Revisiting the Domestication Process of African Vigna Species (Fabaceae): Background, Perspectives and Challenges
Abstract
1. Introduction
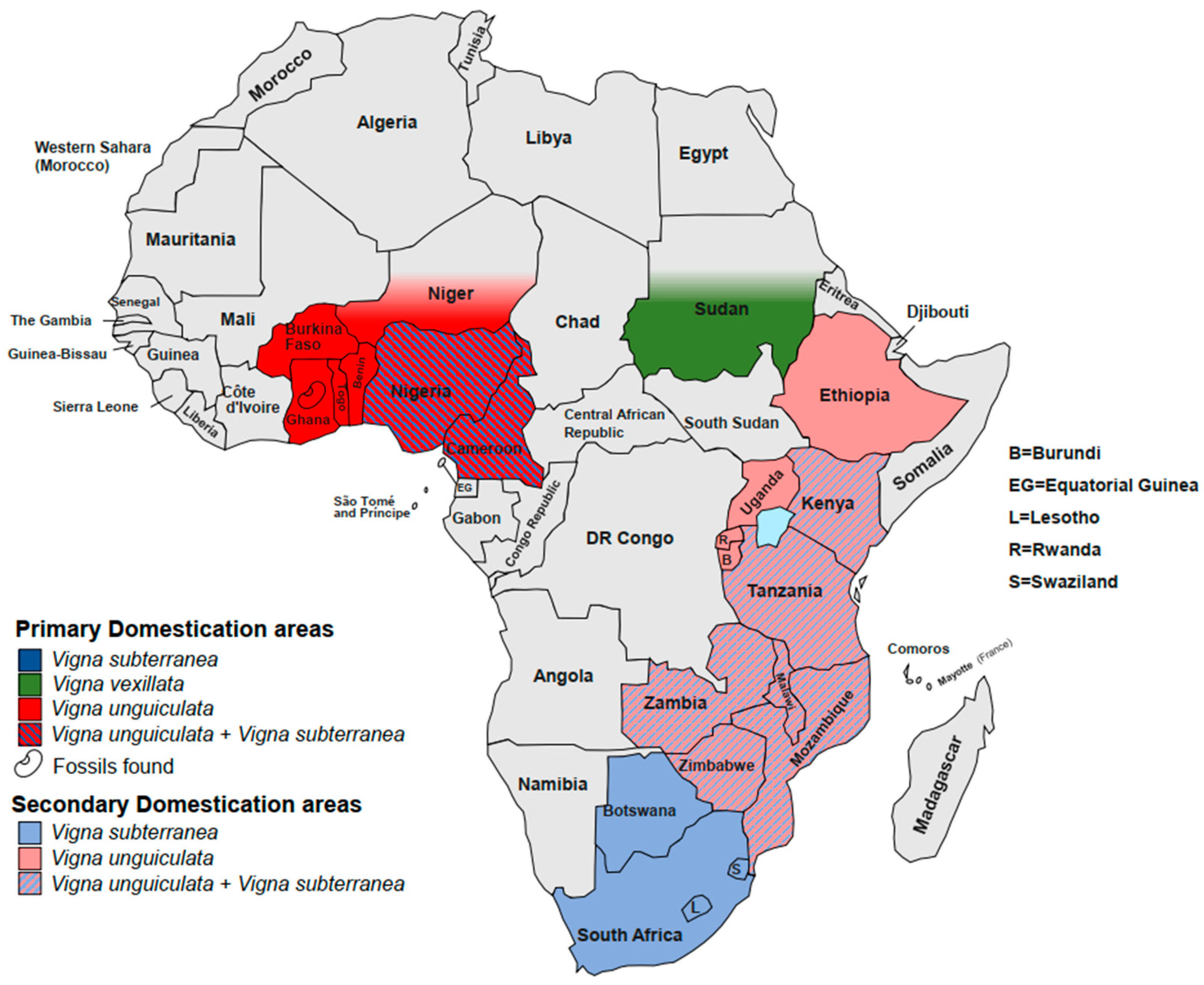
2. Vigna unguiculata (L.) Walp.
3. Vigna subterranea (L.) Verdc.
4. Vigna vexillata (L.) A. Rich.
5. Healthy Natural Compounds for Designing Sustainable Crops
5.1. Antioxidant and Anti-Inflammatory Activity
5.2. Anti-Tumor Compounds
5.3. Anti Hypercholesterolemic
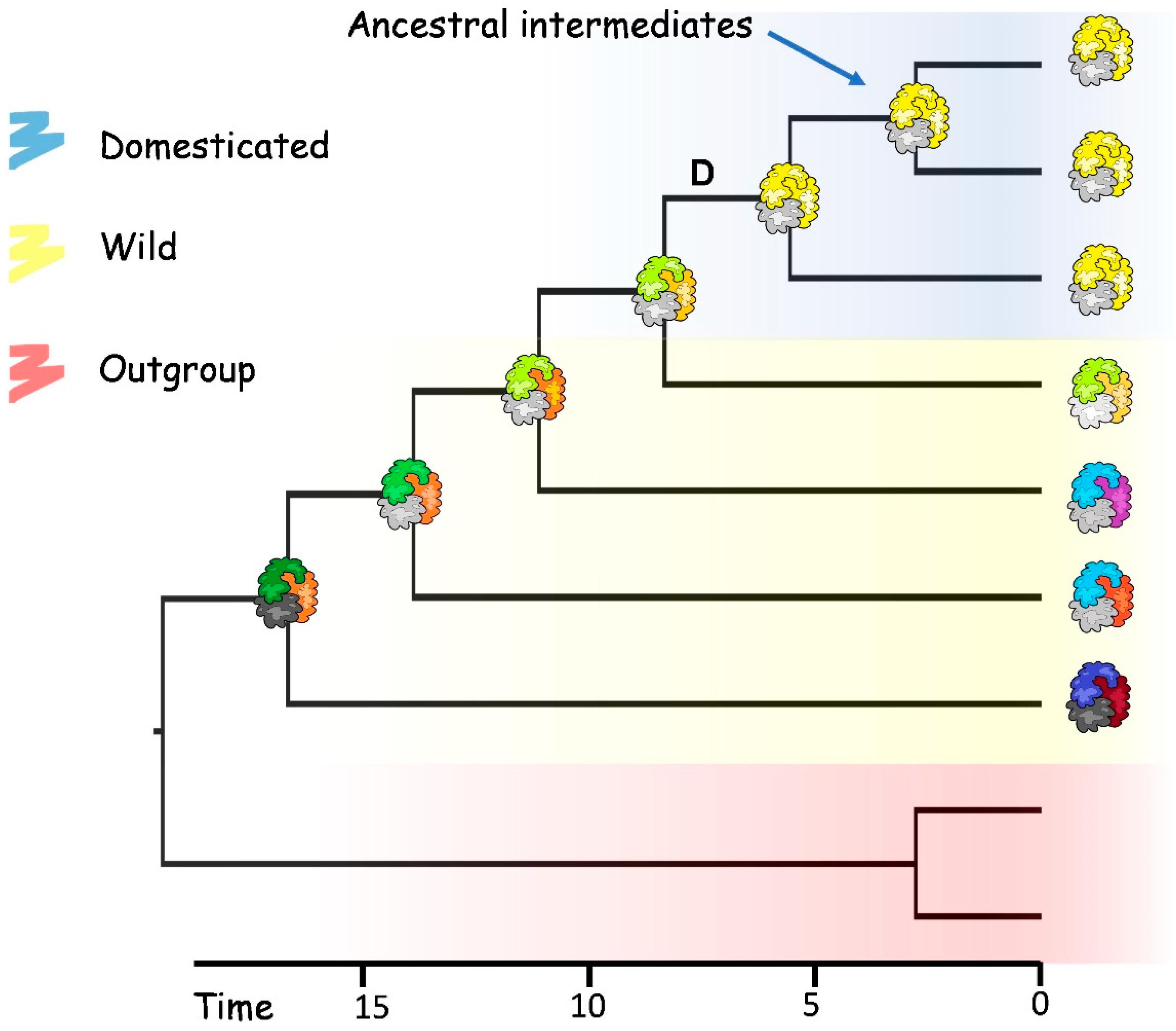
6. Introgression and Feralisation Processes
7. Domestication-Related Traits and De Novo Domestication
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kahane, R.; Hodgkin, T.; Jaenicke, H.; Hoogendoorn, C.; Hermann, M.; d’Arros Hughes, J.; Padulosi, S.; Looney, N. Agrobiodiversity for Food Security, Health and Income. Agron. Sustain. Dev. 2013, 33, 671–693. [Google Scholar] [CrossRef]
- Hu, X.-R.; Chou, G.-X.; Zhang, C.-G. Flavonoids, Alkaloids from the Seeds of Crotalaria Pallida and Their Cytotoxicity and Anti-Inflammatory Activities. Phytochemistry 2017, 143, 64–71. [Google Scholar] [CrossRef]
- Lam, S.-H.; Li, Y.-C.; Kuo, P.-C.; Hwang, T.-L.; Yang, M.-L.; Wang, C.-C.; Tzen, J.T. Chemical Constituents of Vigna luteola and Their Anti-Inflammatory Bioactivity. Molecules 2019, 24, 1371. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sakai, H.; Yoshitsu, Y.; Muto, C.; Anai, T.; Pandiyan, M.; Senthil, N.; Tomooka, N.; Naito, K. Domesticating Vigna stipulacea: A Potential Legume Crop with Broad Resistance to Biotic Stresses. Front. Plant Sci. 2019, 10, 1607. [Google Scholar] [CrossRef]
- Ku, Y.-S.; Contador, C.A.; Ng, M.-S.; Yu, J.; Chung, G.; Lam, H.-M. The Effects of Domestication on Secondary Metabolite Composition in Legumes. Front. Genet. 2020, 11, 581357. [Google Scholar] [CrossRef] [PubMed]
- Marconi, E.; Ruggeri, S.; Carnovale, E. Chemical Evaluation of Wild Under-Exploited Vigna spp. Seeds. Food Chem. 1997, 59, 203–212. [Google Scholar] [CrossRef]
- Duranti, M. Grain Legume Proteins and Nutraceutical Properties. Fitoterapia 2006, 77, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Tomooka, N.; Naito, K.; Kaga, A.; Sakai, H.; Isemura, T.; Ogiso-Tanaka, E.; Iseki, K.; Takahashi, Y. Evolution, Domestication and Neo-Domestication of the Genus Vigna. Plant Genet. Resour. 2014, 12, S168–S171. [Google Scholar] [CrossRef]
- Harouna, D.V.; Venkataramana, P.B.; Ndakidemi, P.A.; Matemu, A.O. Under-Exploited Wild Vigna Species Potentials in Human and Animal Nutrition: A Review. Glob. Food Secur. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Boukar, O.; Belko, N.; Chamarthi, S.; Togola, A.; Batieno, J.; Owusu, E.; Haruna, M.; Diallo, S.; Umar, M.L.; Olufajo, O. Cowpea (Vigna unguiculata): Genetics, Genomics and Breeding. Plant Breed. 2019, 138, 415–424. [Google Scholar] [CrossRef]
- Vigna Savi|Plants of the World Online|Kew Science. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:325971-2 (accessed on 17 January 2022).
