Polyphenol Composition and Antioxidant Activity of Tapirira guianensis Aubl. (Anarcadiaceae) Leaves
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yield and Total Polyphenol Contents in Crude Tapirira guianensis Extracts
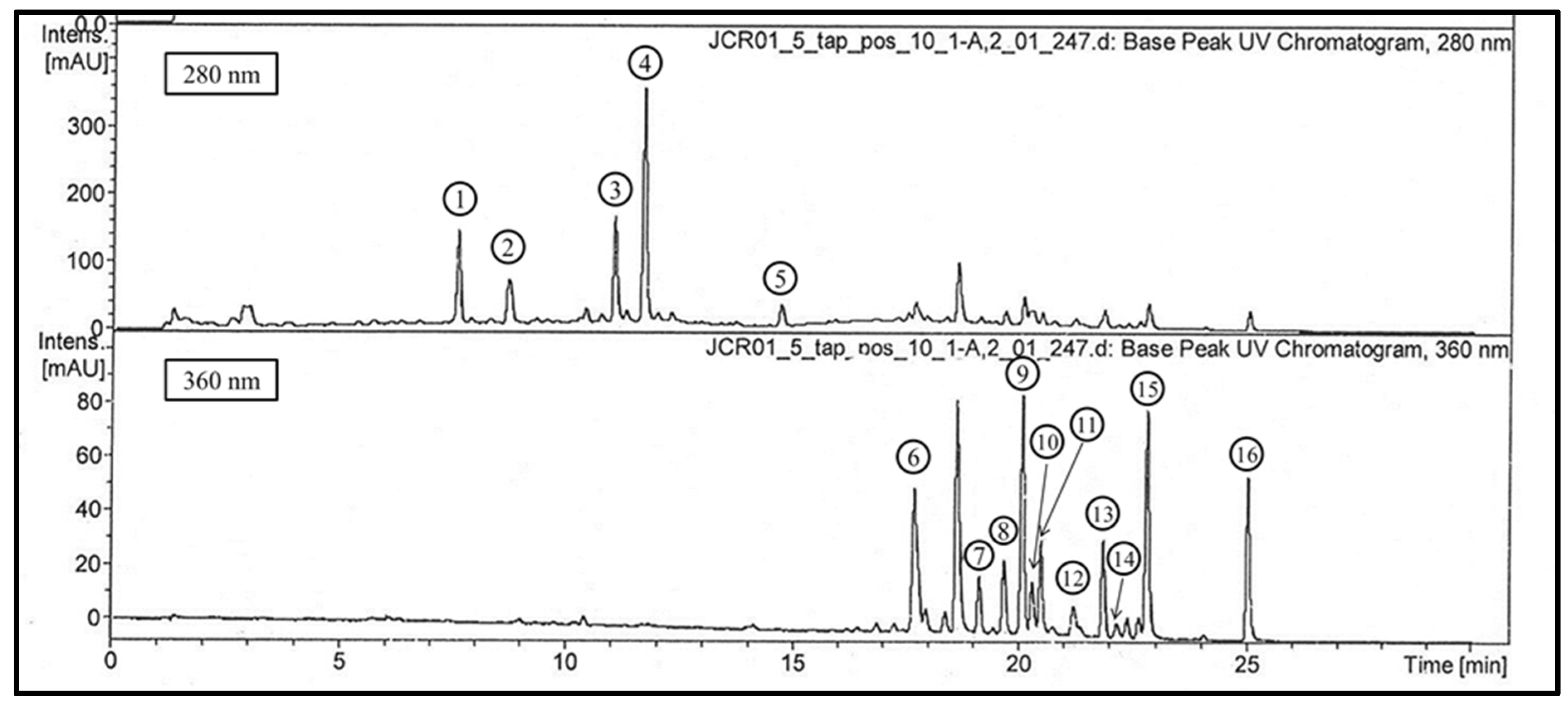
2.2. UPLC-DAD-ESI-IT-MSn Analysis of the 85% MeOH Extract
2.3. AO Activities in Crude Tapirira guianensis Extracts
2.4. Fractionation of the 85% MeOH Extract
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material and Extraction Procedures
3.3. Total Polyphenol, Flavonoid, and Flavanols Contents
3.4. Fractionation of Crude Extract
3.5. UPLC-DAD-ESI-IT-MSn Analysis
3.6. DPPH, TEAC and ORAC Assays
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARA | Antiradical Activity |
| AO | Antioxidant |
| CE | Catechin Equivalent |
| DAD | Diode Array Detector |
| DE | Dry Extract |
| DM | Dry Matter |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ESI | Electrospray Ionization |
| FRS | Free Radical Scavengers |
| GAE | Gallic Acid Equivalent |
| IT | Ion Trap |
| MS | Mass Spectrometer |
| MW | Molecular Weight |
| NMR | Nuclear Magnetic Resonance |
| ORAC | Oxygen Radical Absorbance Capacity |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| TPC | total polyphenol content |
| TFC | total flavonoid content |
| TE | Trolox Equivalent |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| UAE | ultrasound-assisted extraction |
| UPLC | Ultra-Performance Liquid Chromatography |
| UV | Ultraviolet |
| TG | T. guianensis |
References
- Mannino, G.; Di Stefano, V.; Lauria, A.; Pitonzo, R.; Gentile, C. Vaccinium Macrocarpon (Cranberry)-Based Dietary Supplements: Variation in Mass Uniformity, Proanthocyanidin Dosage and Anthocyanin Profile Demonstrates Quality Control Standard Needed. Nutrients 2020, 12, 992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, G.S.; Matos, F.J.; Vieira, J.E.; Sousa, M.P.; Medeiros, M.C. Pharmacological Screening of Some Brazilian Plants. J. Pharm. Pharmacol. 1970, 22, 116–122. [Google Scholar] [CrossRef] [PubMed]
- DeFilipps, R.A.; Maina, S.L.; Crepin, J. Medicinal Plants of the Guianas (Guyana, Surinam, French Guiana); Department of Botany, National Museum of Natural History, Smithsonian Institution: Washington, DC, USA, 2004. [Google Scholar]
- Roumy, V.; Fabre, N.; Portet, B.; Bourdy, G.; Acebey, L.; Vigor, C.; Valentin, A.; Moulis, C. Four Anti-Protozoal and Anti-Bacterial Compounds from Tapirira Guianensis. Phytochemistry 2009, 70, 305–311. [Google Scholar] [CrossRef] [PubMed]
