Ecological Networks in Urban Forest Fragments Reveal Species Associations between Native and Invasive Plant Communities
Abstract
1. Introduction
2. Results
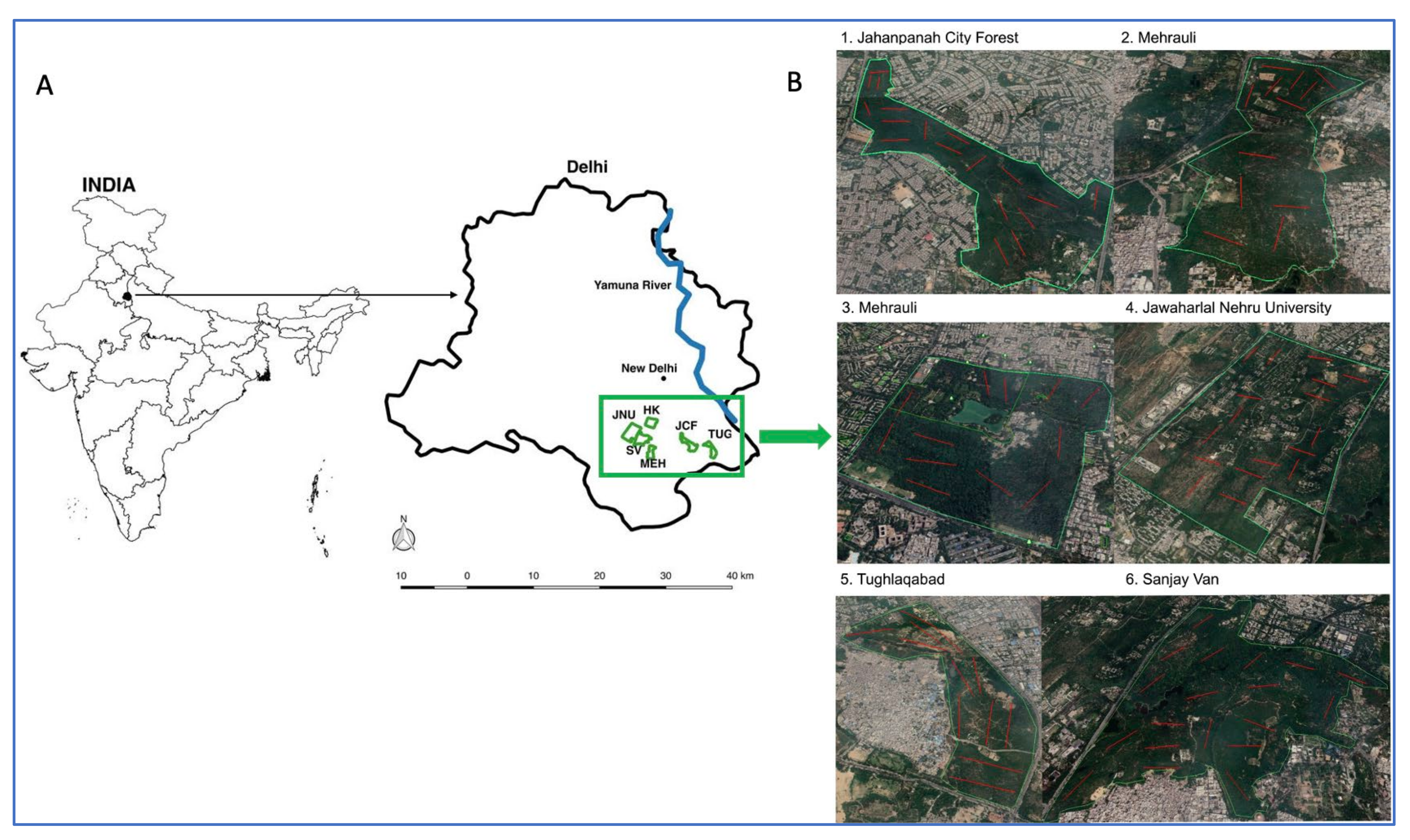
2.1. Study Area Networks
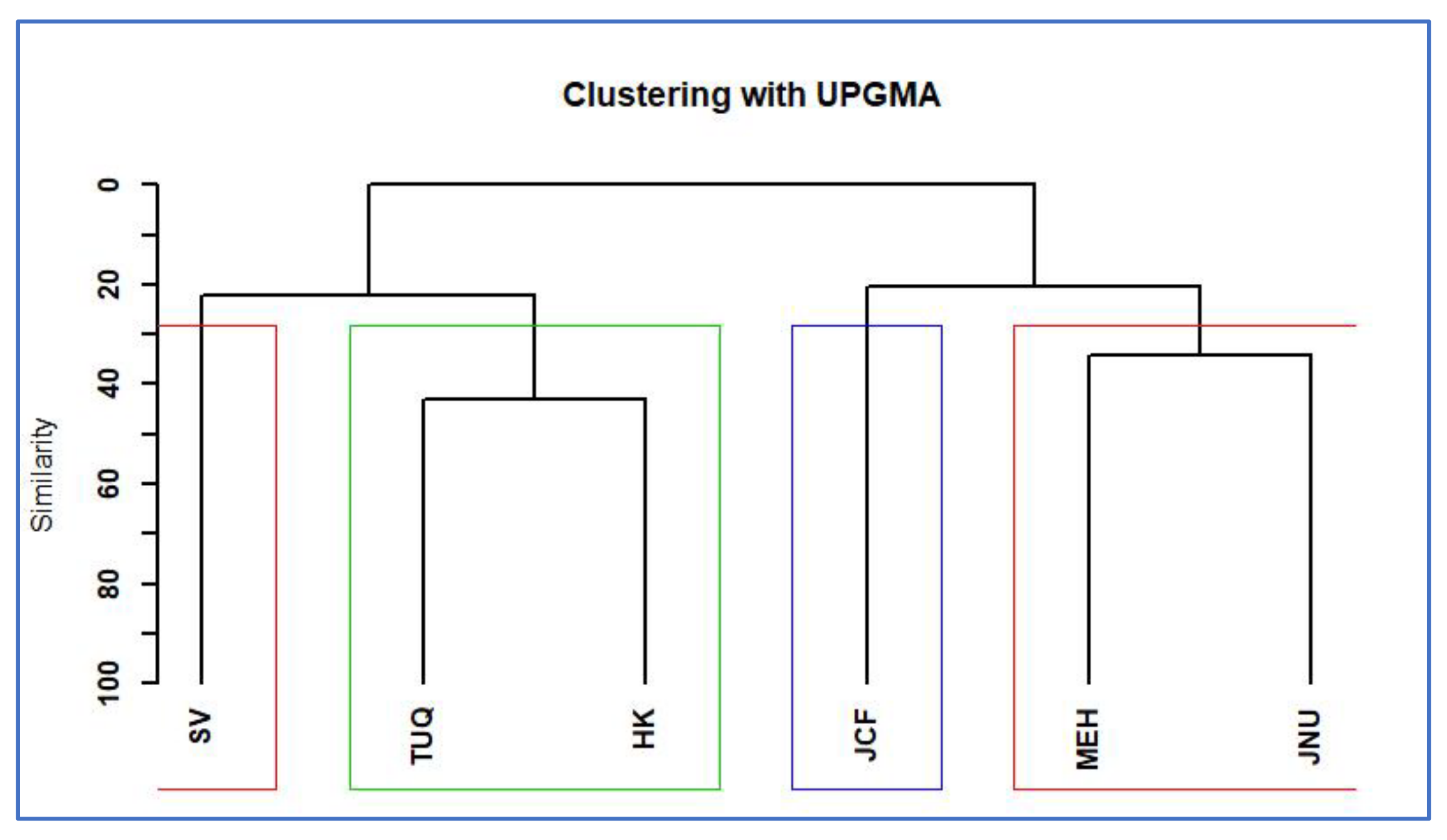
2.2. Distinct Native–Invasive Communities
2.3. Patterns in Species Association
3. Discussion
4. Material and Methods
4.1. Site Selection
4.2. Vegetation Sampling
4.3. Species Identification
4.4. Multivariate Data Analyses
4.5. Network Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Champion, S.H.G.; Seth, S.K. A Revised Survey of the Forest Types of India; The Manager of Publications: Delhi, India, 1968. [Google Scholar]
- Hobbs, R.J.; Higgs, E.; Harris, J.A. Novel ecosystems: Implications for conservation and restoration. Trends Ecol. Evol. 2009, 24, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.; Sehrawat, S. A City With a View: The Afforestation of the Delhi Ridge, 1883–1913. Mod. Asian Stud. 2009, 43, 543–570. [Google Scholar] [CrossRef][Green Version]
- Bowe, P. the Genius of an Artist’: William R. Mustoe and the Planting of the City of New Delhi and Its Gardens. Gard. Hist. Soc. 2009, 37, 68–79. [Google Scholar]
- Sud, M. Political Ecology of the Ridge: The Establishment and Contestation of Urban Forest Conservation in Delhi; Franz Steiner Verlag: Stuttgart, Germany, 2015. [Google Scholar]