- Maréchal, R. Etude Taxonomique d’un Groupe Complexe d’espèces Des Genres Phaseolus et Vigna (Papilionaceae) Sur La Base de Données Morphologiques et Polliniques, Traitées Par l’analyse Informatique. Boissiera 1978, 28, 1–273. [Google Scholar]
- Thulin, M.; Lavin, M.; Pasquet, R.; Delgado-Salinas, A. Phylogeny and Biogeography of Wajira (Leguminosae): A Monophyletic Segregate of Vigna Centered in the Horn of Africa Region. Syst. Bot. 2004, 29, 903–920. [Google Scholar] [CrossRef]
- Javadi, F.; Tun, Y.T.; Kawase, M.; Guan, K.; Yamaguchi, H. Molecular Phylogeny of the Subgenus Ceratotropis (Genus Vigna, Leguminosae) Reveals Three Eco-Geographical Groups and Late Pliocene–Pleistocene Diversification: Evidence from Four Plastid DNA Region Sequences. Ann. Bot. 2011, 108, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Salinas, A.; Thulin, M.; Pasquet, R.; Weeden, N.; Lavin, M. Vigna (Leguminosae) Sensu Lato: The Names and Identities of the American Segregate Genera. Am. J. Bot. 2011, 98, 1694–1715. [Google Scholar] [CrossRef] [PubMed]
- Lavin, M.; Herendeen, P.S.; Wojciechowski, M.F. Evolutionary Rates Analysis of Leguminosae Implicates a Rapid Diversification of Lineages during the Tertiary. Syst. Biol. 2005, 54, 575–594. [Google Scholar] [PubMed]
- Li, H.; Wang, W.; Lin, L.; Zhu, X.; Zhu, X.; Li, J.; Chen, Z. Diversification of the Phaseoloid Legumes: Effects of Climate Change, Range Expansion and Habit Shift. Front. Plant Sci. 2013, 4, 386. [Google Scholar] [CrossRef] [PubMed]
- Singh, B. Cowpea: The Food Legume of the 21st Century; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 164. [Google Scholar]
- Maxted, N.; Mabuza-Diamini, P.; Moss, H.; Padulosi, S.; Jarvis, A.; Guarino, L. An Ecogeographic Study African Vigna; International Plant Genetic Resources Institute (IPGRI): Rome, Italy, 2004; ISBN 978-92-9043-637-9. [Google Scholar]
- Tateishi, Y. Systematics of the Species of Vigna Subgenus Ceratotropis. Mungbean Germplasm Collect. Util. Breed. Program. 1996, 9–24. [Google Scholar]
- Tomooka, N.; Maxted, N.; Thavarasook, C.; Jayasuriya, A.H.M. Two New Species, Sectional Designations and New Combinations in Vigna Subgenus Ceratotropis (Piper) Verdc. (Leguminosae, Phaseoleae). Kew Bull. 2002, 57, 613–624. [Google Scholar] [CrossRef]
- Tomooka, N.; Yoon, M.S.; Doi, K.; Kaga, A.; Vaughan, D. AFLP Analysis of Diploid Species in the Genus Vigna Subgenus Ceratotropis. Genet. Resour. Crop. Evol. 2002, 49, 521–530. [Google Scholar] [CrossRef]
- Pienaar, B.J. The Vigna vexillata Complex (Fabaceae) in Southern Africa. South Afr. J. Bot. 1991, 57, 236–245. [Google Scholar] [CrossRef][Green Version]
- Ladizinsky, G. Seed Dispersal in Relation to the Domestication of Middle East Legumes. Econ. Bot. 1979, 33, 284–289. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kongjaimun, A.; Muto, C.; Kobayashi, Y.; Kumagai, M.; Sakai, H.; Satou, K.; Teruya, K.; Shiroma, A.; Shimoji, M. Same Locus for Non-Shattering Seed Pod in Two Independently Domesticated Legumes, Vigna angularis and Vigna unguiculata. Front. Genet. 2020, 11, 748. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Elliott, K.A.; Gonzalez-Carranza, Z.H. Abscission, Dehiscence, and Other Cell Separation Processes. Annu. Rev. Plant Biol. 2002, 53, 131–158. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.; Parker, T.; Muñoz-Amatriaín, M.; Berny-Mier y Teran, J.C.; Jernstedt, J.; Close, T.J.; Gepts, P. Genetic, Anatomical, and Environmental Patterns Related to Pod Shattering Resistance in Domesticated Cowpea (Vigna unguiculata [L.] Walp.). J. Exp. Bot. 2021, 72, 6219–6229. [Google Scholar] [CrossRef] [PubMed]
- Parker, T.A.; Berny Mier y Teran, J.C.; Palkovic, A.; Jernstedt, J.; Gepts, P. Pod Indehiscence Is a Domestication and Aridity Resilience Trait in Common Bean. New Phytol. 2020, 225, 558–570. [Google Scholar] [CrossRef]
- Meyer, R.S.; Purugganan, M.D. Evolution of Crop Species: Genetics of Domestication and Diversification. Nat. Rev. Genet. 2013, 14, 840–852. [Google Scholar] [CrossRef]
- Herniter, I.A.; Muñoz-Amatriaín, M.; Lo, S.; Guo, Y.-N.; Close, T.J. Identification of Candidate Genes Controlling Black Seed Coat and Pod Tip Color in Cowpea (Vigna unguiculata [L.] Walp.). G3 Genes Genomes Genet. 2018, 8, 3347–3355. [Google Scholar] [CrossRef]
- Lo, S.; Muñoz-Amatriaín, M.; Hokin, S.A.; Cisse, N.; Roberts, P.A.; Farmer, A.D.; Xu, S.; Close, T.J. A Genome-Wide Association and Meta-Analysis Reveal Regions Associated with Seed Size in Cowpea [Vigna unguiculata (L.) Walp.]. Theor. Appl. Genet. 2019, 132, 3079–3087. [Google Scholar] [CrossRef]
- Seo, E.; Kim, K.; Kang, R.; Kim, G.; Park, A.; Kim, W.J.; Sun, H.; Ha, B.-K. Genome-Wide Association Study for Flowering Time in Korean Cowpea Germplasm. Plant Breed. Biotechnol. 2020, 8, 413–425. [Google Scholar] [CrossRef]
- Amkul, K.; Somta, P.; Laosatit, K.; Wang, L. Identification of QTLs for Domestication-Related Traits in Zombi Pea [Vigna vexillata (L.) A. Rich.], a Lost Crop of Africa. Front. Genet. 2020, 11, 803. [Google Scholar] [CrossRef]
- Lu, J.; Tang, T.; Tang, H.; Huang, J.; Shi, S.; Wu, C.-I. The Accumulation of Deleterious Mutations in Rice Genomes: A Hypothesis on the Cost of Domestication. Trends Genet. 2006, 22, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mittal, N.; Leamy, L.J.; Barazani, O.; Song, B.-H. Back into the Wild—Apply Untapped Genetic Diversity of Wild Relatives for Crop Improvement. Evol. Appl. 2017, 10, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, Y.; Morrell, P.L.; Gaut, B.S. Deleterious Variants in Asian Rice and the Potential Cost of Domestication. Mol. Biol. Evol. 2017, 34, 908–924. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Tomooka, N.; Okabayashi, M.; Kaga, A.; Boonkerd, N.; Vaughan, D.A. Variation in the Nod Gene RFLPs, Nucleotide Sequences of 16S RRNA Genes, Nod Factors, and Nodulation Abilities of Bradyrhizobium Strains Isolated from Thai Vigna Plants. Can. J. Microbiol. 2006, 52, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Chankaew, S.; Isemura, T.; Naito, K.; Ogiso-Tanaka, E.; Tomooka, N.; Somta, P.; Kaga, A.; Vaughan, D.A.; Srinives, P. QTL Mapping for Salt Tolerance and Domestication-Related Traits in Vigna marina subsp. oblonga, a Halophytic Species. Theor. Appl. Genet. 2014, 127, 691–702. [Google Scholar] [CrossRef]
- Tomooka, N.; Kaga, A.; Isemura, T.; Vaughan, D.V. Wild Crop Relatives: Genomic and Breeding Resources: Legume Crops and Forages; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Takahashi, Y.; Somta, P.; Muto, C.; Iseki, K.; Naito, K.; Pandiyan, M.; Natesan, S.; Tomooka, N. Novel Genetic Resources in the Genus Vigna Unveiled from Gene Bank Accessions. PLoS ONE 2016, 11, e0147568. [Google Scholar]
- Yoshida, J.; Tomooka, N.; Yee Khaing, T.; Shantha, P.S.; Naito, H.; Matsuda, Y.; Ehara, H. Unique Responses of Three Highly Salt-Tolerant Wild Vigna Species against Salt Stress. Plant Prod. Sci. 2020, 23, 114–128. [Google Scholar] [CrossRef]
- van Zonneveld, M.; Rakha, M.; Tan, S.y.; Chou, Y.-Y.; Chang, C.-H.; Yen, J.-Y.; Schafleitner, R.; Nair, R.; Naito, K.; Solberg, S.Ø. Mapping Patterns of Abiotic and Biotic Stress Resilience Uncovers Conservation Gaps and Breeding Potential of Vigna Wild Relatives. Sci. Rep. 2020, 10, 1–11. [Google Scholar]
- Carvalho, M.d.; Halecki, W. Modeling of Cowpea (Vigna unguiculata) Yield and Control Insecticide Exposure in a Semi-Arid Region. Plants 2021, 10, 1074. [Google Scholar] [CrossRef]