- França, F.; Lago, E.L.; Marsden, P.D. Plants Used in the Treatment of Leishmanial Ulcers Due to Leishmania (Viannia) Braziliensis in an Endemic Area of Bahia, Brazil. Rev. Soc. Bras. Med. Trop. 1996, 29, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Grenand, P.; Moretti, C.; Jacquemin, H.; Prévost, M.-F. (Eds.) Pharmacopées Traditionnelles en Guyane: Créoles, Wayãpi, Palikur; Guides Illustrés; IRD Éditions: Marseille, France, 2004; ISBN 978-2-7099-2344-6. [Google Scholar]
- de A. Mello, M.O.; da Costa, C.F.; da S. Barbosa, M.M. Catalogo das plantas toxicas e medicinais no Estado da Bahia. Bol. Inst. Biol. Bahia 1971, 1, 39–66. [Google Scholar]
- Lorenzi, H. Brazilian Trees: A Guide to the Identification and Cultivation of Brazilian Native Trees, 4th ed.; Instituto Plantarum de Estudos da Flora: Nova Odessa, Brazil, 2002; ISBN 978-85-86714-17-7. [Google Scholar]
- Berni, C.A.; Bolza, E.; Christensen, F.J. South American Timbers: The Characteristics, Properties and Uses of 190 Species (Algunas maderas de America del Sur); Division of Building Research, Commonwealth Scientific and Industrial Research Organization: Melbourne, Australia, 1979; ISBN 978-0-643-02582-0. [Google Scholar]
- Longatti, T.R.; Cenzi, G.; Lima, L.A.R.S.; Oliveira, R.J.S.; Oliveira, V.N.; Silva, S.L.D.; Ribeiro, R.I.M.A. Inhibition of Gelatinases by Vegetable Extracts of the Species Tapirira Guianensis (Stick Pigeon). British journal of pharmaceutical Research. J. 2011, 1, 133–140. [Google Scholar] [CrossRef]
- Mahmoud, T.S.; Marques, M.R.; do Ó. Pessoa, C.; Lotufo, L.V.C.; Magalhães, H.I.F.; de Moraes, M.O.; de Lima, D.P.; Tininis, A.G.; de Oliveira, J.E. In Vitro Cytotoxic Activity of Brazilian Middle West Plant Extracts. Rev. Bras. Farmacogn. 2011, 21, 456–464. [Google Scholar] [CrossRef] [Green Version]
- Silva-Oliveira, R.J.; Lopes, G.F.; Camargos, L.F.; Ribeiro, A.M.; dos Santos, F.V.; Severino, R.P.; Severino, V.G.P.; Terezan, A.P.; Thomé, R.G.; dos Santos, H.B.; et al. Tapirira Guianensis Aubl. Extracts Inhibit Proliferation and Migration of Oral Cancer Cells Lines. Int. J. Mol. Sci. 2016, 17, 1839. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.M.G.; Guimarães, D.O.; Konno, T.U.P.; Tinoco, L.W.; Barth, T.; Aguiar, F.A.; Lopes, N.P.; Leal, I.C.R.; Raimundo, J.M.; Muzitano, M.F. Phytochemical Study of Tapirira Guianensis Leaves Guided by Vasodilatory and Antioxidant Activities. Molecules 2017, 22, 304. [Google Scholar] [CrossRef] [Green Version]
