The Response of Spore Germination of Sphagnum Mosses to Single and Combined Fire-Related Cues
Abstract
1. Introduction
2. Results
2.1. Effects of Heat on Spore Germination
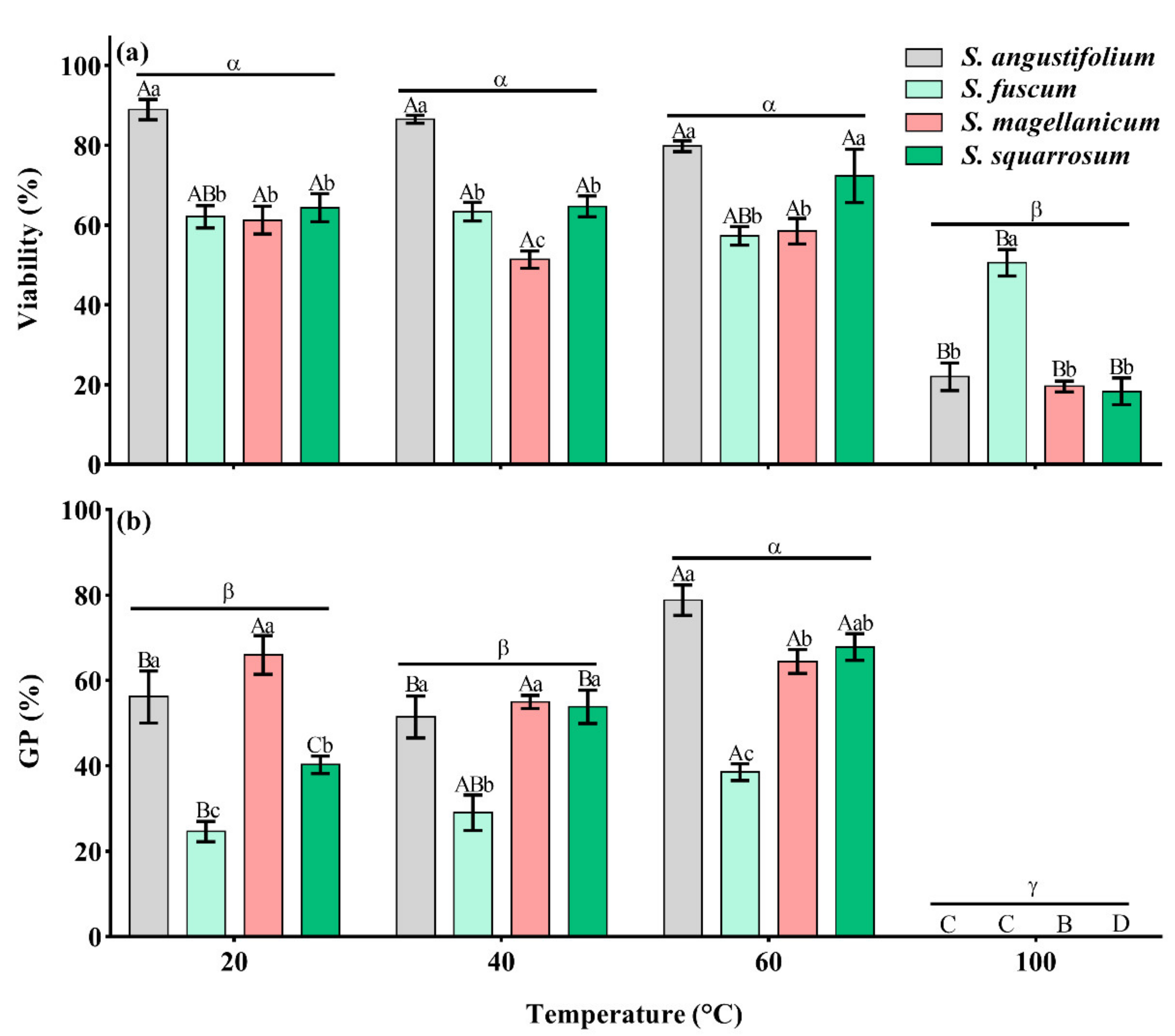
2.1.1. Germination Percentage (GP)
2.1.2. Germination Speed (GS) and Heat Treatment Effect Index (TEI)
2.1.3. Spore Viability
2.2. Effects of Heat + Smoke on Spore Germination
2.2.1. Germination Percentage
2.2.2. Germination Speed and Heat Treatment Effect Index
3. Discussion
3.1. Heat and Spore Germination
3.2. Effect of Smoke on Spore Germination
3.3. Spore Germination in Relation to Heat and Smoke–Water
3.4. Fire-Responsive Germination and Species Habitat Preference
3.5. Fire Disturbance and Establishment of Sphagnum Spores
4. Materials and Methods
4.1. Study Species, Spore Capsule Collection and Spore Suspension Preparation
4.2. Experimental Design
4.2.1. Heat Treatments
4.2.2. Heat + Smoke Treatments
4.3. Germination Experiment
4.4. Spore Viability Test
4.5. Data Processing and Statistical Analysis
4.5.1. Data Processing
GS = GP11d/GP21d;
V (%) = (ns/N) × 100%.