- Peña-Valdivia, C.B.; García-Nava, J.R.; Aguirre R, J.R.; Ybarra-Moncada, M.C.; López H, M. Variation in Physical and Chemical Characteristics of Common Bean (Phaseolus vulgaris L.) Grain along a Domestication Gradient. Chem. Biodivers. 2011, 8, 2211–2225. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Marín, B.; Milla, R.; Martín-Robles, N.; Arc, E.; Kranner, I.; Becerril, J.M.; García-Plazaola, J.I. Side-Effects of Domestication: Cultivated Legume Seeds Contain Similar Tocopherols and Fatty Acids but Less Carotenoids than Their Wild Counterparts. BMC Plant Biol. 2014, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Larson, G.; Piperno, D.R.; Allaby, R.G.; Purugganan, M.D.; Andersson, L.; Arroyo-Kalin, M.; Barton, L.; Vigueira, C.C.; Denham, T.; Dobney, K. Current Perspectives and the Future of Domestication Studies. Proc. Natl. Acad. Sci. USA 2014, 111, 6139–6146. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.C.; Kahlheber, S.; Logan, A.L.; Watson, D.J. Early Domesticated Cowpea (Vigna unguiculata) from Central Ghana. Antiquity 2007, 81, 686–698. [Google Scholar] [CrossRef]
- Allaby, R.G.; Fuller, D.Q.; Brown, T.A. The Genetic Expectations of a Protracted Model for the Origins of Domesticated Crops. Proc. Natl. Acad. Sci. USA 2008, 105, 13982–13986. [Google Scholar] [CrossRef] [PubMed]
- Purugganan, M.D.; Fuller, D.Q. Archaeological Data Reveal Slow Rates of Evolution during Plant Domestication. Evol. Int. J. Org. Evol. 2011, 65, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Engels, J.M.M.; Ebert, A.W.; Thormann, I.; De Vicente, M.C. Centres of Crop Diversity and/or Origin, Genetically Modified Crops and Implications for Plant Genetic Resources Conservation. Genet. Resour. Crop. Evol. 2006, 53, 1675–1688. [Google Scholar] [CrossRef]
- Huynh, B.; Close, T.J.; Roberts, P.A.; Hu, Z.; Wanamaker, S.; Lucas, M.R.; Chiulele, R.; Cissé, N.; David, A.; Hearne, S. Gene Pools and the Genetic Architecture of Domesticated Cowpea. Plant Genome 2013, 6, 1–8. [Google Scholar] [CrossRef]
- Herniter, I.A.; Muñoz-Amatriaín, M.; Close, T.J. Genetic, Textual, and Archeological Evidence of the Historical Global Spread of Cowpea (Vigna unguiculata [L.] Walp.). Legume Sci. 2020, 2, e57. [Google Scholar] [CrossRef]
- Vijaykumar, A.; Saini, A.; Jawali, N. Assessment of Hybridization among Wild and Cultivated Vigna unguiculata Subspecies Revealed by Arbitrarily Primed Polymerase Chain Reaction Analysis. AoB Plants 2012, 2012, pls012. [Google Scholar] [CrossRef]
- Small, E. Top 100 Food Plants: The World’s Most Important Culinary Crops; NRC Research Press: Ottawa, ON, Canada, 2009. [Google Scholar]
- Timko, M.P.; Ehlers, J.D.; Roberts, P.A. Cowpea. In Pulses, Sugar and Tuber Crops; Springer: Berlin/Heidelberg, Germany, 2007; pp. 49–67. [Google Scholar]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 17 January 2022).
- Smith, M.R.; Veneklaas, E.; Polania, J.; Rao, I.M.; Beebe, S.E.; Merchant, A. Field Drought Conditions Impact Yield but Not Nutritional Quality of the Seed in Common Bean (Phaseolus vulgaris L.). PLoS ONE 2019, 14, e0217099. [Google Scholar] [CrossRef]
- Adjei-Nsiah, S.; Kuyper, T.W.; Leeuwis, C.; Abekoe, M.K.; Cobbinah, J.; Sakyi-Dawson, O.; Giller, K.E. Farmers’ Agronomic and Social Evaluation of Productivity, Yield and N 2-Fixation in Different Cowpea Varieties and Their Subsequent Residual N Effects on a Succeeding Maize Crop. Nutr. Cycl. Agroecosyst. 2008, 80, 199–209. [Google Scholar] [CrossRef]
- Chikowo, R.; Mapfumo, P.; Nyamugafata, P.; Giller, K.E. Woody Legume Fallow Productivity, Biological N2-Fixation and Residual Benefits to Two Successive Maize Crops in Zimbabwe. Plant Soil 2004, 262, 303–315. [Google Scholar] [CrossRef]
- Singh, B.B.; Sharma, B. Restructuring Cowpea for Higher Yield. Indian J. Genet. 1996, 56, 389–405. [Google Scholar]
- Boukar, O.; Fatokun, C.A.; Huynh, B.-L.; Roberts, P.A.; Close, T.J. Genomic Tools in Cowpea Breeding Programs: Status and Perspectives. Front. Plant Sci. 2016, 7, 757. [Google Scholar] [CrossRef]
- Horn, L.; Shimelis, H.; Laing, M. Participatory Appraisal of Production Constraints, Preferred Traits and Farming System of Cowpea in the Northern Namibia: Implications for Breeding. Legume Res. Int. J. 2015, 38, 691–700. [Google Scholar] [CrossRef]
- Boukar, O.; Massawe, F.; Muranaka, S.; Franco, J.; Maziya-Dixon, B.; Singh, B.; Fatokun, C. Evaluation of Cowpea Germplasm Lines for Protein and Mineral Concentrations in Grains. Plant Genet. Resour. 2011, 9, 515–522. [Google Scholar] [CrossRef]
- Abadassi, J. Cowpea (Vigna unguiculata (L.) Walp.) Agronomic Traits Needed in Tropical Zone. Int. J. Pure Appl. Biosci. 2015, 3, 158–165. [Google Scholar]
- Dakora, F.D.; Belane, A.K. Evaluation of Protein and Micronutrient Levels in Edible Cowpea (Vigna unguiculata L. Walp.) Leaves and Seeds. Front. Sustain. Food Syst. 2019, 3, 70. [Google Scholar] [CrossRef]
- Weng, Y.; Shi, A.; Ravelombola, W.S.; Yang, W.; Qin, J.; Motes, D.; Moseley, D.O.; Chen, P. A Rapid Method for Measuring Seed Protein Content in Cowpea (Vigna unguiculata (L.) Walp.). Am. J. Plant Sci. 2017, 8, 2387. [Google Scholar] [CrossRef]
- Zuluaga, D.L.; Lioi, L.; Delvento, C.; Pavan, S.; Sonnante, G. Genotyping-by-Sequencing in Vigna unguiculata Landraces and Its Utility for Assessing Taxonomic Relationships. Plants 2021, 10, 509. [Google Scholar] [CrossRef]
- Padulosi, S.; Ng, N.Q. Origin, Taxonomy, and Morphology of Vigna unguiculata (L.) Walp. Adv. Cowpea Res. 1997, 1–12. [Google Scholar]
- Padulosi, S. Genetic Diversity, Taxonomy and Ecogeographic Survey of the Wild Relatives of Cowpea (Vigna unguiculata (L.) Walpers). Ph.D. Thesis, University of Louvain La Neuve, Ottignies-Louvain-la-Neuve, Belgium, 1993. [Google Scholar]
- Pasquet, R.S. Classification Infraspécifique des Formes Spontanées de Vigna unguiculata (L.) Walp. (Fabaceae) à Partir de Données Morphologiques. Bull. Du Jard. Bot. Natl. Belg. Bull. Natl. Plantentuin Belg. 1993, 62, 127–173. [Google Scholar] [CrossRef]
- Pasquet, R.S. Wild Cowpea (Vigna unguiculata) Evolution. Adv. Legume Syst. 1996, 8, 95–100. [Google Scholar]
- Pasquet, R. A New Subspecies of Vigna unguiculata (Leguminosae: Papilionoideae). Kew Bull. 1997, 52, 840. [Google Scholar] [CrossRef]
- Pasquet, R.S. Genetic Relationships among Subspecies of Vigna unguiculata (L.) Walp. Based on Allozyme Variation. Theor. Appl. Genet. 1999, 98, 1104–1119. [Google Scholar] [CrossRef]
- Coulibaly, S.; Pasquet, R.S.; Papa, R.; Gepts, P. AFLP Analysis of the Phenetic Organization and Genetic Diversity of Vigna unguiculata L. Walp. Reveals Extensive Gene Flow between Wild and Domesticated Types. Theor. Appl. Genet. 2002, 104, 358–366. [Google Scholar] [CrossRef]
- Pasquet, R. Genus Vigna and Cowpea (V. unguiculata [L.] Walp.) Taxonomy: Current Status and Prospects. In Proceedings of the Fifth World Cowpea Conference on Improving Livelihoods in the Cowpea Value Chain through Advancement in Science, Dakar, Senegal, 27 September–1 October 2010. [Google Scholar]
- Pasquet, R.S.; Feleke, Y.; Gepts, P. Cowpea [Vigna unguiculata (L.) Walp.] Maternal Lineages, Chloroplast Captures, and Wild Cowpea Evolution. Genet. Resour. Crop. Evol. 2021, 68, 2799–2812. [Google Scholar] [CrossRef]
- Lush, W.M.; Evans, L.T.; Wien, H.C. Environmental Adaptation of Wild and Domesticated Cowpeas (Vigna unguiculata (L.) Walp.). Field Crop. Res. 1980, 3, 173–187. [Google Scholar] [CrossRef]
- Faris, D.G. The Origin and Evolution of the Cultivated Forms of Vigna sinensis. Can. J. Genet. Cytol. 1965, 7, 433–452. [Google Scholar] [CrossRef]
- Vaillancourt, R.E.; Weeden, N.F. Chloroplast DNA Polymorphism Suggests Nigerian Center of Domestication for the Cowpea, Vigna unguiculata (Leguminosae). Am. J. Bot. 1992, 79, 1194–1199. [Google Scholar] [CrossRef]
