In-Vivo Biophoton Emission, Physiological and Oxidative Responses of Biostimulant-Treated Winter Wheat (Triticum eastivum L.) as Seed Priming Possibility, for Heat Stress Alleviation
Abstract
:1. Introduction
2. Results
2.1. Estimation of Fresh/Dry Weight Ratio (FDWR) and Chlorophyll Content (SPAD) in Wheat Seedlings
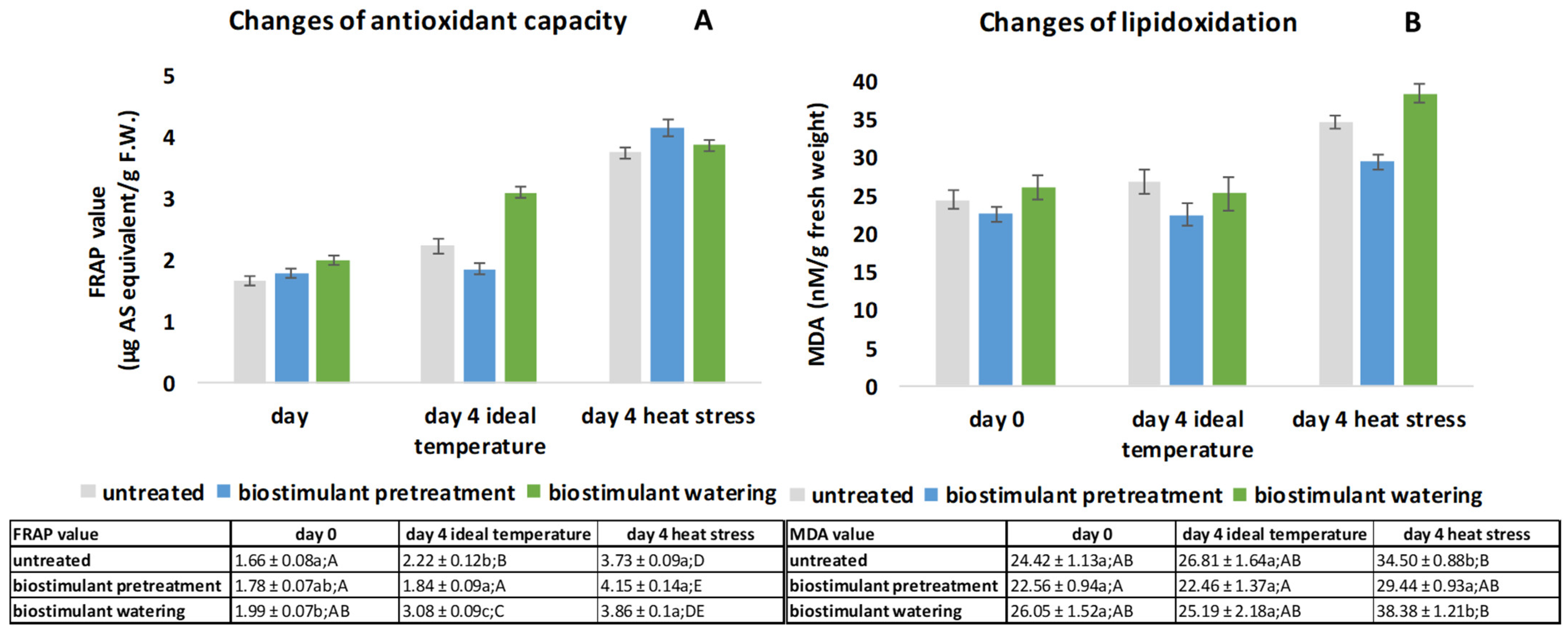
2.2. Analyses of Antioxidant Capacity (FRAP) Results Based on Iron Reducing Ability and Lipid Oxidation Based on MDA Quantification
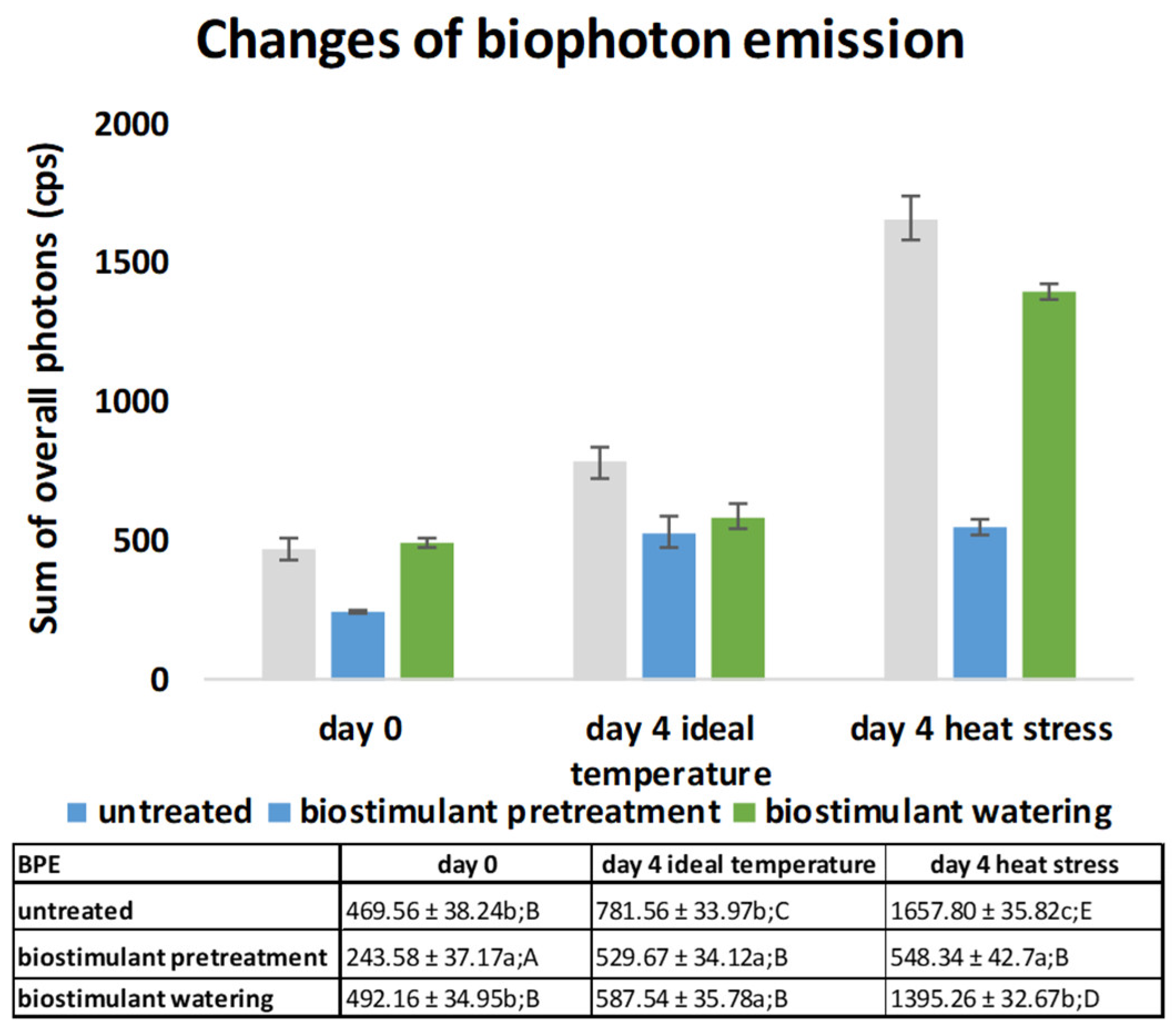
2.3. Biophoton Emission Measurement Results and Their Evaluation
3. Discussion
4. Materials and Methods
4.1. Sowing and Germination
- day parameters: 22 °C [1]; 120 μM m−2 s−1; 16 h
- night parameters: 16 °C; 0 μM m−2 s−1; 8 h
4.2. Biostimulant Treatments
- Untreated (UT): the first group included the untreated seedlings that did not receive biostimulatory treatment. The watering of UT plants was carried out with distilled water.
- Biostimulant pretreatment (BPT): the seedlings in the second group were pretreated with the biostimulant as indicated above, but after sowing and 4 days of germination, they were only watered with distilled water.
- Biostimulant watering (BW): the seedlings in the third group were exposed to biostimulant watering. The germination of these seedlings was carried out in distilled water overnight, while subsequently, after 4 days of germination they received a watering of 1% biostimulant (Figure 5).
