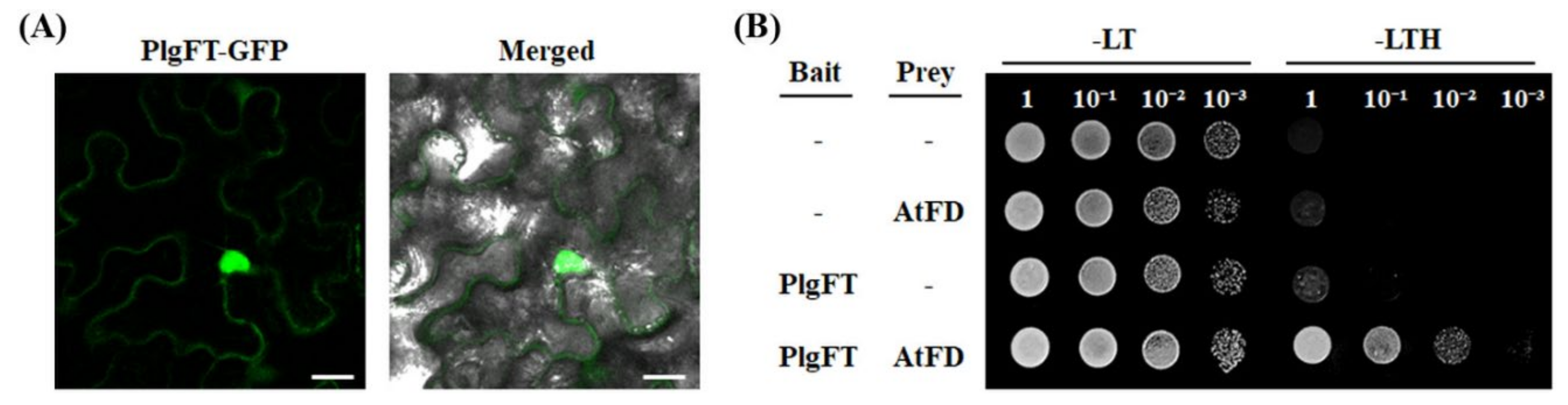
2.5. Ectopic Overexpression of PlgFT in P. grandiflorus Accelerates flowering
3. Materials and Methods
3.1. Plant Materials and Experimental Treatment
3.2. Identification FT Gene in P. grandiflorus
3.3. Plasmid Construction
3.4. RNA Extraction and Quantitative Real-Time PCR
3.5. Subcellular Localization of PlgFT in Tobacco Leaves
3.6. Yeast Two-Hybrid Assay
3.7. Agrobacterium Mediated Transformation
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fornara, F.; de Montaigu, A.; Coupland, G. SnapShot: Control of flowering in Arabidopsis. Cell 2010, 141, 550. [Google Scholar] [CrossRef] [Green Version]
- Sang, N.; Cai, D.; Li, C.; Sun, Y.; Huang, X. Characterization and activity analyses of the FLOWERING LOCUS T promoter in Gossypium Hirsutum. Int. J. Mol. Sci. 2019, 20, 4769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turck, F.; Fornara, F.; Coupland, G. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu. Rev. Plant Biol. 2008, 59, 573–594. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.H.; Ito, S.; Imaizumi, T. Flowering time regulation: Photoperiod-and temperature-sensing in leaves. Trends Plant Sci. 2013, 18, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Abe, M.; Kobayashi, Y.; Yamamoto, S.; Daimon, Y.; Yamaguchi, A.; Ikeda, Y.; Ichinoki, H.; Notaguchi, M.; Goto, K.; Araki, T. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 2005, 309, 1052–1056. [Google Scholar] [CrossRef]
- Collani, S.; Neumann, M.; Yant, L.; Schmid, M. FT Modulates genome-wide DNA-binding of the bZIP transcription factor FD. Plant Physiol. 2019, 180, 367–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Kumimoto, R.W.; Gnesutta, N.; Calogero, A.M.; Mantovani, R.; Holt III, B.F. A distal CCAAT/NUCLEAR FACTOR Y complex promotes chromatin looping at the FLOWERING LOCUS T promoter and regulates the timing of flowering in Arabidopsis. Plant Cell 2014, 26, 1009–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayama, R.; Yokoi, S.; Tamaki, S.; Yano, M.; Shimamoto, K. Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature 2003, 422, 719–722. [Google Scholar] [CrossRef]
- Hayama, R.; Agashe, B.; Luley, E.; King, R.; Coupland, G. A circadian rhythm set by dusk determines the expression of FT homologs and the short-day photoperiodic flowering response in Pharbitis. Plant Cell 2007, 19, 2988–3000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samach, A.; Lotan, H. The transition to flowering in tomato. Plant Biotechnol. J. 2007, 24, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Lifschitz, E.; Eviatar, T.; Rozman, A.; Shalit, A.; Goldshmidt, A.; Amsellem, Z.; Alvarez, J.P.; Eshed, Y. The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc. Natl. Acad. Sci. USA 2006, 103, 6398–6403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClung, C.; Lou, P.; Hermand, V.; Kim, J.A. The importance of ambient temperature to growth and the induction of flowering. Front. Plant Sci. 2016, 7, 1266. [Google Scholar] [CrossRef]
- Kumar, S.V.; Lucyshyn, D.; Jaeger, K.E.; Alós, E.; Alvey, E.; Harberd, N.P.; Wigge, P.A. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature 2012, 484, 242–245. [Google Scholar] [CrossRef]