- Calassara, L.L.; Pinto, S.C.; Condack, C.P.M.; Leite, B.F.; do Nery, E.S.L.C.; Tinoco, L.W.; Aguiar, F.A.; Leal, I.C.R.; Martins, S.M.; da Silva, L.L.; et al. Isolation and Characterization of Flavonoids from Tapirira guianensis Leaves with Vasodilatory and Myeloperoxidase-Inhibitory Activities. Nat. Prod. Res. 2020, 35, 5480–5483. [Google Scholar] [CrossRef]
- de Correia, J.S.; DavidI, J.M.; da Silva, E.P.; David, J.P.; Lopes, L.M.X.; Guedes, M.L.S. Flavonóides, norisoprenóides e outros terpenos das folhas de Tapirira guianensis. Quím. Nova 2008, 31, 2056–2059. [Google Scholar] [CrossRef] [Green Version]
- Taylor, P.G.; Cesari, I.M.; Arsenak, M.; Ballen, D.; Abad, M.J.; Fernández, A.; Milano, B.; Ruiz, M.-C.; Williams, B.; Michelangeli, F. Evaluation of Venezuelan Medicinal Plant Extracts for Antitumor and Antiprotease Activities. Pharm. Biol. 2006, 44, 349–362. [Google Scholar] [CrossRef]
- da Silva, E.P.; David, J.M.; David, J.P.; Garcia, G.H.T.; Silva, M.T. Chemical Composition of Biological Active Extracts of “Tapirira Guianensis” (Anacardiaceae). Quím. Nova 2020, 43, 1216–1219. [Google Scholar] [CrossRef]
- Bartosz, G. Generation of Reactive Oxygen Species in Biological Systems. Comments Toxicol. 2003, 9, 5–21. [Google Scholar] [CrossRef]
- Beckman, K.B.; Ames, B.N. The Free Radical Theory of Aging Matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef] [Green Version]
- Pincemail, J.; Bonjean, K.; Cayeux, K.; Defraigne, J.-O. Physiological Action of Antioxidant Defences. Nutr. Clin. Metab. 2002, 16, 233–239. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A Clean, Green Extraction Technology. Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; da F. Machado, A.P.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids from Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Vural, N.; Algan Cavuldak, Ö.; Akay, M.A.; Anlı, R.E. Determination of the Various Extraction Solvent Effects on Polyphenolic Profile and Antioxidant Activities of Selected Tea Samples by Chemometric Approach. J. Food Meas. Charact. 2020, 14, 1286–1305. [Google Scholar] [CrossRef]
- Clifford, M.N.; Stoupi, S.; Kuhnert, N. Profiling and Characterization by LC-MSn of the Galloylquinic Acids of Green Tea, Tara Tannin, and Tannic Acid. J. Agric. Food Chem. 2007, 55, 2797–2807. [Google Scholar] [CrossRef]
- Ma, Y.L.; Li, Q.M.; Van den Heuvel, H.; Claeys, M. Characterization of Flavone and Flavonol Aglycones by Collision-Induced Dissociation Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 1997, 11, 1357–1364. [Google Scholar] [CrossRef]