- Pasha, S.V.; Satish, K.V.; Reddy, C.S.; Rao, P.P.; Jha, C.S. Satellite image based quantification of invasion and patch dynamics of mesquite (Prosopis juliflora) in Great Rann of Kachchh, Kachchh Biosphere Reserve, Gujarat, India. J. Earth Syst. Sci. 2014, 123, 1481–1490. [Google Scholar] [CrossRef]
- Robbins, P. Tracking Invasive Land Covers in India, or Why Our Landscapes Have Never Been Modern. Annals of the Association of American Geographers. 2001, 91, 637–659. [Google Scholar] [CrossRef]
- Kaur, R.; Gonzáles, W.L.; Llambi, L.D.; Soriano, P.J.; Callaway, R.M.; Rout, M.E.; Gallaher, T.J. Inderjit. Community Impacts of Prosopis juliflora Invasion: Biogeographic and Congeneric Comparisons. PLoS ONE 2012, 7, e44966. [Google Scholar] [CrossRef]
- Dakshini, K.M.M. Vegetation of Delhi State: Mehrauli Region. Proc. Natl. Inst. Sci. India Biol. Sci. 1966, 34, 127–133. [Google Scholar]
- Robbins, P. Comparing Invasive Networks: Cultural and Political Biographies of Invasive Species. Geogr. Rev. 2004, 94, 139–156. [Google Scholar] [CrossRef]