When T < C, TEI = T/C − 1;
4.5.2. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keeley, J.E.; Fotheringham, C.J.; Baer-Keeley, M. Determinants of postfire recovery and succession in Mediterranean climate shrublands of California. Ecol. Appl. 2005, 15, 1515–1534. [Google Scholar] [CrossRef]
- Bowman, D.M.; Balch, J.K.; Artaxo, P.; Bond, W.J.; Carlson, J.M.; Cochrane, M.A.; D’Antonio, C.M.; DeFries, R.S.; Doyle, J.C.; Harrison, S.P.; et al. Fire in the Earth system. Science 2009, 324, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Pausas, J.G.; Ribeiro, E. Fire and plant diversity at the global scale. Glob. Ecol. Biogeogr. 2017, 26, 889–897. [Google Scholar] [CrossRef]
- Keeley, J.E.; Pausas, J.G.; Rundel, P.W.; Bond, W.J.; Bradstock, R.A. Fire as an evolutionary pressure shaping plant traits. Trends Plant Sci. 2011, 16, 406–411. [Google Scholar] [CrossRef]
- Koch, J.M.; Bell, D.T. Leaf scorch in Xanthorrhoea gracilis as an index of fire intensity. Aust. For. Res. 1980, 10, 113–119. [Google Scholar]
- Lamont, B.B.; He, T. Fire-proneness as a prerequisite for the evolution of fire-adapted traits. Trends Plant Sci. 2017, 22, 278–288. [Google Scholar] [CrossRef]
- Zacharias, P.J.K. The effect of fire on germination in five common veld grasses. J. Grassl. Soc. S. Afr. 1988, 5, 229–230. [Google Scholar] [CrossRef]
- Reyes, O.; Trabaud, L. Germination behavior of 14 Mediterranean species in relation to fire factors: Smoke and heat. Plant Ecol. 2008, 202, 113–121. [Google Scholar] [CrossRef]
- Nelson, D.C.; Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Regulation of seed germination and seedling growth by chemical signals from burning vegetation. Annu. Rev. Plant Biol. 2012, 63, 107–130. [Google Scholar] [CrossRef]
- Van Staden, J.; Brown, N.A.; Jäger, A.K.; Johnson, T.A. Smoke as a germination cue. Plant Species Biol. 2000, 15, 167–178. [Google Scholar] [CrossRef]
- Herranz, J.M.; Ferrandis, P.; Martínez Sánchez, J.J. Influence of heat on seed germination of nine woody Cistaceae species. Int. J. Wildland Fire 1998, 9, 173–182. [Google Scholar] [CrossRef]
- Moreira, B.; Tormo, J.; Estrelles, E.; Pausas, J.G. Disentangling the role of heat and smoke as germination cues in Mediterranean Basin flora. Ann. Bot. 2010, 105, 627–635. [Google Scholar] [CrossRef]
- De Lange, J.H.; Boucher, C. Autecological studies on Audouinia capitata (Bruniaceae). I. Plant-derived smoke as a seed germination cue. S. Afr. J. Bot. 1990, 56, 700–703. [Google Scholar] [CrossRef]
- Dixon, K.W.; Roche, S.; Pate, J.S. The promotive effect of smoke derived from burnt native vegetation on seed germination of Western Australian plants. Oecologia 1995, 101, 185–192. [Google Scholar] [CrossRef]
- Brown, N.A.C.; Van Staden, J.; Daws, M.I.; Johnson, T. Patterns in the seed germination response to smoke in plants from the Cape Floristic Region, South Africa. S. Afr. J. Bot. 2003, 69, 514–525. [Google Scholar] [CrossRef]
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D. A compound from smoke that promotes seed germination. Science 2004, 305, 977. [Google Scholar] [CrossRef]
- Van Staden, J.; Jager, A.K.; Light, M.E.; Burger, B.V. Isolation of the major germination cue from plant-derived smoke. S. Afr. J. Bot. 2004, 70, 654–659. [Google Scholar] [CrossRef]
- Flematti, G.R.; Merritt, D.J.; Piggott, M.J.; Trengove, R.D.; Smith, S.M.; Dixon, K.W.; Ghisalberti, E.L. Burning vegetation produces cyanohydrins that liberate cyanide and stimulate seed germination. Nat. Commun. 2011, 2, 1–6. [Google Scholar] [CrossRef]