- Li, X.F.; Jia, L.Y.; Xu, J.; Deng, X.J.; Wang, Y.; Zhang, W.; Zhang, X.P.; Fang, Q.; Zhang, D.M.; Sun, Y.; et al. FT-like NFT1 gene may play a role in flower transition induced by heat accumulation in Narcissus tazetta var. chinensis. Plant Cell Physiol. 2013, 54, 270–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Teranishi, Y.; Ueda, S.; Suzuki, H.; Kawano, N.; Yoshimatsu, K.; Saito, K.; Kawahara, N.; Muranaka, T.; Seki, H. Cytochrome P450 monooxygenase CYP716A141 is a unique β-amyrin C-16β oxidase involved in triterpenoid saponin biosynthesis in Platycodon grandiflorus. Plant Cell Physiol. 2017, 58, 874–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon Sun, H.; Hee Doo, L.; Joung Kwan, L.; Bo Goo, K.; Ki Yeol, L. Effect of pinching time and position on growth and flowering of Platycodon grandiflorum var. duplex Makino in cut flower cultivation. Flower Res. J. 2015, 23, 25–30. [Google Scholar]
- Cho, J.T. Physiological and ecological studies of Chinese bellflower Platycodon grandiflorum DC. J. Korean Soc. Hortic. Sci. 1984, 23, 194–200. [Google Scholar]
- Halevy, A.H.; Shlomo, E.; Ziv, O. Improving cut flower production of balloon flower. HortScience 2002, 37, 759–761. [Google Scholar] [CrossRef] [Green Version]
- Ben-Naim, O.; Eshed, R.; Parnis, A.; Teper-Bamnolker, P.; Shalit, A.; Coupland, G.; Samach, A.; Lifschitz, E. The CCAAT binding factor can mediate interactions between CONSTANS-like proteins and DNA. Plant J. 2006, 46, 462–476. [Google Scholar] [CrossRef] [Green Version]
- Bennett, T.; Dixon, L.E. Asymmetric expansions of FT and TFL1 lineages characterize differential evolution of the EuPEBP family in the major angiosperm lineages. BMC Biol. 2021, 19, 181. [Google Scholar] [CrossRef]
- Wickland, D.P.; Hanzawa, Y. The FLOWERING LOCUS T/TERMINAL FLOWER 1 gene family: Functional evolution and molecular mechanisms. Mol. Plant 2015, 8, 983–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, S.Y.; Kardailsky, I.; Lee, J.S.; Weigel, D.; Ahn, J.H. Acceleration of flowering by overexpression of MFT (MOTHER OF FT AND TFL1). Mol. Cells 2004, 17, 95–101. [Google Scholar]
- Ho, W.W.H.; Weigel, D. Structural features determining flower-promoting activity of Arabidopsis FLOWERING LOCUS T. Plant Cell 2014, 26, 552–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Kang, S.H.; Park, S.G.; Yang, T.J.; Lee, Y.; Kim, O.T.; Chung, O.; Lee, J.; Choi, J.P.; Kwon, S.J.; et al. Whole-genome, transcriptome, and methylome analyses provide insights into the evolution of platycoside biosynthesis in Platycodon grandiflorus, a medicinal plant. Hortic. Res. 2020, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Miller, D.; Winter, V.J.; Banfield, M.J.; Lee, J.H.; Yoo, S.Y.; Henz, S.R.; Brady, R.L.; Weigel, D. A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J. 2006, 25, 605–614. [Google Scholar] [CrossRef]
- Manoharan, R.K.; Han, J.S.H.; Vijayakumar, H.; Subramani, B.; Thamilarasan, S.K.; Park, J.-I.; Nou, I.-S. Molecular and functional characterization of FLOWERING LOCUS T homologs in Allium cepa. Molecules 2016, 21, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanzawa, Y.; Money, T.; Bradley, D. A single amino acid converts a repressor to an activator of flowering. Proc. Natl. Acad. Sci. USA 2005, 102, 7748–7753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imamura, T.; Nakatsuka, T.; Higuchi, A.; Nishihara, M.; Takahashi, H. The gentian orthologs of the FT/TFL1 gene family control floral initiation in Gentiana. Plant Cell Physiol. 2011, 52, 1031–1041. [Google Scholar] [CrossRef] [Green Version]