- Hvattum, E.; Ekeberg, D. Study of the Collision-Induced Radical Cleavage of Flavonoid Glycosides Using Negative Electrospray Ionization Tandem Quadrupole Mass Spectrometry. J. Mass Spectrom. 2003, 38, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of Flavone, Flavonol, and Flavanone Aglycones by Negative Ion Liquid Chromatography Electrospray Ion Trap Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef] [Green Version]
- Yokozawa, T.; Dong, E.; Nakagawa, T.; Kashiwagi, H.; Nakagawa, H.; Takeuchi, S.; Chung, H.Y. In Vitro and in Vivo Studies on the Radical-Scavenging Activity of Tea. J. Agric. Food Chem. 1998, 46, 2143–2150. [Google Scholar] [CrossRef]
- Wojcikowski, K.; Stevenson, L.; Leach, D.; Wohlmuth, H.; Gobe, G. Antioxidant Capacity of 55 Medicinal Herbs Traditionally Used to Treat The Urinary System: A Comparison Using A Sequential Three-Solvent Extraction Process. J. Altern. Complementary Med. 2007, 13, 103–110. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.; Jaiswal, A.; Condon, K.; García-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef]
- Arnous, A.; Makris, D.P.; Kefalas, P. Correlation of Pigment and Flavanol Content with Antioxidant Properties in Selected Aged Regional Wines from Greece. J. Food Compos. Anal. 2002, 15, 655–665. [Google Scholar] [CrossRef]
- Leba, L.-J.; Brunschwig, C.; Saout, M.; Martial, K.; Bereau, D.; Robinson, J.-C. Oenocarpus Bacaba and Oenocarpus Bataua Leaflets and Roots: A New Source of Antioxidant Compounds. Int. J. Mol. Sci. 2016, 17, 1014. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant Capacity of Phenolic Phytochemicals from Various Cultivars of Plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- de Villiers, A.; Lynen, F.; Crouch, A.; Sandra, P. Development of a Solid-Phase Extraction Procedure for the Simultaneous Determination of Polyphenols, Organic Acids and Sugars in Wine. Chromatographia 2004, 59, 403–409. [Google Scholar] [CrossRef]
- Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B.; Laureano, O.; Ricardo da Silva, J.M. Monomeric, Oligomeric, and Polymeric Flavan-3-Ol Composition of Wines and Grapes from Vitis Vinifera L. Cv. Graciano, Tempranillo, and Cabernet Sauvignon. J. Agric. Food Chem. 2003, 51, 6475–6481. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.M.; Platat, C.; Meudec, E.; Cheynier, V.; Ibrahim, W.H. Polyphenolic Compounds in Date Fruit Seed (Phoenix Dactylifera): Characterisation and Quantification by Using UPLC-DAD-ESI-MS. J. Sci. Food Agric. 2014, 94, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Cakir, A.; Mavi, A.; Kilic, H.; Yildirim, A. Screening of Chemical Composition and Antifungal and Antioxidant Activities of the Essential Oils from Three Turkish Artemisia Species. J. Agric. Food Chem. 2005, 53, 1408–1416. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Rezende, C.; Rigotto, C.; Caneschi, W.; Rezende, C.; Hyaric, M.; Couri, M.; Simões, C.; Almeida, M. Anti-HSV-1 and Antioxidant Activities of Dicaffeoyl and Digalloyl Esters of Quinic Acid. Biomed. Prev. Nutr. 2014, 4, 35–38. [Google Scholar] [CrossRef]