- Auld, T.D.; Denham, A.J. How much seed remains in the soil after a fire? Plant Ecol. 2006, 187, 15–24. [Google Scholar] [CrossRef]
- Hu, F.; Tang, X.R.; Yang, J.; Chen, Y.F.; Kong, C.H. Ecophysiological effects of plant-derived smoke and heat on plants. Acta Ecol. Sin. 2006, 26, 594–600. [Google Scholar]
- Jefferson, L.; Pennacchio, M.; Havens-Young, K. Ecology of Plant-Derived Smoke: Its Use in Seed Germination; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Tieu, A.; Dixon, K.W.; Meney, K.A.; Sivasithamparam, K. The interaction of heat and smoke in the release of seed dormancy in seven species from southwestern Western Australia. Ann. Bot. 2001, 88, 259–265. [Google Scholar] [CrossRef]
- Jeffery, D.J.; Holmes, P.M.; Rebelo, A.G. Effects of dry heat on seed germination in selected indigenous and alien Legume species in South Africa. S. Afr. J. Bot. 1988, 54, 28–34. [Google Scholar] [CrossRef]
- Williams, P.R.; Congdon, R.A.; Grice, A.C.; Clarke, P.J. Fire-related cues break seed dormancy of legumes in tropical eucalypt savannas of north-eastern Australia. Austral Ecol. 2003, 28, 507–514. [Google Scholar] [CrossRef]
- Lukenbach, M.C.; Devito, K.J.; Kettridge, N.; Petrone, R.M.; Waddington, J.M. Hydrogeological controls on post-fire moss recovery in peatlands. J. Hydrol. 2015, 530, 405–418. [Google Scholar] [CrossRef]
- Gabriel, M.; Martin, L.; Serge, P. Impact of fire on long-term vegetation dynamics of ombrotrophic peatlands in northwestern Québec, Canada. Quat. Res. 2012, 77, 110–121. [Google Scholar]
- Marcisz, K.; Galka, M.; Pietrala, P.; Miotk-Szpiganowicz, G.; Obremska, M.; Tobolski, K.; Lamentowicz, M. Fire activity and hydrological dynamics in the past 5700 years reconstructed from Sphagnum peatlands along the oceanic-continental climatic gradient in northern Poland. Quat. Sci. Rev. 2017, 177, 145–157. [Google Scholar] [CrossRef]
- Clymo, R.S.; Hayward, P.M. The ecology of Sphagnum. In Bryophyte Ecology; Springer: Dordrecht, The Netherlands, 1982; pp. 229–289. [Google Scholar]
- During, H.J. Diaspore banks. Bryologist 2001, 104, 92–97. [Google Scholar] [CrossRef]
- Sundberg, S.; Rydin, H. Habitat requirements for establishment of Sphagnum from spores. J. Ecol. 2002, 90, 268–278. [Google Scholar] [CrossRef]
- Bu, Z.J.; Sundberg, S.; Feng, L.; Li, H.K.; Zhao, H.Y.; Li, H.C. The Methuselah of plant diaspores: Sphagnum spores can survive in nature for centuries. New Phytol. 2017, 214, 1398–1402. [Google Scholar] [CrossRef]
- Guo, H.B.; Xu, X.Y.; Bu, Z.J.; Feng, L.; Lu, X.Y.; Wang, J.Y.; Chen, Y.D.; Yusup, S.; Lu, F. Effects of high temperature during burning on Sphagnum spore germinability: A simulated experimental study. Chin. J. Ecol. 2019, 38, 2369–2376. [Google Scholar]
- Johnson, P.N. Vegetation recovery after fire on a southern New Zealand peatland. N. Z. J. Bot. 2001, 39, 251–267. [Google Scholar] [CrossRef]
- Benscoter, B.W. Post-fire bryophyte establishment in a continental bog. J. Veg. Sci. 2006, 17, 647–652. [Google Scholar] [CrossRef]
- Clarke, P.J.; Keith, D.A.; Vincent, B.E.; Letten, A.D. Post-grazing and post-fire vegetation dynamics: Long-term changes in mountain bogs reveal community resilience. J. Veg. Sci. 2015, 26, 278–290. [Google Scholar] [CrossRef]