4.3. Growing Period of Wheat Seedlings
4.4. Experiment I: The Ideal Temperature
- day parameters: 22 °C [1]; 120 μM m−2·s−1; 16 h
- night parameters: 16 °C; 0 μM m−2·s−1; 8 h
4.5. Experiment II: Heat Stress
- heat stress parameters: 35 °C [1]; 120 μM m−2·s−1; 8 h
- day parameters: 28 °C; 0 μM m−2·s−1; 8 h
- night parameters: 22 °C; 0 μM m−2·s−1; 8 h
4.6. Sampling
4.7. Determination of Fresh/Dry Weight Ratio (FDWR)
4.8. Chlorophyll Content Estimation (SPAD Index Measurement)
4.9. Measurement of Lipid Oxidation
4.10. Ferric Reducing Antioxidant Power Assay
4.11. Biophoton Emission (BPE) Measurement
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hura, T. Wheat and Barley: Acclimatization to Abiotic and Biotic Stress. Int. J. Mol. Sci. 2020, 21, 7423. [Google Scholar] [CrossRef] [PubMed]
- Macholdt, J.; Honermeier, B. Impact of climate change on cultivar choice. Adaptation strategies of farmers and advisors in German cereal production. Agronomy 2016, 6, 40. [Google Scholar] [CrossRef] [Green Version]
- Abenavoli, L.; Milanovic, M.; Procopio, A.C.; Spampinato, G.; Maruca, G.; Perrino, E.V.; Mannino, G.C.; Fagoonee, S.; Luzza, F.; Musarella, C.M. Ancient wheats: Beneficial effects on insulin resistance. Min. Med. 2021, 12, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, S.; Ishikawa, N.; Takahashi, H.; Okuno, R.; Moriya, K. Interactions of cultivar, sowing date, and growing environment differentially alter wheat phenology under climate warming. Agron. J. 2021, 113, 4982–4992. [Google Scholar] [CrossRef]
- Lei, L.; Zhu, X.; Wang, S.; Zhu, M.; Carver, B.F.; Yan, L. TaMFT-A1 Is Associated with Seed Germination Sensitive to Temperature in Winter Wheat. PLoS ONE 2013, 8, e73330. [Google Scholar] [CrossRef] [Green Version]
- Sayed, O.H.; Earnshaw, M.J.; Emes, M.J. Photosynthetic Responses of Different Varieties of Wheat to High Temperature: II. Effect of heat stress on photosynthetic electron transport. J. Exp. Bot. 1989, 40, 633–638. [Google Scholar] [CrossRef]
- Végh, B.; Marček, T.; Karsai, I.; Janda, T.; Darkó, É. Heat acclimation of photosynthesis in wheat genotypes of different origin. S. Afr. J. Bot. 2018, 117, 184–192. [Google Scholar] [CrossRef]
- Wassie, M.; Zhang, W.; Zhang, Q.; Ji, K.; Chen, L. Effect of Heat Stress on Growth and Physiological Traits of Alfalfa (Medicago sativa L.) and a Comprehensive Evaluation for Heat Tolerance. Agronomy 2019, 9, 597. [Google Scholar] [CrossRef] [Green Version]
- Kováčik, J.; Klejdus, B.; Babula, P.; Hedbavny, J. Ascorbic acid affects short-term response of Scenedesmus quadricauda to cadmium excess. Algal Res. 2017, 24, 354–359. [Google Scholar] [CrossRef]
- Kocsy, G.; Szalai, G.; Sutka, J.; Páldi, E.; Galiba, G. Heat tolerance together with heat stress-induced changes in glutathione and hydroxymethylglutathione levels is affected by chromosome 5A of wheat. Plant Sci. 2004, 166, 451–458. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Phys. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xing, D.; Tan, S.; Tangy, Y.; He, Y. Imaging of ultraweak biochemiluminescence and singlet oxygen generation in germinating soybean in response to wounding. Luminescence 2003, 18, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Gallep, C.M. Ultraweak, spontaneous photon emission in seedlings: Toxicological and chronobiological applications. J. Biol. Chem. Lumin. 2014, 29, 963–968. [Google Scholar]
- Pospišil, P. Molecular mechanisms of production and scavenging of reactiveoxygen species by photosystem II. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 218–231. [Google Scholar] [CrossRef] [Green Version]
