Developmental Stages Affect the Capacity to Produce Aldehyde Green Leaf Volatiles in Zea mays and Vigna radiata
Abstract
:1. Introduction
2. Results
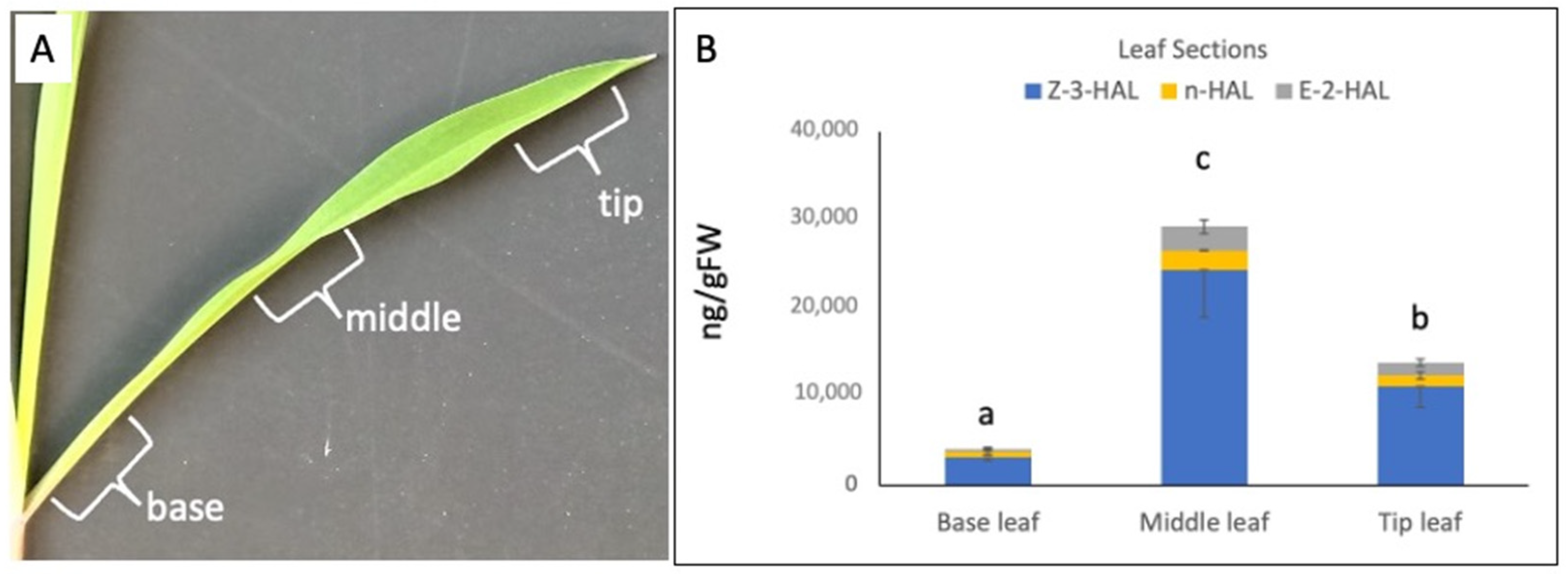
2.1. Zea mays
2.2. Vigna radiata
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Leaf Damage and Analysis of Green Leaf Volatile Synthesis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green leaf volatiles: A plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2017, 220, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Shiojiri, K.; Kishimoto, K.; Ozawa, R.; Kugimiya, S.; Urashimo, S.; Arimura, G.; Horiuchi, J.; Nishioka, T.; Matsui, K.; Takabayashi, J. Changing green leaf volatile biosynthesis in plants: An approach for improving plant resistance against both herbivores and pathogens. Proc. Natl. Acad. Sci. USA 2006, 103, 16672–16676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jardine, K.J.; Chambers, J.Q.; Holm, J.; Jardine, A.B.; Fontes, C.G.; Zorzanelli, R.F.; Meyers, K.T.; de Souza, V.F.; Garcia, S.; Giminez, B.O.; et al. Green leaf volatile emissions during high temperature and drought stress in a Central Amazon rainforest. Plants 2010, 4, 678–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cofer, T.M.; Engelberth, M.J.; Engelberth, J. Green leaf volatiles protect maize (Zea mays) seedlings against damage from cold stress. Plant Cell Environ. 2018, 41, 1673–1682. [Google Scholar] [CrossRef]
- Engelberth, M.; Selman, S.M.; Engelberth, J. In-cold exposure to Z-3-hexenal provides protection against ongoing cold stress in Zea mays. Plants 2019, 8, E165. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, Y.; Kunishima, M.; Mizutani, M.; Sugimoto, Y. Reactive short-chain leaf volatiles act as powerful inducers of abiotic stress-related gene expression. Sci. Rep. 2015, 5, 8030. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Guo, R.; Zou, X.; Zhang, X.; Yu, X.; Zhan, Y.; Ci, D.; Wang, M.; Si, T. Priming with the green leaf volatile (Z)-3-hexeny-1-yl acetate enhances salinity stress tolerance in peanut (Arachis hypogaea L.) seedlings. Front. Plant Sci. 2019, 10, 785. [Google Scholar] [CrossRef]
- Lee, K.; Seo, P.J. Airborne signals from salt-stressed Arabidopsis plants trigger salinity tolerance in neighboring plants. Plant Signal. Behav. 2014, 9, e28392. [Google Scholar] [CrossRef] [Green Version]
- Engelberth, J.; Alborn, H.T.; Schmelz, E.A.; Tumlinson, J.H. Airborne signals prime plants against herbivore attack. Proc. Natl. Acad. Sci. USA 2004, 101, 1781–1785. [Google Scholar] [CrossRef] [Green Version]
- Hatanaka, A. The biogeneration of green odour by green leaves. Phytochem 1993, 34, 1201–1218. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ikeda, A.; Shiojiri, K.; Ozawa, R.; Shiki, K.; Nagai-Kunihiro, N.; Fujita, K.; Sugimoto, K.; Yamato, K.T.; Dohra, H.; et al. Identification of a hexenal reductase that modulates the composition of green leaf volatiles. Plant Physiol. 2018, 178, 552–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Auria, J.C.; Pichersky, E.; Schaub, A.; Hansel, A.; Gershenzon, J. Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J. 2007, 49, 194–207. [Google Scholar] [CrossRef] [Green Version]