- Sharma, R.; Dakshini, K.M.M. A Comparative Assessment of the Ecological Effects of Prosopis cineraria and P. juliflora on the Soil of Revegetated Spaces. Vegetio 1991, 96, 87–96. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poor, M. 100 of the World’s Worst Invasive Alien Species A selection from the Global Invasive Species Database.The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN). 2000. Available online: http://www.issg.org/pdf/publications/worst_100/english_100_worst.pdf (accessed on 20 December 2021).
- Babu, S.; Love, A.; Babu, C.R. Ecological Restoration of Lantana-Invaded Landscapes in Corbett Tiger Reserve, India. Ecol. Restorration 2009, 27, 467–477. [Google Scholar] [CrossRef]
- Love, A.; Babu, S.; Babu, C.R. Management of Lantana, an invasive alien weed, in forest ecosystems of India. Curr. Sci. 2009, 97, 1421–1429. [Google Scholar]
- Sundaram, B.; Hiremath, A.J.; Krishnaswamy, J. Factors influencing the local scale colonisation and change in density of a widespread invasive plant species, Lantana camara, in South India. NeoBiota 2015, 25, 27–46. [Google Scholar] [CrossRef][Green Version]
- “Lantana camara L.” GBIF Secretariat. GBIF Backbone Taxonomy. 2021. Available online: https://www.gbif.org/species/2925303 (accessed on 31 January 2022).
- “Prosopis juliflora (Sw.) DC.” GBIF Secretariat. GBIF Backbone Taxonomy. 2021. Available online: https://www.gbif.org/species/5358460 (accessed on 31 January 2022).