- Cifra, M.; Pospišil, P. Ultra-weak photon emission from biological samples: Definition, mechanisms, properties, detection and applications. J. Photochem. Photobiol. B 2014, 139, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Hideg, É.; Inaba, H. Biophoton Emission (Ultraweak Photoemission) from dark adapted spinach chloroplasts. Photochem. Photobiol. 1991, 53, 137–142. [Google Scholar] [CrossRef]
- Prasad, A.; Sedlářová, M.; Kale, R.S.; Pospíšil, P. Lipoxygenase in singlet oxygen generation as a response to wounding: In vivo imaging in Arabidopsis thaliana. Sci. Rep. 2017, 7, 9831. [Google Scholar] [CrossRef] [PubMed]
- Oros, C.L.; Alves, F. Leaf wound induced ultraweak photon emission is suppressed under anoxic stress: Observations of Spathiphyllum under aerobic and anaerobic conditions using novel in vivo methodology. PLoS ONE 2018, 13, e0198962. [Google Scholar] [CrossRef] [Green Version]
- Oszlányi, R.; Mirmazloum, I.; Pónya, Z.; Szegő, A.; Jamal, S.; Bat-Erdene, O.; Papp, I. Oxidative stress level and dehydrin gene expression pattern differentiate two contrasting cucumber F1 hybrids under high fertigation treatment. Int. J. Biol. Macromol. 2020, 161, 864–874. [Google Scholar] [CrossRef]
- Iida, T.; Yoshiki, Y.; Akiyama, Y.; Okubo, K. Photon emission properties of roasted soybean as related to reactive oxygen scavenging activities. Food Chem. 2002, 77, 471–477. [Google Scholar] [CrossRef]
- Kobayashi, K.; Okabe, H.; Kawano, S.; Hidaka, Y.; Hara, K. Biophoton Emission Induced by Heat Shock. PLoS ONE 2014, 9, e105700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamal, A.H.M.; Komatsu, S. Proteins involved in biophoton emission and flooding-stress responses in soybean under light and dark conditions. Mol. Biol. Rep. 2016, 43, 73. [Google Scholar] [CrossRef] [PubMed]
- Jócsák, I.; Malgwi, I.; Rabnecz, G.; Szegő, A.; Varga-Visi, É.; Végvári, G.; Pónya, Z. Effect of cadmium stress on certain physiological parameters, antioxidative enzyme activities and biophoton emission of leaves in barley (Hordeum vulgare L.) seedlings. PLoS ONE 2020, 15, e0240470. [Google Scholar] [CrossRef] [PubMed]
- Cornetti, U. Antioxidant use in nutraceuticals. Clin. Dermatol. 2009, 27, 175–194. [Google Scholar]
- Hassan, S.M.; Ashour, M.; Sakai, N.; Zhang, L.; Hassanien, H.A.; Gaber, A.; Ammar, G. Impact of Seaweed Liquid Extract Biostimulant on Growth, Yield, and Chemical Composition of Cucumber (Cucumis sativus). Agriculture 2021, 11, 320. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Galvão, Í.M.; dos Santos, O.F.; de Souza, M.L.C.; de Jesus Guimarães, J.; Kühn, I.E.; Broetto, F. Biostimulants action in common bean crop submitted to water deficit. Agric. Water Manag. 2019, 225, 105762. [Google Scholar] [CrossRef]
- Hammad, S.A.R. Physiological and anatomical studies on drought tolerance of pea plants by application of some natural extracts. Ann. Agric. Sci. 2008, 53, 285–305. [Google Scholar]
- Choudhary, S.K.; Kumar, V.; Singhal, R.K.; Bose, B.; Chauhan, J.; Alamri, S.; Siddiqui, M.H.; Javed, T.; Shabbir, R.; Rajendran, K.; et al. Seed Priming with Mg(NO3)2 and ZnSO4 Salts Triggers the Germination and Growth Attributes Synergistically in Wheat Varieties. Agronomy 2021, 11, 2110. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed Priming with Phytohormones: An Effective Approach for the Mitigation of Abiotic Stress. Plants 2021, 10, 37. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Barutçular, C.; Yıldırım, M.; Koc, M.; Akıncı, C.; Toptaş, I.; Albayrak, O.; Tanrıkulu, A.; El Sabagh, A. Evaluation of SPAD chlorophyll in spring wheat genotypes under different environments. Fresenius Environ. Bull. 2016, 25, 1258–1266. [Google Scholar]
- Lobell, D.; Sibley, A.; Ivan Ortiz-Monasterio, J. Extreme heat effects on wheat senescence in India. Nat. Clim. Chang. 2012, 2, 186–189. [Google Scholar] [CrossRef]