- Lush, W.M.; Evans, L.T. The Domestication and Improvement of Cowpeas (Vigna unguiculata (L.) W Alp.). Euphytica 1981, 30, 579–587. [Google Scholar] [CrossRef]
- Champion, L.; Fuller, D.Q.; Ozainne, S.; Huysecom, É.; Mayor, A. Agricultural Diversification in West Africa: An Archaeobotanical Study of the Site of Sadia (Dogon Country, Mali). Archaeol. Anthropol. Sci. 2021, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Vavilov, N.I. Centers of Origin of Cultivated Plants. In Origin and Geography of Cultivated Plants; Cambridge University Press: Cambridge, UK, 1926. [Google Scholar]
- Faris, D.G. Evidence for the West African Origin of Vigna sinensis (L.) Savi; University of California: Davis, CA, USA, 1963. [Google Scholar]
- Rawal, K.M. Natural Hybridization among Wild, Weedy and Cultivated Vigna unguiculata (L.) Walp. Euphytica 1975, 24, 699–707. [Google Scholar] [CrossRef]
- Ba, F.S.; Pasquet, R.S.; Gepts, P. Genetic Diversity in Cowpea [Vigna unguiculata (L.) Walp.] as Revealed by RAPD Markers. Genet. Resour. Crop. Evol. 2004, 51, 539–550. [Google Scholar] [CrossRef]
- Fang, J.; Chao, C.-C.T.; Roberts, P.A.; Ehlers, J.D. Genetic Diversity of Cowpea [Vigna unguiculata (L.) Walp.] in Four West African and USA Breeding Programs as Determined by AFLP Analysis. Genet. Resour. Crop. Evol. 2007, 54, 1197–1209. [Google Scholar] [CrossRef]
- Xiong, H.; Shi, A.; Mou, B.; Qin, J.; Motes, D.; Lu, W.; Ma, J.; Weng, Y.; Yang, W.; Wu, D. Genetic Diversity and Population Structure of Cowpea (Vigna unguiculata L. Walp.). PLoS ONE 2016, 11, e0160941. [Google Scholar] [CrossRef]
- Muñoz-Amatriaín, M.; Lo, S.; Herniter, I.; Boukar, O.; Fatokun, C.; Carvalho, E.; Castro, I.; Guo, Y.; Huynh, B.; Roberts, P.A. The UCR Minicore: A Valuable Resource for Cowpea Research and Breeding. Legume Sci. 2021, 3, e95. [Google Scholar] [CrossRef]
- Sarr, A.; Bodian, A.; Gbedevi, K.M.; Ndir, K.N.; Ajewole, O.O.; Gueye, B.; Foncéka, D.; Diop, E.A.; Diop, B.M.; Cissé, N. Genetic Diversity and Population Structure Analyses of Wild Relatives and Cultivated Cowpea (Vigna unguiculata (L.) Walp.) from Senegal Using Simple Sequence Repeat Markers. Plant Mol. Biol. Rep. 2021, 39, 112–124. [Google Scholar] [CrossRef]
- Kongjaimun, A.; Kaga, A.; Tomooka, N.; Somta, P.; Vaughan, D.A.; Srinives, P. The Genetics of Domestication of Yardlong Bean, Vigna unguiculata (L.) Walp. ssp. unguiculata Cv.-Gr. sesquipedalis. Ann. Bot. 2012, 109, 1185–1200. [Google Scholar] [CrossRef]
- Xu, P.; Wu, X.; Wang, B.; Luo, J.; Liu, Y.; Ehlers, J.D.; Close, T.J.; Roberts, P.A.; Lu, Z.; Wang, S. Genome Wide Linkage Disequilibrium in Chinese Asparagus Bean (Vigna unguiculata ssp. sesquipedialis) Germplasm: Implications for Domestication History and Genome Wide Association Studies. Heredity 2012, 109, 34–40. [Google Scholar] [CrossRef]
- Xu, P.; Wu, X.; Wang, B.; Liu, Y.; Qin, D.; Ehlers, J.D.; Close, T.J.; Hu, T.; Lu, Z.; Li, G. Development and Polymorphism of Vigna Unguiculata ssp. Unguiculata Microsatellite Markers Used for Phylogenetic Analysis in Asparagus Bean (Vigna unguiculata ssp. sesquipedialis (L.) Verdc.). Mol. Breed. 2010, 25, 675–684. [Google Scholar] [CrossRef]
- Pasquet, R.S. Morphological Study of Cultivated Cowpea Vigna unguiculata (L.) Walp. Importance of Ovule Number and Definition of Cv Gr Melanophthalmus. Agronomie 1998, 18, 61–70. [Google Scholar] [CrossRef]
- Pasquet, R.S. Allozyme Diversity of Cultivated Cowpea Vigna unguiculata (L.) Walp. Theor. Appl. Genet. 2000, 101, 211–219. [Google Scholar] [CrossRef]
- Kongjaimun, A.; Kaga, A.; Tomooka, N.; Somta, P.; Shimizu, T.; Shu, Y.; Isemura, T.; Vaughan, D.A.; Srinives, P. An SSR-Based Linkage Map of Yardlong Bean (Vigna unguiculata (L.) Walp. subsp. unguiculata Sesquipedalis Group) and QTL Analysis of Pod Length. Genome 2012, 55, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Mubaiwa, J.; Fogliano, V.; Chidewe, C.; Linnemann, A.R. Hard-to-Cook Phenomenon in Bambara Groundnut (Vigna subterranea (L.) Verdc.) Processing: Options to Improve Its Role in Providing Food Security. Food Rev. Int. 2017, 33, 167–194. [Google Scholar] [CrossRef]
- Aliyu, S.; Massawe, F.; Mayes, S. Genetic Diversity and Population Structure of Bambara Groundnut (Vigna subterranea (L.) Verdc.): Synopsis of the Past Two Decades of Analysis and Implications for Crop Improvement Programmes. Genet. Resour. Crop. Evol. 2016, 63, 925–943. [Google Scholar] [CrossRef]
- Olayide, O.E.; Donkoh, S.A.; Ansah, I.G.K.; Adzawla, W.; O’Reilly, P.J.; Mayes, S.; Feldman, A.; Halimi, R.A.; Nyarko, G.; Ilori, C.O. Assessing Socioeconomic Factors Influencing Production and Commercialization of Bambara Groundnut as an Indigenous Climate Resilient Crop in Nigeria; Springer Nature: Cham, Switzerland, 2018. [Google Scholar]
- Azam-Ali, S.; Azam-Ali, S.N.; Aguilar-Manjarrez, J.; Bannayan-Avval, M. A Global Mapping System for Bambara Groundnut Production; Food and Agriculture Organization: Rome, Italy, 2001; Volume 1. [Google Scholar]
- Pasquet, R.S.; Schwedes, S.; Gepts, P. Isozyme Diversity in Bambara Groundnut. Crop. Sci. 1999, 39, 1228–1236. [Google Scholar] [CrossRef]
- Doku, E.V.; Karikari, S.K. Operational Selection in Wild Bambara Groundnuts. Ghana J. Sci. 1971, 11, 45–56. [Google Scholar]
- Majola, N.G.; Gerrano, A.S.; Shimelis, H. Bambara Groundnut (Vigna subterranea [L.] Verdc.) Production, Utilisation and Genetic Improvement in Sub-Saharan Africa. Agronomy 2021, 11, 1345. [Google Scholar] [CrossRef]
- Mohammed, S.M. Pre-Breeding of Bambara Groundnut (Vigna subterranea [L.] Verdc.). Ph.D. Thesis, Abubakar Tafawa Balewa University, Bauchi, Nigeria, 2014. [Google Scholar]
- Dalziel, J.M. The Useful Plants of West Tropical Africa; Royal Botanic Gardens: London, UK, 1937. [Google Scholar]
- Begemann, F. Ecogeographic Differentiation of Bambarra Groundnut (Vigna subterranea) in the Collection of the International Institute of Tropical Agriculture (IITA); Wissenschaftlicher Fachverlag; Wissenschaftlicher Fachverlag: Berlin, Germany, 1988. [Google Scholar]
- Goli, A.E.; Begemann, F.; Ng, N.Q. Characterisation and Evaluation of IITA’S Bambara Groundnut (Vigna subterranea (L) Verdc). Promoting the Conservation and Use of Underutilized and Neglected Crops; International Plant Genetic Resources Institute: Rome, Italy, 1997; Volume 9, pp. 101–118. [Google Scholar]
- Somta, P.; Chankaew, S.; Rungnoi, O.; Srinives, P. Genetic Diversity of the Bambara Groundnut (Vigna subterranea (L.) Verdc.) as Assessed by SSR Markers. Genome 2011, 54, 898–910. [Google Scholar] [CrossRef]
- Olukolu, B.A.; Mayes, S.; Stadler, F.; Ng, N.Q.; Fawole, I.; Dominique, D.; Azam-Ali, S.N.; Abbott, A.G.; Kole, C. Genetic Diversity in Bambara Groundnut (Vigna subterranea (L.) Verdc.) as Revealed by Phenotypic Descriptors and DArT Marker Analysis. Genet. Resour. Crop. Evol. 2012, 59, 347–358. [Google Scholar] [CrossRef]
- Molosiwa, O.O.; Aliyu, S.; Stadler, F.; Mayes, K.; Massawe, F.; Kilian, A.; Mayes, S. SSR Marker Development, Genetic Diversity and Population Structure Analysis of Bambara Groundnut [Vigna subterranea (L.) Verdc.] Landraces. Genet. Resour. Crop. Evol. 2015, 62, 1225–1243. [Google Scholar] [CrossRef]
- Tanimu, B.; Aliyu, L. Bambara Groundnut, Vigna subterranea (L.) verdc. In Proceedings of the Workshop on Conservation and Improvement of Bambara Groundnut, Harare, Zimbabwe, 14–16 November 1995; Country Report: Northern Nigeria; Promoting the Conservation and Use of Underutilized and Neglected Crops. International Plant Genetic Resources Institute: Rome, Italy, 1997. [Google Scholar]