- Sukopp, H. Urban Ecology-Scientific and Practical Aspects. In Urban Ecology; Breuste, J.U.O., Feldmann, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Rowntree, R.A. Ecology Of The Urban Forest-Introduction To Part I. Urban Ecol. 1984, 8, 1–11. [Google Scholar] [CrossRef]
- Labatore, A.C.; Spiering, D.J.; Potts, D.L.; Warren, R.J. Canopy trees in an urban landscape—viable forests or long-lived gardens? Urban Ecosyst. 2017, 20, 393–401. [Google Scholar] [CrossRef]
- Barton, J.; Pretty, J. Urban Ecology. In Urban Ecology; Gaston, K., Ed.; Cambridge University Press: Cambridge, UK, 2013; pp. 202–229. [Google Scholar]
- Bolund, P.; Hunhammar, S. Ecosystem services in urban areas. Ecol. Econ. 1999, 29, 293–301. [Google Scholar] [CrossRef]
- Cox, D.T.C.; Shanahan, D.F.; Hudson, H.L.; Fuller, R.A.; Gaston, K.J. The impact of urbanisation on nature dose and the implications for human health. Landsc. Urban Plan. 2018, 179, 72–80. [Google Scholar] [CrossRef]
- Albert, R.; Barabási, A.L. Statistical mechanics of complex networks. Rev. Mod. Phys. 2002, 74, 47–97. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P. Plant-animal mutualistic networks: The architecture of biodiversity. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 567–593. [Google Scholar] [CrossRef]
- Qu, X.; Peng, W.; Liu, Y.; Zhang, M.; Ren, Z.; Wu, N.; Liu, X. Networks and ordination analyses reveal the stream community structures of fish, macroinvertebrate and benthic algae, and their responses to nutrient enrichment. Ecol. Indic. 2019, 101, 501–511. [Google Scholar] [CrossRef]
- Ramaswami, G.; Somnath, P.; Quader, S. Plant-disperser mutualisms in a semi-arid habitat invaded by Lantana camara L. Plant Ecol. 2017, 218, 935–946. [Google Scholar] [CrossRef]
- Lusseau, D. Evidence for social role in a dolphin social network. Evol. Ecol. 2007, 21, 357–366. [Google Scholar] [CrossRef]
- Ellenberg, H.; Mueller-Dombois, D. Aims and Methods of Vegetation Ecology; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Tansley, A.G. The Use and Abuse of Vegetational Concepts and Terms. Ecology 1935, 16, 284–307. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F. Ordination. In Data Analysis in Community and Landscape Ecology; Cambridge University Press: Cambridge, UK, 1995; pp. 91–173. [Google Scholar]
- McCune, B.; Grace, J.B.; Urban, D.L. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002; Volume 28. [Google Scholar]
- Podani, J. Multivariate exploratory analysis of ordinal data in ecology: Pitfalls, problems and solutions. J. Veg. Sci. 2005, 16, 497–510. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; Handel, S.N.; la Puma, I.P.; Clemants, S.E. Urbanization promotes non-native woody species and diverse plant assemblages in the New York metropolitan region. Urban Ecosyst. 2015, 18, 31–45. [Google Scholar] [CrossRef]
- Rebelle, F. Urban Ecology and Special Features of Urban Ecosystems. Glob. Ecol. Biogeogr. Lett. 1994, 4, 173–187. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Dar, P.A.; Bhat, M.S.; Shah, M.A.; Andrabi, S.M. Urbanization and Its Impact on Biodiversity in the Kashmir Himalaya. In Biodiversity of the Himalaya: Jammu and Kashmir State; Topics in Biodiversity and Conservation; Dar, G.K.A., Ed.; Springer: Singapore, 2020; pp. 1011–1028. [Google Scholar]
- Von Der Lippe, M.; Kowarik, I. Long-distance dispersal of plants by vehicles as a driver of plant invasions. Conserv. Biol. 2007, 21, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.; Stevens, R.A.; Zhang, L. Urban plant ecology patterns and processes: A case study of the flora of the City of Plymouth, Devon, UK. J. Biogeogr. 1999, 26, 1281–1298. [Google Scholar] [CrossRef]