- Christensen, S.A.; Nemchenko, A.; Borrego, E.; Sobhy, I.; Bosak, L.; Erb, M.; Roberts, C.; Vaughn, K.A.; Göbel, C.; Tumlinson, J.; et al. The multi-tasking lipoxygenase, ZmLOX10, regulates GLV, JA, and HIPV production for defense against insect attack. Plant J. 2012, 74, 59–73. [Google Scholar] [CrossRef]
- Kunishima, M.; Yamauchi, Y.; Mizutani, M.; Kuse, M.; Takikawa, H.; Sugimoto, Y. Identification of (Z)-3:(E)-2-hexenal isomerases essential to the production of the leaf aldehyde in plants. J. Biol. Chem. 2016, 291, 14023–14033. [Google Scholar] [CrossRef] [Green Version]
- Spyropoulou, E.A.; Dekker, H.L.; Steemers, L.; van Maarseveen, J.H.; de Koster, C.G.; Haring, M.A.; Schuurink, R.C.; Allmann, S. Identification and characterization of (3Z):(2E)-hexenal isomerases from cucumber. Front. Plant Sci. 2017, 8, 1342. [Google Scholar] [CrossRef] [Green Version]
- Matsui, K.; Sugimoto, K.; Mano, J.; Ozawa, R.; Takabayashi, J. Differential metabolism of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. PLoS ONE 2012, 7, e36433. [Google Scholar]
- Engelberth, M.; Engelberth, J. Variability of damage-induced green leaf volatile release among different plant species provides novel insights into biosynthetic diversity. Plants 2020, 9, 213. [Google Scholar] [CrossRef] [Green Version]
- Savchenko, T.; Pearse, I.S.; Ignatia, L.; Karban, R.; Dehesh, K. Insect herbivores selectively suppress the HPL branch of the oxylipin pathway in host plants. Plant J. 2012, 73, 653–662. [Google Scholar] [CrossRef]
- Savchenko, T.; Dehesh, K. Insect herbivores selectively mute GLV production in plants. Plant Signal. Behav. 2013, 8, e24136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takai, H.; Ozawa, R.; Takabayashi, J.; Fujii, S.; Arai, K.; Ichiki, R.T.; Koeduka, T.; Dohra, H.; Ohnishi, T.; Taketazu, S.; et al. Silkworms suppress the release of green leaf volatiles by mulberry leaves with an enzyme from their spinnerets. Sci. Rep. 2018, 8, 11942. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Seidl-Adams, I.; Engelberth, J.; Hunter, C.T.; Alborn, H.; Tumlinson, J.H. Herbivorous caterpillars can utilize three mechanisms to alter green leaf volatile emission. Environ. Entomol. 2019, 48, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Cofer, T.M.; Engelberth, J.; Tumlinson, J.H. Herbivorous caterpillars and the green leaf volatile (GLV) quandary. J. Chem. Ecol. 2021. [Google Scholar] [CrossRef]
- Curtius, T.; Franzen, H. Aldehyde aus gruenen Pflanzenteilen. Chem. Zentr. 1911, 2, 1142–1143. [Google Scholar]
- Curtius, T.; Franzen, H. Über die chemischen Bestandteile grüner Pflanzen: Über den Blätteraldehyd. Ann. Chem. 1912, 390, 89–121. [Google Scholar] [CrossRef] [Green Version]
- Bate, N.J.; Rothstein, S.J. C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J. 1998, 16, 561–569. [Google Scholar] [CrossRef]
- Vancanneyt, G.; Sanz, C.; Farmaki, T.; Paneque, M.; Ortego, F. Hydroperoxide lyase depletion in transgenic potato plants leads to an increase in aphid performance. Proc. Natl. Acad. Sci. USA 2001, 98, 8139–8144. [Google Scholar] [CrossRef] [Green Version]