- Muhammad, I.; Rafii, M.Y.; Ramlee, S.I.; Nazli, M.H.; Harun, A.R.; Oladosu, Y.; Musa, I.; Arolu, F.; Chukwu, S.C.; Sani Haliru, B. Exploration of Bambara Groundnut (Vigna subterranea (L.) Verdc.), an Underutilized Crop, to Aid Global Food Security: Varietal Improvement, Genetic Diversity and Processing. Agronomy 2020, 10, 766. [Google Scholar] [CrossRef]
- Molosiwa, O.; Basu, S.M.; Stadler, F.; Azam-Ali, S.; Mayes, S. Assessment of Genetic Variability of Bambara Groundnut (Vigna subterranean (L.) Verdc.) Accessions Using Morphological Traits and Molecular Markers. In Proceedings of the II International Symposium on Underutilized Plant Species: Crops for the Future-Beyond Food Security 979, Kuala Lumpur, Malaysia, 27 June1 July 2011; pp. 779–790. [Google Scholar]
- Molosiwa, O.O. Genetic Diversity and Population Structure Analysis of Bambara Groundnuts (Vigna subterranea (L.) Verdc.) Landraces Using Morpho-Agronomic Characters and SSR Markers. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2012. [Google Scholar]
- Verdcourt, B. Studies in the Leguminosae-Papilionoïdeae for the’Flora of Tropical East Africa’: III. Kew Bull. 1970, 24, 379–447. [Google Scholar] [CrossRef]
- Vanderborght, T. Some Observations on Seedlings of Vigna vexillata (L.) A. Rich. (Fabaceae). Bull. Du Jard. Bot. Natl. Belg. Bull. Natl. Plantentuin Belg. 1989, 59, 179–187. [Google Scholar] [CrossRef]
- Damayanti, F.; Lawn, R.J.; Bielig, L.M. Genotypic Variation in Domesticated and Wild Accessions of the Tropical Tuberous Legume Vigna vexillata (L.) A. Rich. Crop. Pasture Sci. 2010, 61, 771–784. [Google Scholar] [CrossRef]
- Dachapak, S.; Somta, P.; Poonchaivilaisak, S.; Yimram, T.; Srinives, P. Genetic Diversity and Structure of the Zombi Pea (Vigna vexillata (L.) A. Rich.) Gene Pool Based on SSR Marker Analysis. Genetica 2017, 145, 189–200. [Google Scholar] [CrossRef]
- Cosmas, P.; Agathar, K.; Ronald, M.; John, C.T.; Simon, M. Preliminary Evaluation of Different Seed Dormancy Breaking Methods In Wild Tuber Cowpea (Vigna vexillata). Can. J. Agric. Crop. 2019, 4, 33–40. [Google Scholar] [CrossRef]
- Tripathi, K.; Gore, P.G.; Pandey, A.; Nayar, E.R.; Gayacharan, C.; Pamarthi, R.K.; Bhardwaj, R.; Kumar, A. Morphological and Nutritional Assessment of Vigna Vexillata (L.) A. Rich.: A Potential Tuberous Legume of India. Genet. Resour. Crop. Evol. 2021, 68, 397–408. [Google Scholar] [CrossRef]
- Ferguson, H. The Food Crops of the Sudan and Their Relationship to Environment; McCorquodale & Co., Ltd.: London, UK, 1954. [Google Scholar]
- Bhattacharyya, P.K.; Ghosh, A.K.; Sanyal, B.; Deb Ray, G. Grow Vigna vexillata for Protein-Rich Tuber-Cum-Pulse Crop in North-Eastern Hill Region. Seeds Farms 1984, 10, 33–36. [Google Scholar]
- Wong, K.C. Vigna vexillata (L.) A. Richard. PROSEA, Plant Resources of South-East Asia. Aux. Plants 1997, 11, 261–263. [Google Scholar]
- Asati, B.S.; Yadav, D.S. Diversity of Horticultural Crops in North Eastern Region. ENVIS Bull. Himal. Ecol. 2004, 12, 1. [Google Scholar]
- Dachapak, S.; Tomooka, N.; Somta, P.; Naito, K.; Kaga, A.; Srinives, P. QTL Analysis of Domestication Syndrome in Zombi Pea (Vigna vexillata), an Underutilized Legume Crop. PLoS ONE 2018, 13, e0200116. [Google Scholar] [CrossRef] [PubMed]
- Garba, M.; Pasquet, R.S. Isozyme Diversity in Vigna vexillata (L.) A. Rich. (Fabaceae) Complex. S. Afr. J. Bot. 1998, 64, 163–175. [Google Scholar] [CrossRef][Green Version]
- Lawn, R.J.; Watkinson, A.R. Habitats, Morphological Diversity, and Distribution of the Genus Vigna Savi in Australia. Aust. J. Agric. Res. 2002, 53, 1305–1316. [Google Scholar] [CrossRef]
- Karuniawan, A.; Iswandi, A.; Kale, P.R.; Heinzemann, J.; Grüneberg, W.J. Vigna vexillata (L.) A. Rich. Cultivated as a Root Crop in Bali and Timor. Genet. Resour. Crop. Evol. 2006, 53, 213–217. [Google Scholar] [CrossRef]
- Butsayawarapat, P.; Juntawong, P.; Khamsuk, O.; Somta, P. Comparative Transcriptome Analysis of Waterlogging-Sensitive and Tolerant Zombi Pea (Vigna vexillata) Reveals Energy Conservation and Root Plasticity Controlling Waterlogging Tolerance. Plants 2019, 8, 264. [Google Scholar] [CrossRef]
- Chiang, H.S.; Singh, S.R. Pod Hairs as a Factor in Vigna vexillata Resistance to the Pod-Sucking Bug, Clavigralla tomentosicollis. Entomol. Exp. Et Appl. 1988, 47, 195–199. [Google Scholar] [CrossRef]
- Jackai, L.E.N.; Oghiakhe, S. Pod Wall Trichomes and Resistance of Two Wild Cowpea, Vigna vexillata, Accessions to Maruca testualis (Geyer) (Lepidoptera: Pyralidae) and Clavigralla tomentosicollis Stål (Hemiptera: Coreidae). Bull. Entomol. Res. 1989, 79, 595–605. [Google Scholar] [CrossRef]
- Thottappilly, G.; Ng, N.Q.; Rossel, H.W. Screening Germplasm of Vigna vexillata for Resistance to Cowpea Mottle Virus. Int. J. Trop. Plant Dis. 1994, 12, 75–80. [Google Scholar]
- Ogundiwin, E.A.; Thottappilly, G.; AkenOva, M.E.; Ekpo, E.J.A.; Fatokun, C.A. Resistance to Cowpea Mottle Carmovirus in Vigna vexillata. Plant Breed. 2002, 121, 517–520. [Google Scholar] [CrossRef]
- Amkul, K.; Wang, L.; Somta, P.; Wang, S.; Cheng, X. Construction of a High Density Linkage Map and Genome Dissection of Bruchid Resistance in Zombi Pea (Vigna vexillata (L.) A. Rich.). Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sodedji, F.A.K.; Agbahoungba, S.; Nguetta, S.-P.A.; Agoyi, E.E.; Ayenan, M.A.T.; Sossou, S.H.; Mamadou, C.; Assogbadjo, A.E.; Kone, D. Resistance to Legume Pod Borer (Maruca vitrata Fabricius) in Cowpea: Genetic Advances, Challenges, and Future Prospects. J. Crop. Improv. 2020, 34, 238–267. [Google Scholar] [CrossRef]
- Ojwang, L.O.; Dykes, L.; Awika, J.M. Ultra Performance Liquid Chromatography–Tandem Quadrupole Mass Spectrometry Profiling of Anthocyanins and Flavonols in Cowpea (Vigna unguiculata) of Varying Genotypes. J. Agric. Food Chem. 2012, 60, 3735–3744. [Google Scholar] [CrossRef]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martinez-Augustin, O.; Medina, F.S.D. Effects of Flavonoids and Other Polyphenols on Inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef]
- Sombié, P.A.E.D.; Compaoré, M.; Coulibaly, A.Y.; Ouédraogo, J.T.; Tignégré, J.-B.D.L.S.; Kiendrébéogo, M. Antioxidant and Phytochemical Studies of 31 Cowpeas (Vigna unguiculata (L. Walp.)) Genotypes from Burkina Faso. Foods 2018, 7, 143. [Google Scholar] [CrossRef]
- Adedayo, B.C.; Anyasi, T.A.; Taylor, M.J.; Rautenbauch, F.; Roes-Hill, L.; Jideani, V.A. Phytochemical Composition and Antioxidant Properties of Methanolic Extracts of Whole and Dehulled Bambara Groundnut (Vigna subterranea) Seeds. Sci. Rep. 2021, 11, 14116. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Siddhuraju, P.; Manian, S. Antioxidant and Free Radical Scavenging Capacity of the Underutilized Legume, Vigna vexillata (L.) A. Rich. J. Food Compos. Anal. 2011, 24, 160–165. [Google Scholar] [CrossRef]
- Leu, Y.-L.; Hwang, T.-L.; Kuo, P.-C.; Liou, K.-P.; Huang, B.-S.; Chen, G.-F. Constituents from Vigna vexillata and Their Anti-Inflammatory Activity. Int. J. Mol. Sci. 2012, 13, 9754–9768. [Google Scholar] [CrossRef] [PubMed]
- Arise, A.K.; Alashi, A.M.; Nwachukwu, I.D.; Ijabadeniyi, O.A.; Aluko, R.E.; Amonsou, E.O. Antioxidant Activities of Bambara Groundnut (Vigna subterranea) Protein Hydrolysates and Their Membrane Ultrafiltration Fractions. Food Funct. 2016, 7, 2431–2437. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein–Polyphenol Conjugates: Antioxidant Property, Functionalities and Their Applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- James, A.M. Evolutionary Insights into Two Plant Protein Families: Bowman-Birk Inhibitors and Asparaginyl Endopeptidases. Ph.D. Thesis, University of Victoria, Victoria, BC, Canada, 2017. [Google Scholar]