- Niemelä, J. Ecology and urban planning. Biodivers. Conserv. 1999, 8, 119–131. [Google Scholar] [CrossRef]
- Kowarik, I. Cities and Wilderness-A New Perspective. Int. J. Wilderness 2013, 19, 32–36. [Google Scholar]
- El-Keblawy, A.; Al-Rawai, A. Impacts of the invasive exotic Prosopis juliflora (Sw.) D.C. on the native flora and soils of the UAE. Plant Ecol. 2007, 190, 23–35. [Google Scholar] [CrossRef]
- Kumar, S.; Mathur, M. Impact of invasion by Prosopis juliflora on plant communities in arid grazing lands. Trop. Ecol. 2014, 55, 33–46. [Google Scholar]
- Pasiecznik, N.; Felker, P.; Harris, P.; Harsh, L.; Cruz, G.; Tewari, J.; Cadoret, K.; Maldonado, L. The Prosopis Juliflora-Prosopis Pallida Complex: A Monograph; HDRA: Conventery, UK, 2002; Volume 12, p. 229. ISBN 0-905343-30-1. [Google Scholar]
- Mosbah, M.; Taieb, T.; Habib, K. Invasive character of Prosopis juliflora facilitated by its Allelopathy and a wide Mutualistic interaction with soil microorganisms. J. Biol. Sci. 2018, 18, 115–123. [Google Scholar] [CrossRef]
- Gaertner, M.; Wilson, J.R.U.; Cadotte, M.W.; MacIvor, J.S.; Zenni, R.D.; Richardson, D.M. Non-native species in urban environments: Patterns, processes, impacts and challenges. Biol. Invasions 2017, 19, 3461–3469. [Google Scholar] [CrossRef]
- Novel Ecosystems: Intervening in the New Ecological World Order; Wiley Online Books; Hobbs, R.J., Higgs, E.S., Hall, C.M., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; ISBN 9781118354186. [Google Scholar]
- Richardson, D.M.; Ricciardi, A. Misleading criticisms of invasion science: A field guide. Divers. Distrib. 2013, 19, 1461–1467. [Google Scholar] [CrossRef]
- Lee, L.S.H.; Zhang, H.; Jim, C.Y. Serviceable tree volume: An alternative tool to assess ecosystem services provided by ornamental trees in urban forests. Urban For. Urban Green. 2021, 59, 127003. [Google Scholar] [CrossRef]
- Vila, M.; Weiner, J. Are invasive plant species better competitors than native plant species?–evidence from pair-wise experiments. Oikos 2004, 105, 229–238. [Google Scholar] [CrossRef]
- Richardson, D.M.; Ek, P.P.Y.S.; Rejmánek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants: Concepts and definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Simberloff, D.; Von Holle, B. Positive interactions of nonindigenous species: Invasional meltdown? Biol. Invasions 1999, 1, 21–32. [Google Scholar] [CrossRef]
- Geological Survey of India. Copyright Government of India.Geological, Geomorphological and Mineral Map of Delhi. 2000. 30, 16. Available online: https://www.indiawaterportal.org/sites/default/files/iwp2/Map_Delhi_State_Geology_and_Mineral_Maps_Geological_Survey_of_India.pdf (accessed on 20 December 2021).
- Chauhan, S. Analysis of Woody Species in Delhi Ridge: Community Dynamics in Urban Context. Master’s Thesis, School of Human Ecology, Ambedkar University Delhi, Delhi, India, 2013. [Google Scholar]
- Krebs, C.J. Ecological Methodology. In Ecological Methodology, 2nd ed.; A. Wesley Longman: New York, NY, USA, 1999; pp. 157–168. [Google Scholar]
- Maheshwari, J.K. Flora of Delhi; Council of Scientific and Industrial Research: New Delhi, India, 1963. [Google Scholar]
- Sandilyan, S. Invasive Species of India Report; National Biodiversity Authority, Ministry of Environment Forests and Climate Change Government of India: Chennai, India, 2005; Available online: http://nbaindia.org/uploaded/pdf/Iaslist.pdf (accessed on 20 December 2021).
- Kassambara, A. Package ‘ggpubr’; 2017; pp. 37–40. Available online: https://rpkgs.datanovia.com/ggpubr/ (accessed on 20 December 2021).