- de Sousa, L.R.F.; Ramalho, S.D.; Fernandes, J.B.; das G.F. da Silva, M.F.; da C. Iemma, M.R.; Corrêa, C.J.; de Souza, D.H.F.; Lima, M.I.S.; Vieira, P.C. Leishmanicidal Galloylquinic Acids Are Noncompetitive Inhibitors of Arginase. J. Braz. Chem. Soc. 2014, 25, 1832–1838. [Google Scholar] [CrossRef]
| Sample | Extraction Yield 1 (%) | Total Polyphenol Contents 2 (mg GAE/g DM) | Flavonoid Contents 3 (mg CE/g DM) | Flavanol Contents 3 (mg CE/g DM) |
|---|---|---|---|---|
| H2O extract | 28.0 ± 1.6 a | 40.5 ± 14.2 a | 16.7 ± 6.3 a | 14.7 ± 8.6 a |
| MeOH extract | 28.9 ± 0.4 a | 69.2 ± 1.9 b | 25.6 ± 0.8 b | 43.0 ± 5.5 b |
| MeOH/H2O (85/15) extract | 28.1 ± 1.6 a | 112.7 ± 5.8 c | 17.9 ± 0.5 a | 49.0 ± 5.6 b |
| Product | tR | λmax (nm) | [M−H]− (m/z) | MS2 (m/z) Negative Mode | [M+H]+ (m/z) | MS2 (m/z) Positive Mode | MS3 (m/z) Positive Mode | Structural Hypothesis (Class) | Sources |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 7.7 | 280 | 495 | 343(100) 325(2) 191(3) 169(6) | 497 | 479(100) | 309 | 3,5-Digalloylquinic acid | 24 |
| 2 | 8.8 | 280 | 495 | 343(100) 325(38) 193(18) 169(14) | 497 | 479(100) | 309 | 4,5-Digalloylquinic acid | 24 |
| 3 | 11 | 275 | 647 | 495(100) 477(13) 343(12) 325(7) | 649 | 479(100) | 309 | 3,4,5-Trigalloylquinic acid | 24 |
| 4 | 11.6 | 275 | 799 | 601(100) 629(32) 477(12) | 801 | 631 461 | 461 (from m/z 631) 291(from m/z 461) | 1,3,4,5-Tetragalloylquinic acid | 24 |
| 5 | 14.7 | 279 (tailling) | 441 | 289(100) 169(11) 125(4) | 443 | 273 | epicatechin gallate (flavanol) | ||
| 6 | 17.7 | 266 352 | 463 | 317(100) 316(95) 271(3) 179(6) | 465 | 319 | 301(59) 291(10) 273(100) 263(24) 245(33) | Myricetin deoxyhexoside (flavonol) | 14, 26, 27 |
| 7 | 19.1 | 256 353 | 433 | 301(100) | 435 | 303 | 285(30) 275(9) 257(100) 247(60) 229(67) | Quercetin pentoside (flavonol) | 14 |
| 8 | 19.6 | 265 346 | 365 | 285 | 367 | 287 | 269(77) 259(56) 231(22) 213(61) | Kaempferol derivative * (flavonol) | 25 |
| 9 | 20.1 | 256 353 | 433 | 301(100) | 435 | 303 | 285(30) 275(9) 257(100) 247(60) 229(67) | Quercetin pentoside (flavonol) | 14 |
| 10 | 20.4 | 265 355 | 447 | 315 | 449 | 317 | 302(72) 299(6) 289(2) 285(100) 257(4) | Isorhamnetin pentoside (flavonol) | 25 |
| 11 | 20.6 | 265 350 | 447 | 301 | 449 | 303 | 285(30) 275(9) 257(100) 247(60) 229(67) | Quercetin deoxyhexoside (flavonol) | 14 |