- Clemente, A.; del Carmen Arques, M. Bowman-Birk Inhibitors from Legumes as Colorectal Chemopreventive Agents. World J. Gastroenterol. WJG 2014, 20, 10305. [Google Scholar] [CrossRef]
- Clemente, A.; Olias, R. Beneficial Effects of Legumes in Gut Health. Curr. Opin. Food Sci. 2017, 14, 32–36. [Google Scholar] [CrossRef]
- Panzeri, D.; Guzzetti, L.; Sacco, G.; Tedeschi, G.; Nonnis, S.; Airoldi, C.; Labra, M.; Fusi, P.; Forcella, M.; Regonesi, M.E. Effectiveness of Vigna unguiculata Seed Extracts in Preventing Colorectal Cancer. Food Funct. 2020, 11, 5853–5865. [Google Scholar] [CrossRef]
- Mehdad, A.; Brumana, G.; Souza, A.A.; Barbosa, J.; Ventura, M.M.; De Freitas, S. A Bowman–Birk Inhibitor Induces Apoptosis in Human Breast Adenocarcinoma through Mitochondrial Impairment and Oxidative Damage Following Proteasome 20S Inhibition. Cell Death Discov. 2016, 2, 1–10. [Google Scholar] [CrossRef]
- Teixeira-Guedes, C.I.; Oppolzer, D.; Barros, A.I.; Pereira-Wilson, C. Phenolic Rich Extracts from Cowpea Sprouts Decrease Cell Proliferation and Enhance 5-Fluorouracil Effect in Human Colorectal Cancer Cell Lines. J. Funct. Foods 2019, 60, 103452. [Google Scholar] [CrossRef]
- Guo, X.; Li, T.; Tang, K.; Liu, R.H. Effect of Germination on Phytochemical Profiles and Antioxidant Activity of Mung Bean Sprouts (Vigna radiata). J. Agric. Food Chem. 2012, 60, 11050–11055. [Google Scholar] [CrossRef]
- Rejhová, A.; Opattová, A.; Čumová, A.; Slíva, D.; Vodička, P. Natural Compounds and Combination Therapy in Colorectal Cancer Treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef]
- Xavier, C.P.; Lima, C.F.; Preto, A.; Seruca, R.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Luteolin, Quercetin and Ursolic Acid Are Potent Inhibitors of Proliferation and Inducers of Apoptosis in Both KRAS and BRAF Mutated Human Colorectal Cancer Cells. Cancer Lett. 2009, 281, 162–170. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Wang, M.; Qian, Y.; Dong, X.; Gu, H.; Wang, H.; Guo, S.; Hisamitsu, T. Quercetin-Induced Apoptosis of HT-29 Colon Cancer Cells via Inhibition of the Akt-CSN6-Myc Signaling Axis. Mol. Med. Rep. 2016, 14, 4559–4566. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef]
- Fact Sheets—Malnutrition. Available online: https://www.who.int/news-room/fact-sheets/detail/malnutrition (accessed on 17 January 2022).
- Tan, W.-C.; Tan, C.-H.; Nyam, K.-L.; Tan, C.-P.; Julkifle, A. Nutritive Bambara Groundnut Powdered Drink Mix: Characterization and in-Vivo Assessment of the Cholesterol-Lowering Effect. J. Food Sci. Technol. 2021, 58, 2992–3000. [Google Scholar] [CrossRef]
- Kanetro, B. Hypocholesterolemic Properties of Protein Isolate from Cowpeas (Vigna unguiculata) Sprout in Normal and Diabetic Rats. Procedia Food Sci. 2015, 3, 112–118. [Google Scholar] [CrossRef]
- Janeesh, P.A.; Abraham, A. Amelioration of Cholesterol Induced Atherosclerosis by Normalizing Gene Expression, Cholesterol Profile and Antioxidant Enzymes by Vigna unguiculata. Plant Foods Hum. Nutr. 2013, 68, 118–123. [Google Scholar] [CrossRef]
- Lattanzio, V.; Cardinali, A.; Linsalata, V.; Perrino, P.; Ng, N.Q. A Chemosystematic Study of the Flavonoids of Vigna. Genet. Resour. Crop. Evol. 1996, 43, 493–504. [Google Scholar] [CrossRef]
- Lattanzio, V.; Terzano, R.; Cicco, N.; Cardinali, A.; Venere, D.D.; Linsalata, V. Seed Coat Tannins and Bruchid Resistance in Stored Cowpea Seeds. J. Sci. Food Agric. 2005, 85, 839–846. [Google Scholar] [CrossRef]
- Gutiérrez-Uribe, J.A.; Romo-Lopez, I.; Serna-Saldívar, S.O. Phenolic Composition and Mammary Cancer Cell Inhibition of Extracts of Whole Cowpeas (Vigna unguiculata) and Its Anatomical Parts. J. Funct. Foods 2011, 3, 290–297. [Google Scholar] [CrossRef]
- Herniter, I.A.; Lo, R.; Muñoz-Amatriaín, M.; Lo, S.; Guo, Y.-N.; Huynh, B.-L.; Lucas, M.; Jia, Z.; Roberts, P.A.; Lonardi, S. Seed Coat Pattern QTL and Development in Cowpea (Vigna unguiculata [L.] Walp.). Front. Plant Sci. 2019, 10, 1346. [Google Scholar] [CrossRef]
- Gaiero, P.; Speranza, P.; de Jong, H. Introgressive Hybridization in Potato Revealed by Novel Cytogenetic and Genomic Technologies. Am. J. Potato Res. 2018, 95, 607–621. [Google Scholar] [CrossRef]
- Aliyu, B. Crossability of Vigna rhomboidea Burtt. Davy with Cowpea (V. unguiculata (L.) Walp.). Genet. Resour. Crop. Evol. 2005, 52, 447–453. [Google Scholar] [CrossRef]
- Fatokun, C.A.; Ng, Q. Outcrossing in Cowpea. J. Food Agric. Environ. 2007, 5, 334–338. [Google Scholar]
- Pasquet, R.S.; Peltier, A.; Hufford, M.B.; Oudin, E.; Saulnier, J.; Paul, L.; Knudsen, J.T.; Herren, H.R.; Gepts, P. Long-Distance Pollen Flow Assessment through Evaluation of Pollinator Foraging Range Suggests Transgene Escape Distances. Proc. Natl. Acad. Sci. USA 2008, 105, 13456–13461. [Google Scholar] [CrossRef]
- Sakupwanya, S.; Mithen, R.; Mutangandura-Mhlanga, T. Studies on the African Vigna Genepool. II. Hybridization Studies with Vigna unguiculata var. tenuis and var. stenophylla. Bulletin des Ressources Genetiques Vegetales (CIRP/FAO); Noticiario de Recursos Geneticos Vegetales (CIRF/FAO): Roma, Italy, 1989. [Google Scholar]
- Mithen, R.; Kibblewhite, H. Taxonomy and ecology of Vigna unguiculata (Leguminosae—Papilionoideae) in south-central africa. Kirkia 1993, 14, 100–113. [Google Scholar]
- Fatokun, C.A. Wide Hybridization in Cowpea: Problems and Prospects. Euphytica 1991, 54, 137–140. [Google Scholar] [CrossRef]
- Vijaykumar, A.; Saini, A.; Jawali, N. Phylogenetic Analysis of Subgenus Vigna Species Using Nuclear Ribosomal RNA ITS: Evidence of Hybridization among Vigna unguiculata Subspecies. J. Hered. 2010, 101, 177–188. [Google Scholar] [CrossRef]
- Vijaykumar, A.; Saini, A.; Jawali, N. Molecular Characterization of Intergenic Spacer Region of 5S Ribosomal RNA Genes in Subgenus Vigna: Extensive Hybridization among V. Unguiculata Subspecies. Plant Syst. Evol. 2011, 294, 39–55. [Google Scholar] [CrossRef]
- Fatokun, C.A.; Singh, B.B. Interspecific Hybridization between Vigna pubescens and V. unguiculata (L.) Walp. through Embryo Rescue. Plant Cell Tissue Organ Cult. 1987, 9, 229–233. [Google Scholar] [CrossRef]
- Fatokun, C.A.; Perrino, P.; Ng, N.Q. Wide Crossing in African Vigna Species. Adv. Cowpea Res. 1997, 50–57. [Google Scholar]
- Mohammed, M.S.; Russom, Z.; Abdul, S.D. Inheritance of Hairiness and Pod Shattering, Heritability and Correlation Studies in Crosses between Cultivated Cowpea (Vigna unguiculata (L.) Walp.) and Its Wild (var. pubescens) Relative. Euphytica 2010, 171, 397–407. [Google Scholar] [CrossRef]
- Lelou, B.; Diawata, M.; Van Damme, P. A Study of Intraspecific Hybrid Lines Derived from the Reciprocal Crosses between Wild Accessions and Cultivated Cowpeas (Vigna unguiculata (L.) Walp.). Afr. J. Plant Sci. 2011, 5, 337–348. [Google Scholar]
- Amusa, O.D.; Ogunkanmi, L.A.; Adetumbi, J.A.; Akinyosoye, S.T.; Bolarinwa, K.A.; Ogundipe, O.T. Intraspecific-Cross Compatibility in Cowpea (Vigna unguiculata (L.) Walp.). J. Crop. Improv. 2021, 19, 100–119. [Google Scholar] [CrossRef]
- Souleymane, A.; Aken’Ova, M.E.; Fatokun, C.A.; Alabi, O.Y. Screening for Resistance to Cowpea Aphid (Aphis craccivora Koch) in Wild and Cultivated Cowpea (Vigna unguiculata L. Walp.) Accessions. Int. J. Sci. Environ. Technol. 2013, 2, 611–621. [Google Scholar]