- Yadav, G.; Babu, S. Nexcade: Perturbation analysis for complex networks. PLoS ONE 2012, 7, e41827. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 1971, 13, 426. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, K.D.; Hogue, C.W.; Groll, M.; Hartmann, C.; Huber, R.; Fields, S. An automated method for finding molecular complexes in large protein interaction networks. Nucleic Acids Res. 2001, 29, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Kuchinsky, A.; Morris, J.H.; States, D.J.; Meng, F. GLay: Community structure analysis of biological networks. Bioinformatics 2010, 26, 3135–3137. [Google Scholar] [CrossRef] [PubMed]
- Dongen, S.V.A.N. Graph Clustering Via a Discrete Uncoupling Process. SIAM J. Matrix Anal. Appl. 2008, 30, 121–141. [Google Scholar] [CrossRef]



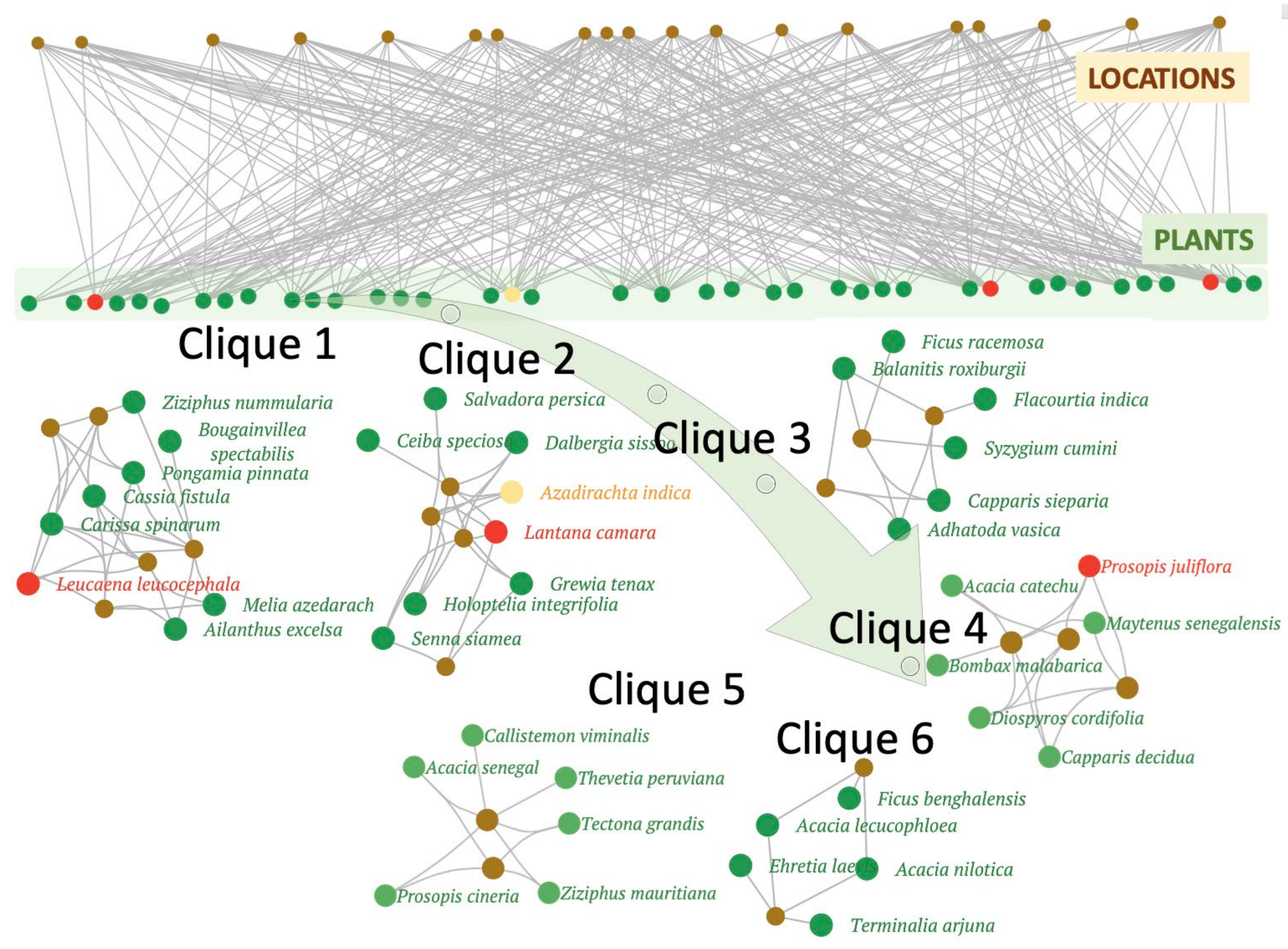

| Site | # Species | Clique | (Hub) Species in Each Community/Clique |
|---|---|---|---|
| SV | 42 | 1 | Leucaena leucocephala, Cassia fistula, Pongamia pinnata, Ailanthus excelsa, Carissa spinarum |
| 2 | Azadirachta indica, Lantana camara, Grewia tenax, Holoptelea integrifolia, Dalbergia sissoo | ||
| 3 | Adhatoda vasica, Capparis sepiaria, Balanites roxiburgii | ||
| 4 | Capparis decidua, Prosopis juliflora, Diospyros cordifolia, Maytenus senegalensis | ||
| 5 | Tectona grandis, Acacia senegal, Ziziphus mauritiana, Prosopis cineria | ||
| TUQ | 31 | 1 | Balanites roxiburgii, Prosopis juliflora, Capparis sepiaria |
| 2 | Acacia nilotica, Cassia fistula, Acacia lecucophloea, Prosopis cineraria | ||
| 3 | Grewia tenax, Holoptelea integrifolia, Pongamia pinnata, Ziziphus nummularia, Diospyros cordifolia | ||
| 4 | Azadirachta indica, Capparis decidua, Adhatoda vasica | ||
| Lantana camara, Bougainvillea spectabilis | |||
| HK | 50 | 1 | Senna siamea, Pongamia pinnata, Capparis sepiaria, Ehretia laevis |
| 2 | Leucaena leucocephala, Morus alba, Adhatoda vasica, Acacia nilotica, Dalbergia sissoo | ||
| 3 | Lantana camara, Diospyros cordifolia, Ziziphus nummularia, Azadirachta indica | ||