- Arimura, G.; Ozawa, R.; Shimoda, T.; Nishioka, T.; Boland, W.; Takabayashi, J. Herbivory-induced volatiles elicit defense genes in lima bean leaves. Nature 2000, 406, 512–515. [Google Scholar] [CrossRef]
- Kessler, A.; Halitschke, R.; Diezel, C.; Baldwin, I.T. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 2006, 148, 280–292. [Google Scholar] [CrossRef]
- Heil, M.; Bueno, J.C. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc. Natl. Acad. Sci. USA 2007, 104, 5467–5472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ton, J.; D’Alessandro, M.; Jourdi, V.; Jakab, G.; Karlen, D.; Held, M.; Mauch-Mani, B.; Turlings, T.C.J. Priming by airborne signals boosts direct and indirect resistance in maize. Plant J. 2007, 49, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Frost, C.J.; Mescher, M.C.; Dervinis, C.; Davis, J.M.; Carlson, J.E.; De Moraes, C.M. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytol. 2008, 180, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Estrada, M.; Kazantsev, T.; Niinemets, Ü. Fading of wound-induced volatile release during Populus tremula leaf expansion. J. Plant Res. 2017, 130, 157–165. [Google Scholar] [CrossRef] [Green Version]
- McKey, D. Distribution of secondary compounds in plants. In Herbivores: Their Association with Secondary Metabolites; Rosenthal, G.A., Janzen, D.H., Eds.; Academic Press: New York, NY, USA, 1979; pp. 56–133. [Google Scholar]
- Rhoades, D.F. Evolution of plant chemical defense against herbivores. In Herbivores: Their Association with Secondary Metabolites; Rosenthal, G.A., Janzen, D.H., Eds.; Academic Press: New York, NY, USA, 1979; pp. 3–54. [Google Scholar]
- Pavia, H.; Toth, G.B.; Aberg, P. Optimal defense theory: Elasticity analysis as a tool to predict intraplant variation in defenses. Ecology 2002, 83, 891–897. [Google Scholar] [CrossRef]
- Loomis, W.E. Growth and differentiation—An introduction and summary. In Growth and Differentiation in Plants; Wareing, P.F., Phillips, I.D.J., Eds.; Pergamon Press: New York, NY, USA, 1981; pp. 1–17. [Google Scholar]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engelberth, J.; Engelberth, M. Developmental Stages Affect the Capacity to Produce Aldehyde Green Leaf Volatiles in Zea mays and Vigna radiata. Plants 2022, 11, 526. https://doi.org/10.3390/plants11040526
Engelberth J, Engelberth M. Developmental Stages Affect the Capacity to Produce Aldehyde Green Leaf Volatiles in Zea mays and Vigna radiata. Plants. 2022; 11(4):526. https://doi.org/10.3390/plants11040526
Chicago/Turabian StyleEngelberth, Jurgen, and Marie Engelberth. 2022. "Developmental Stages Affect the Capacity to Produce Aldehyde Green Leaf Volatiles in Zea mays and Vigna radiata" Plants 11, no. 4: 526. https://doi.org/10.3390/plants11040526
APA StyleEngelberth, J., & Engelberth, M. (2022). Developmental Stages Affect the Capacity to Produce Aldehyde Green Leaf Volatiles in Zea mays and Vigna radiata. Plants, 11(4), 526. https://doi.org/10.3390/plants11040526