- Pantoja-Benavides, A.D.; Garces-Varon, G.; Restrepo-Díaz, H. Foliar Growth Regulator Sprays Induced Tolerance to Combined Heat Stress by Enhancing Physiological and Biochemical Responses in Rice. Front. Plant Sci. 2021, 12, 702892. [Google Scholar] [CrossRef] [PubMed]
- Fatma, M.; Iqbal, N.; Sehar, Z.; Alyemeni, M.N.; Kaushik, P.; Khan, N.A.; Ahmad, P. Methyl Jasmonate Protects the PS II System by Maintaining the Stability of Chloroplast D1 Protein and Accelerating Enzymatic Antioxidants in Heat-Stressed Wheat Plants. Antioxidants 2021, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Ni, Z.; Hu, R.; Lin, L.; Deng, H.; Wang, J.; Tang, Y.; Sun, G.; Wang, X.; Li, H.; et al. Melatonin Alleviates Drought Stress by a Non-Enzymatic and Enzymatic Antioxidative System in Kiwifruit Seedlings. Int. J. Mol. Sci. 2020, 21, 852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lizcano, L.J.; Viloria-Bernal, M.; Vicente, F.; Berrueta, L.A.; Gallo, B.; Martínez-Cañamero, M.; Ruiz-Larrea, M.B.; Ruiz-Sanz, J.I. Lipid Oxidation Inhibitory Effects and Phenolic Composition of Aqueous Extracts from Medicinal Plants of Colombian Amazonia. Int. J. Mol. Sci. 2012, 13, 5454–5467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galić, L.; Špoljarević, M.; Jakovac, E.; Ravnjak, B.; Teklić, T.; Lisjak, M.; Perić, K.; Nemet, F.; Lončarić, Z. Selenium Biofortification of Soybean Seeds Influences Physiological Responses of Seedlings to Osmotic Stress. Plants 2021, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin Suppressed the Heat Stress-Induced Damage in Wheat Seedlings by Modulating the Antioxidant Machinery. Plants 2020, 9, 809. [Google Scholar] [CrossRef]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid, systemic signaling in response to diverse stimuli. Sci. Signal. 2009, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Hayat, S.; Ahmad, H.; Ghani, M.I.; Amin, B.; Atif, M.J.; Cheng, Z. Priming of Solanum melongena L. Seeds Enhances Germination, Alters Antioxidant Enzymes, Modulates ROS, and Improves Early Seedling Growth: Indicating Aqueous Garlic Extract as Seed-Priming Bio-Stimulant for Eggplant Production. Appl. Sci. 2019, 9, 2203. [Google Scholar] [CrossRef] [Green Version]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Szollosi, R.; Varga, I.S. Total antioxidant power in some species of Labiatae (Adaptation of FRAP method). Acta Biol. 2002, 46, 125–127. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jócsák, I.; Gyalog, H.; Hoffmann, R.; Somfalvi-Tóth, K. In-Vivo Biophoton Emission, Physiological and Oxidative Responses of Biostimulant-Treated Winter Wheat (Triticum eastivum L.) as Seed Priming Possibility, for Heat Stress Alleviation. Plants 2022, 11, 640. https://doi.org/10.3390/plants11050640
Jócsák I, Gyalog H, Hoffmann R, Somfalvi-Tóth K. In-Vivo Biophoton Emission, Physiological and Oxidative Responses of Biostimulant-Treated Winter Wheat (Triticum eastivum L.) as Seed Priming Possibility, for Heat Stress Alleviation. Plants. 2022; 11(5):640. https://doi.org/10.3390/plants11050640
Chicago/Turabian StyleJócsák, Ildikó, Henrik Gyalog, Richárd Hoffmann, and Katalin Somfalvi-Tóth. 2022. "In-Vivo Biophoton Emission, Physiological and Oxidative Responses of Biostimulant-Treated Winter Wheat (Triticum eastivum L.) as Seed Priming Possibility, for Heat Stress Alleviation" Plants 11, no. 5: 640. https://doi.org/10.3390/plants11050640
APA StyleJócsák, I., Gyalog, H., Hoffmann, R., & Somfalvi-Tóth, K. (2022). In-Vivo Biophoton Emission, Physiological and Oxidative Responses of Biostimulant-Treated Winter Wheat (Triticum eastivum L.) as Seed Priming Possibility, for Heat Stress Alleviation. Plants, 11(5), 640. https://doi.org/10.3390/plants11050640