- Navarro, C.; Abelenda, J.A.; Cruz-Oró, E.; Cuellar, C.A.; Tamaki, S.; Silva, J.; Shimamoto, K.; Prat, S. Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature 2011, 478, 119–122. [Google Scholar] [CrossRef]
- Niu, L.; Fu, C.; Lin, H.; Wolabu, T.W.; Wu, Y.; Wang, Z.Y.; Tadege, M. Control of floral transition in the bioenergy crop switchgrass. Plant Cell Environ. 2016, 39, 2158–2171. [Google Scholar] [CrossRef]
- Yarur, A.; Soto, E.; León, G.; Almeida, A.M. The sweet cherry (Prunus avium) FLOWERING LOCUS T gene is expressed during floral bud determination and can promote flowering in a winter-annual Arabidopsis accession. Plant Reprod. 2016, 29, 311–322. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, T.; Guo, T.; Ding, W.; Long, R.; Yang, Q.; Wang, Z. Isolation and functional characterization of MsFTa, a FLOWERING LOCUS T homolog from alfalfa (Medicago sativa). Int. J. Mol. Sci. 2019, 20, 1968. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yu, H.; Lin, S.; Gao, Y. Molecular characterization of FT and FD homologs from Eriobotrya deflexa Nakai forma koshunensis. Front. Plant Sci. 2016, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Z.; Zhang, C.; Zhang, H.; Li, X.; Wen, C.; Liang, Y. Molecular cloning, expression analysis, and subcellular localization of FLOWERING LOCUS T (FT) in carrot (Daucus carota L.). Mol. Breed. 2017, 37, 1–9. [Google Scholar] [CrossRef]
- Harig, L.; Beinecke, F.A.; Oltmanns, J.; Muth, J.; Müller, O.; Rüping, B.; Twyman, R.M.; Fischer, R.; Prüfer, D.; Noll, G.A. Proteins from the FLOWERING LOCUS T-like subclade of the PEBP family act antagonistically to regulate floral initiation in tobacco. Plant J. 2012, 72, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Farrona, S.; Thorpe, F.L.; Engelhorn, J.; Adrian, J.; Dong, X.; Sarid-Krebs, L.; Goodrich, J.; Turck, F. Tissue-specific expression of FLOWERING LOCUS T in Arabidopsis is maintained independently of polycomb group protein repression. Plant Cell 2011, 23, 3204–3214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotoda, N.; Hayashi, H.; Suzuki, M.; Igarashi, M.; Hatsuyama, Y.; Kidou, S.; Igasaki, T.; Nishiguchi, M.; Yano, K.; Shimizu, T.; et al. Molecular characterization of FLOWERING LOCUS T-like genes of apple (Malus x domestica Borkh.). Plant Cell Physiol. 2010, 51, 561–575. [Google Scholar] [CrossRef] [Green Version]
- Varkonyi-Gasic, E.; Moss, S.M.A.; Voogd, C.; Wang, T.; Putterill, J.; Hellens, R.P. Homologs of FT, CEN and FD respond to developmental and environmental signals affecting growth and flowering in the perennial vine kiwifruit. New Phytol. 2013, 198, 732–746. [Google Scholar] [CrossRef]