- Lo, S.; Muñoz-Amatriaín, M.; Boukar, O.; Herniter, I.; Cisse, N.; Guo, Y.-N.; Roberts, P.A.; Xu, S.; Fatokun, C.; Close, T.J. Identification of QTL Controlling Domestication-Related Traits in Cowpea (Vigna unguiculata L. Walp.). Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oigiangbe, O.N.; Jackai, L.E.N.; Ewete, F.K.; Hughes, J.d.A.; Lajide, L. Reduced Consumption and Use of Pods of Vigna Species (Leguminosae) by Maruca vitrata (Lepidoptera: Pyralidae). Afr. Entomol. 2002, 10, 333–340. [Google Scholar]
- Koona, P.; Osisanya, E.O.; Jackai, L.E.N.; Tamo, M.; Markham, R.H. Resistance in Accessions of Cowpea to the Coreid Pod-Bug Clavigralla tomentosicollis (Hemiptera: Coreidae). J. Econ. Entomol. 2002, 95, 1281–1288. [Google Scholar] [CrossRef]
- Massawe, F.J.; Schenkel, W.; Basu, S.; Temba, E.M. Artificial Hybridisation in Bambara Groundnut (Vigna subterranea (L.) Verdc.). In Proceedings of the International Symposium on Bambara Groundnut, Botswana College of Agriculture, Gaborone, Botswana, 8–12 September 2003; pp. 8–12. [Google Scholar]
- Suwanprasert, J.; Toojinda, T.; Srinives, P.; Chanprame, S. Hybridization Technique for Bambara Groundnut. Breed. Sci. 2006, 56, 125–129. [Google Scholar] [CrossRef]
- Oyiga, B.C.; Uguru, M.I.; Aruah, C.B. Studies on the Floral Traits and Their Implications on Pod and Seed Yields in Bambara Groundnut [Vigna subterrenea (L.) Verdc]. Aust. J. Crop. Sci. 2010, 4, 91–97. [Google Scholar]
- Onwubiko, N.I.C.; Odum, O.B.; Utazi, C.O.; Poly-Mbah, P.C. Studies on the Adaptation of Bambara Groundnut [Vigna subterranea (L.) Verdc] in Owerri Southeastern Nigeria. N. Y. Sci. J. 2011, 4, 60–67. [Google Scholar] [CrossRef]
- Basu, S.; Mayes, S.; Davey, M.; Roberts, J.A.; Azam-Ali, S.N.; Mithen, R.; Pasquet, R.S. Inheritance of ‘Domestication’Traits in Bambara Groundnut (Vigna subterranea (L.) Verdc.). Euphytica 2007, 157, 59–68. [Google Scholar] [CrossRef]
- James, A.T.; Lawn, R.J. Inheritance of Selected Traits in Accessions of Vigna vexillata (L) a Rich of Australian and African Origin. Aust. J. Bot. 1991, 39, 415–429. [Google Scholar] [CrossRef]
- Damayanti, F.; Lawn, R.J.; Bielig, L.M. Genetic Compatibility among Domesticated and Wild Accessions of the Tropical Tuberous Legume Vigna vexillata (L.) A. Rich. Crop. Pasture Sci. 2010, 61, 785–797. [Google Scholar] [CrossRef]
- Damayanti, F.; Lawn, R.J.; Bielig, L.M. Expression of Qualitative and Quantitative Traits in Hybrids between Domesticated and Wild Accessions of the Tropical Tuberous Legume Vigna vexillata (L.) A. Rich. Crop. Pasture Sci. 2010, 61, 798–811. [Google Scholar] [CrossRef]
- Barone, A.; Del Giudice, A.; Ng, N.Q. Barriers to Interspecific Hybridization between Vigna unguiculata and Vigna vexillata. Sex. Plant Reprod. 1992, 5, 195–200. [Google Scholar] [CrossRef]
- Gomathinayagam, P.; Rathnaswamy, R.; Ramaswamy, N.M. Interspecific Hybridization between Vigna unguiculata (L.) Walp. and V. vexillata (L.) A. Rich. through in Vitro Embryo Culture. Euphytica 1998, 102, 203–209. [Google Scholar] [CrossRef]
- Boukar, O.; Abberton, M.; Oyatomi, O.; Togola, A.; Tripathi, L.; Fatokun, C. Introgression Breeding in Cowpea [Vigna Unguiculata (L.) Walp.]. Front. Plant Sci. 2020, 11, 567425. [Google Scholar] [CrossRef]
- Lexer, C.; Heinze, B.; Alia, R.; Rieseberg, L.H. Hybrid Zones as a Tool for Identifying Adaptive Genetic Variation in Outbreeding Forest Trees: Lessons from Wild Annual Sunflowers (Helianthus spp.). For. Ecol. Manag. 2004, 197, 49–64. [Google Scholar] [CrossRef]
- Huang, H.; Liu, Y. Natural Hybridization, Introgression Breeding, and Cultivar Improvement in the Genus Actinidia. Tree Genet. Genomes 2014, 10, 1113–1122. [Google Scholar] [CrossRef]
- Janzen, G.M.; Wang, L.; Hufford, M.B. The Extent of Adaptive Wild Introgression in Crops. New Phytol. 2019, 221, 1279–1288. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Ma, J. Genomic Introgression through Interspecific Hybridization Counteracts Genetic Bottleneck during Soybean Domestication. Genome Biol. 2019, 20, 1–15. [Google Scholar] [CrossRef]
- Zecca, G.; Labra, M.; Grassi, F. Untangling the Evolution of American Wild Grapes: Admixed Species and How to Find Them. Front. Plant Sci. 2020, 10, 1814. [Google Scholar] [CrossRef]
- Chacón-Sánchez, M.I.; Martínez-Castillo, J.; Duitama, J.; Debouck, D.G. Gene Flow in Phaseolus Beans and Its Role as a Plausible Driver of Ecological Fitness and Expansion of Cultigens. Front. Ecol. Evol. 2021, 9, 312. [Google Scholar] [CrossRef]
- Kovach, M.J.; Calingacion, M.N.; Fitzgerald, M.A.; McCouch, S.R. The Origin and Evolution of Fragrance in Rice (Oryza sativa L.). Proc. Natl. Acad. Sci. USA 2009, 106, 14444–14449. [Google Scholar] [CrossRef]
- Hardigan, M.A.; Laimbeer, F.P.E.; Newton, L.; Crisovan, E.; Hamilton, J.P.; Vaillancourt, B.; Wiegert-Rininger, K.; Wood, J.C.; Douches, D.S.; Farré, E.M. Genome Diversity of Tuber-Bearing Solanum Uncovers Complex Evolutionary History and Targets of Domestication in the Cultivated Potato. Proc. Natl. Acad. Sci. USA 2017, 114, E9999–E10008. [Google Scholar] [CrossRef]
- Burgarella, C.; Barnaud, A.; Kane, N.A.; Jankowski, F.; Scarcelli, N.; Billot, C.; Vigouroux, Y.; Berthouly-Salazar, C. Adaptive Introgression: An Untapped Evolutionary Mechanism for Crop Adaptation. Front. Plant Sci. 2019, 10, 4. [Google Scholar] [CrossRef]
- Pickrell, J.; Pritchard, J. Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data. Nat. Preced. 2012, 8, e1002967. [Google Scholar] [CrossRef]
- Martin, S.H.; Davey, J.W.; Jiggins, C.D. Evaluating the Use of ABBA–BABA Statistics to Locate Introgressed Loci. Mol. Biol. Evol. 2015, 32, 244–257. [Google Scholar] [CrossRef]
- Martin, S.H.; Van Belleghem, S.M. Exploring Evolutionary Relationships across the Genome Using Topology Weighting. Genetics 2017, 206, 429–438. [Google Scholar] [CrossRef]
- Flouri, T.; Jiao, X.; Rannala, B.; Yang, Z. A Bayesian Implementation of the Multispecies Coalescent Model with Introgression for Phylogenomic Analysis. Mol. Biol. Evol. 2020, 37, 1211–1223. [Google Scholar] [CrossRef]
- Setter, D.; Mousset, S.; Cheng, X.; Nielsen, R.; DeGiorgio, M.; Hermisson, J. VolcanoFinder: Genomic Scans for Adaptive Introgression. PLoS Genet. 2020, 16, e1008867. [Google Scholar] [CrossRef]
- Hellenthal, G.; Busby, G.B.; Band, G.; Wilson, J.F.; Capelli, C.; Falush, D.; Myers, S. A Genetic Atlas of Human Admixture History. Science 2014, 343, 747–751. [Google Scholar] [CrossRef]
- Ellstrand, N.C.; Meirmans, P.; Rong, J.; Bartsch, D.; Ghosh, A.; De Jong, T.J.; Haccou, P.; Lu, B.-R.; Snow, A.A.; Neal Stewart Jr, C. Introgression of Crop Alleles into Wild or Weedy Populations. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 325–345. [Google Scholar] [CrossRef]
- Heredia, S.M.; Ellstrand, N.C. Novel Seed Protection in the Recently Evolved Invasive, California Wild Radish, a Hybrid Raphanus sp. (Brassicaceae). Am. J. Bot. 2014, 101, 2043–2051. [Google Scholar] [CrossRef]
- Page, A.M.; Daunay, M.-C.; Aubriot, X.; Chapman, M.A. Domestication of Eggplants: A Phenotypic and Genomic Insight. In The Eggplant Genome; Springer: Berlin/Heidelberg, Germany, 2019; pp. 193–212. [Google Scholar]
- Grimm, A.; Sahi, V.P.; Amann, M.; Vidotto, F.; Fogliatto, S.; Devos, K.M.; Ferrero, A.; Nick, P. Italian Weedy Rice—A Case of de-Domestication? Ecol. Evol. 2020, 10, 8449–8464. [Google Scholar] [CrossRef] [PubMed]
- Grassi, F.; De Lorenzis, G. Back to the Origins: Background and Perspectives of Grapevine Domestication. Int. J. Mol. Sci. 2021, 22, 4518. [Google Scholar] [CrossRef]
- McAlvay, A.C.; Ragsdale, A.P.; Mabry, M.E.; Qi, X.; Bird, K.; Velasco, P.; An, H.; Pires, C.; Emshwiller, E. Brassica Rapa Domestication: Untangling Wild and Feral Forms and Convergence of Crop Morphotypes. bioRxiv 2021. [Google Scholar] [CrossRef]
