Abstract
Macrophomina phaseolina and Rhizoctonia solani are considered two major soil-borne pathogens of Phaseolus vulgaris in Cuba. Their management is difficult, not only due to their intrinsic biology as soil-borne pathogens, but also because the lack of active ingredients available against these pathogens. Actinobacteria, a heterogeneous bacterial group traditionally known as actinomycetes have been reported as promising biological control agents (BCAs) in crop protection. Thus, the main objective of this study was to evaluate the effectiveness of 60 actinobacterial strains as BCAs against M. phaseolina and R. solani in vitro by dual culture assays. The most effective strains were characterized according to their cellulolytic, chitinolytic and proteolytic extracellular enzymatic activity, as well as by their morphological and biochemical characters in vitro. Forty and 25 out of the 60 actinobacteria strains inhibited the mycelial growth of M. phaseolina and R. solani, respectively, and 18 of them showed a common effect against both pathogens. Significant differences were observed on their enzymatic and biochemical activity. The morphological and biochemical characters allow us to identify all our strains as species belonging to the genus Streptomyces. Streptomyces strains CBQ-EA-2 and CBQ-B-8 showed the highest effectiveness in vitro. Finally, the effect of seed treatments by both strains was also evaluated against M. phaseolina and R. solani infections in P. vulgaris cv. Quivicán seedlings. Treatments combining the two Streptomyces strains (CBQ-EA-2 + CBQ-B-8) were able to reduce significantly the disease severity for both pathogen infections in comparison with the non-treated and inoculated control. Moreover, they showed similar effect than that observed for Trichoderma harzianum A-34 and with Celest® Top 312 FS (Syngenta®; Basilea, Switzerland) treatments, which were included for comparative purposes.
1. Introduction
The common bean (Phaseolus vulgaris L.) is one of the most important grain legumes in many areas of the world, providing a diet rich in protein, dietary fiber, essential micronutrients and phytochemicals for more than 500 million people []. The global cultivated surface of P. vulgaris reached 33.1 million hectares in the season 2019/2020, with an annual production of 28.9 million metric tons []. Common bean is the most important plant species for Cuba population within the group of edible legumes with an annual production of 169,900 tons. Together with rice (Oryza sativa L.), they form the basis of the daily diet in this geographic area [].
In countries with a subtropical climate, the environmental conditions are favorable for the development and proliferation of a vast and heterogeneous soil microflora, including complexes of fungal species associated with root rot diseases such as Alternaria alternata (Fr.) Keissl., Colletotrichum truncatum (Schwein.) Andrus & W.D. Moore, Fusarium oxysporum Schltdl., Macrophomina phaseolina (Tassi) Goid., Rhizoctonia solani J.G. Kühn, and Sclerotium rolfsii Sacc. []. In addition, [] pointed out that the incidence and severity of root rot diseases caused by these fungi depend on the climatic factors prevailing at each sowing time, as well as the characteristics of the microclimates existing in each region of the country where common beans are grown. Among them, M. phaseolina and R. solani are considered the most prevalent fungal pathogens associated with root rot diseases of common bean worldwide [,].
Macrophomina phaseolina (Ascomycota), causal agent of ashy stem blight, also affects roots and stems of host species via pycnidiospores and microsclerotia that persist in the soil, where the pathogen establishes the primary inoculum []. Typical symptoms in common bean include dark, irregular lesions on cotyledons, wilting, systemic chlorosis, premature defoliation, epinasty and early maturity or death in adult plants []. Late infections cause the appearance of grey areas on the stems, where microsclerotia and pycnidia of the fungus are produced. The occurrence of M. phaseolina in the seeds has major consequences since it causes the disqualification of legumes as propagation material []. For instance, six tons of common bean and three tons of broad bean to be used as planting material in the province of Villa Clara were disqualified between 2006–2007 because they were affected by M. phaseolina [].