- Lee, R.; Baldwin, S.; Kenel, F.; McCallum, J.; Macknight, R. FLOWERING LOCUS T genes control onion bulb formation and flowering. Nat. Commun. 2013, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Luo, L.; Fu, Q.; Niu, L.; Xu, Z.-F. Isolation and functional characterization of JcFT, a FLOWERING LOCUS T (FT) homologous gene from the biofuel plant Jatropha curcas. BMC Plant Biol. 2014, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sreekantan, L.; Thomas, M.R. VvFT and VvMADS8, the grapevine homologues of the floral integrators FT and SOC1, have unique expression patterns in grapevine and hasten flowering in Arabidopsis. Funct. Plant Biol. 2006, 33, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.B.; Shen, Y.; Chang, H.C.; Hou, Y.; Harris, A.; Ma, S.F.; McPartland, M.; Hymus, G.J.; Adam, L.; Marion, C. The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis-element. New Phytol. 2010, 187, 57–66. [Google Scholar] [CrossRef]
- Park, B.H.; Oliveira, N.; Pearson, S. Temperature affects growth and flowering of the balloon flower [Platycodon grandiflorus (Jacq.) A. DC. cv. Astra Blue]. HortScience 1998, 33, 233–236. [Google Scholar]
- Nakano, Y.; Higuchi, Y.; Yoshida, Y.; Hisamatsu, T. Environmental responses of the FT/TFL1 gene family and their involvement in flower induction in Fragaria × ananassa. J. Plant Physiol. 2015, 177, 60–66. [Google Scholar] [CrossRef]
- Noy-Porat, T.; Cohen, D.; Mathew, D.; Eshel, A.; Kamenetsky, R.; Flaishman, M.A. Turned on by heat: Differential expression of FT and LFY-like genes in Narcissus tazetta during floral transition. J. Exp. Bot. 2013, 64, 3273–3284. [Google Scholar] [CrossRef] [Green Version]
- No, D.H.; Baek, D.; Lee, S.H.; Cheong, M.S.; Chun, H.J.; Park, M.S.; Cho, H.M.; Jin, B.J.; Lim, L.H.; Lee, Y.B.; et al. High-temperature conditions promote soybean flowering through the transcriptional reprograming of flowering genes in the photoperiod pathway. Int. J. Mol. Sci. 2021, 22, 1314. [Google Scholar] [CrossRef]
- Tavora, F.J.A.F.; Melo, F.I.O.; de Pinho, J.L.N.; de Queiroz, G.M. Yield, crop growth rate and assimilatory characteristics of cassava at the coastal area of Ceara. Rev. Bras. Fisiol. Veg. 1995, 7, 81–88. [Google Scholar]
- Chaurasia, A.K.; Patil, H.B.; Krishna, B.; Subramaniam, V.R.; Sane, P.V.; Sane, A.P. Flowering time in banana (Musa spp.), a day neutral plant, is controlled by at least three FLOWERING LOCUS T homologues. Sci. Rep. 2017, 7, 5935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.Y.; Kwon, H.Y.; Kim, N.Y.; Sakuraba, Y.; Paek, N.C. CONSTITUTIVE PHOTOMORPHOGENIC 10 (COP10) contributes to floral repression under non-inductive short days in Arabidopsis. Int. J. Mol. Sci. 2015, 16, 26493–26505. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.; Rim, Y.; Cho, H.; Hyun, T.K. Identification and Functional Characterization of FLOWERING LOCUS T in Platycodon grandiflorus. Plants 2022, 11, 325. https://doi.org/10.3390/plants11030325
Kim G, Rim Y, Cho H, Hyun TK. Identification and Functional Characterization of FLOWERING LOCUS T in Platycodon grandiflorus. Plants. 2022; 11(3):325. https://doi.org/10.3390/plants11030325
Chicago/Turabian StyleKim, Gayeon, Yeonggil Rim, Hyunwoo Cho, and Tae Kyung Hyun. 2022. "Identification and Functional Characterization of FLOWERING LOCUS T in Platycodon grandiflorus" Plants 11, no. 3: 325. https://doi.org/10.3390/plants11030325
APA StyleKim, G., Rim, Y., Cho, H., & Hyun, T. K. (2022). Identification and Functional Characterization of FLOWERING LOCUS T in Platycodon grandiflorus. Plants, 11(3), 325. https://doi.org/10.3390/plants11030325