| 12 | 21.3 | 265 350 | 461 | 315 | 463 | 317 | 302(72) 299(6) 289(2) 285(100) 257(4) | Isorhamnetin deoxyhexoside (flavonol) | 25 |
| 13 | 1.8 | 264 371 | 417 | 285 | 419 | 287 | 269(77) 259(56) 231(22) 213(61) | Kaempferol pentoside (flavonol) | 15 |
| 14 | 22.2 | 264 368 | 431 | 285 | 433 | 287 | 269(77) 259(56) 231(22) 213(61) | Kaempferol deoxyhexoside (flavonol) | 15 |
| 15 | 22.8 | 255 371 | 301 | 303 | 285(30) 275(9) 257(100) 247(60) 229(67) | Quercetin (flavonol) | |||
| 16 | 25 | 265 365 | 285 | 287 | 269(77) 259(56) 231(22) 213(61) | Kaempferol (flavonol) |
| Sample | DPPH Assay 1 (µM TE/g DM) | TEAC Assay 2 (µM TE/g DM) | ORAC Assay 3 (µmol TE/g DM) |
|---|---|---|---|
| H2O extract | 437.5 ± 77.1 a | 356.4 ± 77.3 a | 597.2 ± 114.5 a |
| MeOH extract | 817.2 ± 101.1 b | 708.8 ± 102.5 b | 779.2 ± 148.9 a |
| MeOH/H2O extract | 1050.4 ± 21.9 c | 938.7 ± 110.4 b | 2567.3 ± 476.3 b |
| Green tea extract 3 | 969.7 ± 54.3 | 822.6 ± 87.6 | 2911 ± 221.7 |
| Fractions | Mass Yield (%) 1 | Total Polyphenol Contents 2 | DPPH Assay 3 |
|---|---|---|---|
| Crude extract | 100% | 335 | 100% |
| W1 | 27% | 9.5 | 3% |
| M1 | 17% | 16.5 | 5.5% |
| W2 | 9% | 64 | 20% |
| M2 | 14% | 14.5 | 3.5% |
| W3 | 16% | 96 | 27% |
| M3 | 5% | 37 | 9.5% |
| Total | 88% | 237 | 69% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patient, A.; Jean-Marie, E.; Robinson, J.-C.; Martial, K.; Meudec, E.; Levalois-Grützmacher, J.; Closs, B.; Bereau, D. Polyphenol Composition and Antioxidant Activity of Tapirira guianensis Aubl. (Anarcadiaceae) Leaves. Plants 2022, 11, 326. https://doi.org/10.3390/plants11030326
Patient A, Jean-Marie E, Robinson J-C, Martial K, Meudec E, Levalois-Grützmacher J, Closs B, Bereau D. Polyphenol Composition and Antioxidant Activity of Tapirira guianensis Aubl. (Anarcadiaceae) Leaves. Plants. 2022; 11(3):326. https://doi.org/10.3390/plants11030326
Chicago/Turabian StylePatient, Arnaud, Elodie Jean-Marie, Jean-Charles Robinson, Karine Martial, Emmanuelle Meudec, Joëlle Levalois-Grützmacher, Brigitte Closs, and Didier Bereau. 2022. "Polyphenol Composition and Antioxidant Activity of Tapirira guianensis Aubl. (Anarcadiaceae) Leaves" Plants 11, no. 3: 326. https://doi.org/10.3390/plants11030326
APA StylePatient, A., Jean-Marie, E., Robinson, J.-C., Martial, K., Meudec, E., Levalois-Grützmacher, J., Closs, B., & Bereau, D. (2022). Polyphenol Composition and Antioxidant Activity of Tapirira guianensis Aubl. (Anarcadiaceae) Leaves. Plants, 11(3), 326. https://doi.org/10.3390/plants11030326