- Scossa, F.; Fernie, A.R. When a Crop Goes Back to the Wild: Feralization. Trends Plant Sci. 2021, 26, 543–545. [Google Scholar] [CrossRef]
- Cao, Q.; Lu, B.-R.; Xia, H.U.I.; Rong, J.; Sala, F.; Spada, A.; Grassi, F. Genetic Diversity and Origin of Weedy Rice (Oryza sativa f. spontanea) Populations Found in North-Eastern China Revealed by Simple Sequence Repeat (SSR) Markers. Ann. Bot. 2006, 98, 1241–1252. [Google Scholar] [CrossRef]
- Gering, E.; Incorvaia, D.; Henriksen, R.; Conner, J.; Getty, T.; Wright, D. Getting Back to Nature: Feralization in Animals and Plants. Trends Ecol. Evol. 2019, 34, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Feleke, Y.; Pasquet, R.S.; Gepts, P. Development of PCR-Based Chloroplast DNA Markers That Characterize Domesticated Cowpea (Vigna unguiculata ssp. unguiculata var. unguiculata) and Highlight Its Crop-Weed Complex. Plant Syst. Evol. 2006, 262, 75–87. [Google Scholar] [CrossRef]
- Kouam, E.B.; Pasquet, R.S.; Campagne, P.; Tignegre, J.-B.; Thoen, K.; Gaudin, R.; Ouedraogo, J.T.; Salifu, A.B.; Muluvi, G.M.; Gepts, P. Genetic Structure and Mating System of Wild Cowpea Populations in West Africa. BMC Plant Biol. 2012, 12, 113. [Google Scholar] [CrossRef]
- Kouadio, D.; Echikh, N.; Toussaint, A.; Pasquet, R.S.; Baudoin, J.-P. Organization of the Gene Pool of Vigna unguiculata (L.) Walp.: Crosses between the Wild and Cultivated Forms of Cowpea. Biotechnol. Agron. Soc. Environ. 2007, 11, 47–57. [Google Scholar]
- Darwin’s, C. On the Origin of Species; John Murray: London, UK, 1859. [Google Scholar]
- Ho, W.K.; Chai, H.H.; Kendabie, P.; Ahmad, N.S.; Jani, J.; Massawe, F.; Kilian, A.; Mayes, S. Integrating Genetic Maps in Bambara Groundnut [Vigna subterranea (L) Verdc.] and Their Syntenic Relationships among Closely Related Legumes. BMC Genom. 2017, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Paudel, D.; Dareus, R.; Rosenwald, J.; Munoz-Amatriain, M.; Rios, E. Genome-Wide Association Study Reveals Candidate Genes for Flowering Time in Cowpea (Vigna unguiculata [L.] Walp.). bioRxiv 2021, 12, 667038. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.; Fatokun, C.; Boukar, O.; Gepts, P.; Close, T.J.; Muñoz-Amatriaín, M. Identification of QTL for Perenniality and Floral Scent in Cowpea (Vigna unguiculata [L.] Walp.). PLoS ONE 2020, 15, e0229167. [Google Scholar] [CrossRef] [PubMed]
- Lonardi, S.; Muñoz-Amatriaín, M.; Liang, Q.; Shu, S.; Wanamaker, S.I.; Lo, S.; Tanskanen, J.; Schulman, A.H.; Zhu, T.; Luo, M.-C. The Genome of Cowpea (Vigna unguiculata [L.] Walp.). Plant J. 2019, 98, 767–782. [Google Scholar] [CrossRef]
- González, A.M.; Yuste-Lisbona, F.J.; Saburido, S.; Bretones, S.; De Ron, A.M.; Lozano, R.; Santalla, M. Major Contribution of Flowering Time and Vegetative Growth to Plant Production in Common Bean as Deduced from a Comparative Genetic Mapping. Front. Plant Sci. 2016, 7, 1940. [Google Scholar] [CrossRef]
- Gong, Z. Flowering Phenology as a Core Domestication Trait in Soybean. J. Integr. Plant Biol. 2020, 62, 546–549. [Google Scholar] [CrossRef]
- Levy, Y.Y.; Dean, C. Control of Flowering Time. Curr. Opin. Plant Biol. 1998, 1, 49–54. [Google Scholar] [CrossRef]
- Fernie, A.R.; Yan, J. De Novo Domestication: An Alternative Route toward New Crops for the Future. Mol. Plant 2019, 12, 615–631. [Google Scholar] [CrossRef]
- Smỳkal, P.; Nelson, M.N.; Berger, J.D.; Von Wettberg, E.J. The Impact of Genetic Changes during Crop Domestication. Agronomy 2018, 8, 119. [Google Scholar] [CrossRef]
- Khan, A.W.; Garg, V.; Roorkiwal, M.; Golicz, A.A.; Edwards, D.; Varshney, R.K. Super-Pangenome by Integrating the Wild Side of a Species for Accelerated Crop Improvement. Trends Plant Sci. 2020, 25, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Della Coletta, R.; Qiu, Y.; Ou, S.; Hufford, M.B.; Hirsch, C.N. How the Pan-Genome Is Changing Crop Genomics and Improvement. Genome Biol. 2021, 22, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Leal, D.; Lemmon, Z.H.; Man, J.; Bartlett, M.E.; Lippman, Z.B. Engineering Quantitative Trait Variation for Crop Improvement by Genome Editing. Cell 2017, 171, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.; Wang, Z.; Hu, Y.; Wu, Y.; Luo, Z.; Yang, Z.; Zu, Y.; Li, W.; Huang, P.; Tong, X. Chromosomal Deletions and Inversions Mediated by TALENs and CRISPR/Cas in Zebrafish. Nucleic Acids Res. 2013, 41, e141. [Google Scholar] [CrossRef]
- DeHaan, L.; Larson, S.; López-Marqués, R.L.; Wenkel, S.; Gao, C.; Palmgren, M. Roadmap for Accelerated Domestication of an Emerging Perennial Grain Crop. Trends Plant Sci. 2020, 25, 525–537. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Zhou, Z.; Wu, P.; Fang, M.; Pan, X.; Lin, Q.; Luo, W.; Wu, G.; Li, H. Reassessment of the Four Yield-Related Genes Gn1a, DEP1, GS3, and IPA1 in Rice Using a CRISPR/Cas9 System. Front. Plant Sci. 2016, 7, 377. [Google Scholar] [CrossRef]
- Zsögön, A.; Čermák, T.; Naves, E.R.; Notini, M.M.; Edel, K.H.; Weinl, S.; Freschi, L.; Voytas, D.F.; Kudla, J.; Peres, L.E.P. De Novo Domestication of Wild Tomato Using Genome Editing. Nat. Biotechnol. 2018, 36, 1211–1216. [Google Scholar] [CrossRef]
- Li, T.; Yang, X.; Yu, Y.; Si, X.; Zhai, X.; Zhang, H.; Dong, W.; Gao, C.; Xu, C. Domestication of Wild Tomato Is Accelerated by Genome Editing. Nat. Biotechnol. 2018, 36, 1160–1163. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, C.; Sun, Z.; Wang, L.; Duanmu, D.; Fan, Q. Genome Editing in Cowpea Vigna unguiculata Using CRISPR-Cas9. Int. J. Mol. Sci. 2019, 20, 2471. [Google Scholar] [CrossRef]
- Østerberg, J.T.; Xiang, W.; Olsen, L.I.; Edenbrandt, A.K.; Vedel, S.E.; Christiansen, A.; Landes, X.; Andersen, M.M.; Pagh, P.; Sandøe, P. Accelerating the Domestication of New Crops: Feasibility and Approaches. Trends Plant Sci. 2017, 22, 373–384. [Google Scholar] [CrossRef]
- Catarino, S.; Rangel, J.; Darbyshire, I.; Costa, E.; Duarte, M.C.; Romeiras, M.M. Conservation Priorities for African Vigna Species: Unveiling Angola’s Diversity Hotspots. Glob. Ecol. Conserv. 2021, 25, e01415. [Google Scholar] [CrossRef]
- Tettelin, H.; Masignani, V.; Cieslewicz, M.J.; Donati, C.; Medini, D.; Ward, N.L.; Angiuoli, S.V.; Crabtree, J.; Jones, A.L.; Durkin, A.S. Genome Analysis of Multiple Pathogenic Isolates of Streptococcus agalactiae: Implications for the Microbial “Pan-Genome”. Proc. Natl. Acad. Sci. USA 2005, 102, 13950–13955. [Google Scholar] [CrossRef] [PubMed]
- Bayer, P.E.; Golicz, A.A.; Scheben, A.; Batley, J.; Edwards, D. Plant Pan-Genomes Are the New Reference. Nat. Plants 2020, 6, 914–920. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panzeri, D.; Guidi Nissim, W.; Labra, M.; Grassi, F. Revisiting the Domestication Process of African Vigna Species (Fabaceae): Background, Perspectives and Challenges. Plants 2022, 11, 532. https://doi.org/10.3390/plants11040532
Panzeri D, Guidi Nissim W, Labra M, Grassi F. Revisiting the Domestication Process of African Vigna Species (Fabaceae): Background, Perspectives and Challenges. Plants. 2022; 11(4):532. https://doi.org/10.3390/plants11040532
Chicago/Turabian StylePanzeri, Davide, Werther Guidi Nissim, Massimo Labra, and Fabrizio Grassi. 2022. "Revisiting the Domestication Process of African Vigna Species (Fabaceae): Background, Perspectives and Challenges" Plants 11, no. 4: 532. https://doi.org/10.3390/plants11040532
APA StylePanzeri, D., Guidi Nissim, W., Labra, M., & Grassi, F. (2022). Revisiting the Domestication Process of African Vigna Species (Fabaceae): Background, Perspectives and Challenges. Plants, 11(4), 532. https://doi.org/10.3390/plants11040532