- Blier-Langdeau, A.; Guêné-Nanchen, M.; Hugron, S.; Rocherfort, L. The resistance and short-term resilience of a restored extracted peatland ecosystems post-fire: An opportunistic study after a wildfire. Restor. Ecol. 2021, e13545. [Google Scholar] [CrossRef]
- Yusup, S.; Sundberg, S.; Ooi, M.K.; Zhang, M.M.; Sun, Z.Q.; Rydin, H.; Wang, M.; Feng, L.; Chen, X.; Bu, Z.J. Smoke promotes germination of peatland bryophyte spores. Ecology, 2022; submitted. [Google Scholar]
- Zirondi, H.L.; Silveira, F.A.; Fidelis, A. Fire effects on seed germination: Heat shock and smoke on permeable vs impermeable seed coats. Flora 2019, 253, 98–106. [Google Scholar] [CrossRef]
- Bradstock, R.A.; Auld, T.D. Soil temperatures during experimental bushfires in relation to fire intensity: Consequences for Legume germination and fire management in south-eastern Australia. J. Appl. Ecol. 1995, 32, 76–84. [Google Scholar] [CrossRef]
- Zhang, G.F.; Hua, D.F.; Huang, B.Q.; Zhang, H.H.; Yu, T.; Meng, L.Y.; Su, W.H. Effects of high temperature treatment on seed germination of Dodonaea viscosa (L.) Jacq. Agric. Sci. Technol. 2017, 18, 82–86. [Google Scholar]
- Webster, R.E.; Waterworth, W.M.; Stuppy, W.; West, C.E.; Ennos, R.; Bray, C.M.; Pritchard, H.W. Biomechanical, biochemical, and morphological mechanisms of heat shock-mediated germination in Carica papaya seed. J. Exp. Bot. 2016, 67, 6373–6384. [Google Scholar] [CrossRef]
- Keeley, J.E.; Fotheringham, C.J. Trace gas emissions and smoke-induced seed germination. Science 1997, 276, 1248–1250. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Staszak-Kozinski, L.; Davidson, R. Up in smoke I. Smoke-derived germination cues for post-fire annual, Nicotiana attenuata Toor ex Watson. J. Chem. Ecol. 1994, 20, 2345–2371. [Google Scholar] [CrossRef]
- Gupta, K.J.; Fernie, A.R.; Kaiser, W.M.; van Dongen, J.T. On the origins of nitric oxide. Trends Plant Sci. 2011, 16, 160–168. [Google Scholar] [CrossRef]
- Preston, C.A.; Becker, R.; Baldwin, I.T. Is ‘NO’ news good news? Nitrogen oxides are not components of smoke that elicits germination in two smoke-stimulated species, Nicotiana attenuata and Emmenanthe penduliflora. Seed Sci. Res. 2004, 14, 73–79. [Google Scholar] [CrossRef]
- Light, M.E.; van Staden, J. The nitric oxide specific scavenger carboxy-PTIO does not inhibit smoke stimulated germination of Grand Rapids lettuce seeds. S. Afr. J. Bot. 2003, 69, 217–219. [Google Scholar] [CrossRef][Green Version]
- Footitt, S.; Cohn, M.A. Developmental arrest: From sea urchins to seeds. Seed Sci. Res. 2001, 11, 3–16. [Google Scholar] [CrossRef]
- Abella, S.; Springer, J.; Covington, W. Seed banks of an Arizona Pinus ponderosa landscape: Responses to environmental gradients and fire cues. Can. J. For. Res. 2007, 37, 552–567. [Google Scholar] [CrossRef]
- Figueroa, J.; Cavieres, L.; Gomez-Gonzalez, S.; Molina-Montenegro, M.; Jaksic, F. Do heat and smoke increase emergence of exotic and native plants in the matorral of central Chile? Acta Oecologica 2009, 35, 335–340. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Mojzes, A.; Csontos, P.; Kalapos, T. Is the positive response of seed germination to plant-derived smoke associated with plant traits? Acta Oecologica 2015, 65, 24–31. [Google Scholar] [CrossRef]
- Carthey, A.J.; Tims, A.; Geedicke, I.; Leishman, M.R. Broad-scale patterns in smoke-responsive germination from the south-eastern Australian flora. J. Veg. Sci. 2018, 29, 737–745. [Google Scholar] [CrossRef]