| 4 | Prosopis cineraria, Cassia fistula, Grewia tenax, Capparis decidua, Murraya koenigii, Drypetes roxburghii | ||
| 5 | Prosopis juliflora, Bombax malabarica, Milletia peguensis, Ailanthus excelsa, Terminalia arjuna | ||
| JCF | 49 | 1 | Prosopis juliflora, Capparis sepiaria, Carissa spinarum, Azadirachta indica |
| 2 | Holoptelea integrifolia, Pongamia pinnata, Albizia amara, Cassia fistula, Senna siamea, Tectona grandis, Prosopis cineraria | ||
| 3 | Lantana camara, Ziziphus nummularia, Bombax malabarica | ||
| MEH | 37 | 1 | Lantana camara, Adhatoda vasica, Acacia nilotica, Holoptelea integrifolia, Pongamia pinnata, Cassia fistula |
| 2 | Cassia tora, Ziziphus nimmularia, Acacia lecucophloea, Grewia tenax | ||
| 3 | Abutilon, Capparis sepiaria, Capparis decidua | ||
| 4 | Azadirachta indica, Prosopis juliflora | ||
| JNU | 42 | 1 | Balanites roxiburgii, Lantana camara, Ziziphus nummularia, Ziziphus mauritiana |
| 2 | Capparis decidua, Adhatoda vasica, Azadirachta indica, Acacia lecucophloea | ||
| 3 | Prosopis juliflora, Capparis sepiaria, Diospyros cordifolia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, S.; Yadav, G.; Babu, S. Ecological Networks in Urban Forest Fragments Reveal Species Associations between Native and Invasive Plant Communities. Plants 2022, 11, 541. https://doi.org/10.3390/plants11040541
Chauhan S, Yadav G, Babu S. Ecological Networks in Urban Forest Fragments Reveal Species Associations between Native and Invasive Plant Communities. Plants. 2022; 11(4):541. https://doi.org/10.3390/plants11040541
Chicago/Turabian StyleChauhan, Sonali, Gitanjali Yadav, and Suresh Babu. 2022. "Ecological Networks in Urban Forest Fragments Reveal Species Associations between Native and Invasive Plant Communities" Plants 11, no. 4: 541. https://doi.org/10.3390/plants11040541
APA StyleChauhan, S., Yadav, G., & Babu, S. (2022). Ecological Networks in Urban Forest Fragments Reveal Species Associations between Native and Invasive Plant Communities. Plants, 11(4), 541. https://doi.org/10.3390/plants11040541