- Nungesser, M.K. Modelling microtopography in boreal peatlands: Hummocks and hollows. Ecol. Model. 2003, 165, 175–207. [Google Scholar] [CrossRef]
- Rydin, H.; Jeglum, J.K. The Biology of Peatlands, 2nd ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Clymo, R.S.; Duckett, J.G. Regeneration of Sphagnum. New Phytol. 1986, 102, 589–614. [Google Scholar] [CrossRef]
- Sundberg, S.; Rydin, H. Experimental evidence for a persistent spore bank in Sphagnum. New Phytol. 2000, 148, 105–116. [Google Scholar] [CrossRef]
- Thompson, D.K.; James, M. Wildfire effects on vadose zone hydrology in forested boreal peatland microforms. J. Hydrol. 2013, 486, 48–56. [Google Scholar] [CrossRef]
- Smith, S.M.; Newman, S.; Garrett, P.B.; Leeds, J.A. Differential effects of surface and peat fire on soil constituents in a degraded wetland of the northern Florida Everglades. J. Environ. Qual. 2001, 30, 1998–2005. [Google Scholar] [CrossRef]
- Ramírez-Trejo, M.D.R.; Pérez-García, B.; Orozco-Segovia, A. Analysis of fern spore banks from the soil of three vegetation types in the central region of Mexico. Am. J. Bot. 2004, 91, 682–688. [Google Scholar] [CrossRef]
- Rudolph, H.; Kirchhoff, M.; Gliesmann, S. Sphagnum culture techniques. In Methods in Bryology, Proceedings of the Bryological Methods Workshop, Mainz, Germany, 1988; Hattori Botanical Laboratory: Nichinan, Japan, 1988; pp. 25–34. [Google Scholar]
- Bai, X.S.; Bu, Z.J.; Liu, W.J.; Shuayib, Y.; Xu, X.Y. A preliminary comparative study on rapid viability determination of peatland bryophyte spores. Guihaia 2021. [Google Scholar] [CrossRef]



| Index | Species | Heat | Species × Heat | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| GP | 49.08 | <0.001 | 23.58 | <0.001 | 4.36 | 0.004 |
| V | 33.58 | <0.001 | 165.39 | <0.001 | 14.52 | <0.001 |
| Species | Index | Heat | Heat + Smoke | ||
|---|---|---|---|---|---|
| F | p | F | p | ||
| S. angustifolium | GP | 8.67 | 0.017 | 27.75 | <0.001 |
| GS | 1.55 | 0.287 | 4.23 | 0.046 | |
| TEI | 6.90 | 0.028 | 10.48 | 0.004 | |
| S. fuscum | GP | 5.66 | 0.042 | 8.58 | 0.007 |
| GS | 6.50 | 0.031 | 2.34 | 0.150 | |
| TEI | 1.702 | 0.260 | 3.03 | 0.093 | |
| S. magellanicum | GP | 3.47 | 0.100 | 23.14 | <0.001 |
| GS | 0.10 | 0.904 | 2.70 | 0.116 | |
| TEI | 4.85 | 0.056 | 14.12 | 0.001 | |
| S. squarrosum | GP | 19.70 | 0.002 | 20.42 | <0.001 |
| GS | 0.738 | 0.517 | 1.73 | 0.238 | |
| TEI | 34.88 | <0.001 | 36.25 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusup, S.; Sundberg, S.; Fan, B.; Sulayman, M.; Bu, Z.-J. The Response of Spore Germination of Sphagnum Mosses to Single and Combined Fire-Related Cues. Plants 2022, 11, 485. https://doi.org/10.3390/plants11040485
Yusup S, Sundberg S, Fan B, Sulayman M, Bu Z-J. The Response of Spore Germination of Sphagnum Mosses to Single and Combined Fire-Related Cues. Plants. 2022; 11(4):485. https://doi.org/10.3390/plants11040485
Chicago/Turabian StyleYusup, Shuayib, Sebastian Sundberg, Beibei Fan, Mamtimin Sulayman, and Zhao-Jun Bu. 2022. "The Response of Spore Germination of Sphagnum Mosses to Single and Combined Fire-Related Cues" Plants 11, no. 4: 485. https://doi.org/10.3390/plants11040485
APA StyleYusup, S., Sundberg, S., Fan, B., Sulayman, M., & Bu, Z.-J. (2022). The Response of Spore Germination of Sphagnum Mosses to Single and Combined Fire-Related Cues. Plants, 11(4), 485. https://doi.org/10.3390/plants11040485