On the other hand, R. solani (Basidiomycota) is the causal agent of rhizoctonia blight, also commonly known as damping off []. This soil-borne pathogen can affect more than 500 plant species, including cultivated and wild plants, and causes damping off in stands, necrotic lesions in roots, seeds and stems, as well as foliar lesions with a worldwide distribution [,]. This fungus affects young seedlings much more than adult plant tissues. On the stem and hypocotyl of affected plants, reddish-brown cankers of various sizes appear, usually delimited by a dark border, which later become rough, dry up and destroy plant tissues []. It also attacks the roots causing foot rot of the plants []. The management of soil-borne pathogens including both R. solani and M. phaseolina is usually difficult, not only due to their intrinsic biology, but also because the lack of effective active ingredients. Thus, the use and extension of eco-friendly control methods such as biological control is required, not only to prevent plant diseases, but also contributing markedly to soil preservation and conservation [].
Microorganisms belonging to genera Bacillus (bacteria) and Trichoderma (fungi) are the most commonly used biological control agents (BCAs) against soil-borne plant pathogenic fungi ([,]. Within this context, species belonging to Trichoderma fungal genus have been studied since 1930, and their use has been successfully applied directly to the soil or by seed treatments []. On the other hand, since the last century, bacteria belonging to the genus Bacillus have been used as BCAs due to their ability to colonize the rhizosphere of plants and inhibit the growth and development of plant pathogens. In addition, they are used as plant growth promoters. []. At the same time, the ability of these bacteria to form endospores gives them resistance to climatic changes, which is an important characteristic for inoculum production []. In addition to these well-known BCAs, research in the last decades highlights the benefits of the actinobacteria (Streptomyces spp. mainly) and their potential as BCAs (e.g., Streptomyces griseoviridis, S. lydicus) against soil-borne pathogens, such as species of Rhizoctonia, Phytophthora, Fusarium, and Pythium in legumes and other crops []. Actinobacteria, which have been traditionally known as actinomycetes, are a heterogeneous group of aerobic, filamentous and Gram-positive bacteria. Traditionally, the main genera isolated from soil samples are Micromonospora, Nocardia, and Streptomyces. The genus Streptomyces is represented in nature by the largest number of species among the family Actinomycetaceae []. This genus, as a colonizer of the rhizosphere, is able to: (i) act as BCA of plant pathogenic fungi, (ii) produce siderophores, (iii) produce plant growth promoting substances, (iv) promote nodulation, (v) produce biodegradative enzymes such as chitinases, cellulases, glucanases, peroxidases, and (vi) assist Rhizobium bacteria in iron assimilation, or in nitrogen fixation in legumes, which indirectly contributes to the promotion of plant growth [].
As we mentioned above, ashy stem blight and rhizoctonia blight are considered the main diseases of P. vulgaris in Cuba since they are associated in a complex disease of this crop that causes root rot and plant death. The control management strategies already available against this complex disease are not enough for its optimum control in the frame of the sustainable agriculture. Thus, it is necessary to explore new alternatives towards biological control of these diseases. Therefore, actinobacteria could play an important role as BCAs against the main causal agents of the disease, M. phaseolina and R. solani. However, the effect of actinobacteria as BCAs against plant pathogenic fungi is still uncertain. Consequently, no biological based compounds on actinobacteria have been developed so far. Likewise, the ‘Centro de Bioactivos Químicos’ Universidad Central “Marta Abreu” de Las Villas (Cuba) has a wide collection of actinobacterial strains isolated in the central region of the country, which may be explored as a new biological alternative to be included in the integrated disease management program against soil-borne plant pathogens in the common bean crop. Therefore, the main goal of this study was to evaluate 60 actinobacterial strains for their effectiveness as BCAs against M. phaseolina and R. solani by in vitro dual-cultures assays and finally to select several actinobacterial strains with high efficiency of reduction the viability of both pathogens in vitro, and the disease progress in planta. We expect to select several actinobacterial strains with high efficacy on reducing the viability of M. phaseolina and R. solani in vitro, and the disease progress in planta.
2. Results
2.1. In Vitro Effect of Actinobacterial Strains against Macrophomina phaseolina and Rhizoctonia solani: Dual Culture Assays
For both fungal pathogens M. phaseolina and R. solani, significant differences between actinobacterial strains were observed on their effectiveness in the Mycelial Growth Inhibition (MGI; %) (p < 0.001 in both cases). Regarding their effect against M. phaseolina isolate CCIBP-Mp1, 40 out of the 60 strains tested showed antagonistic activity against the pathogen. For this group of 40 strains, the MGI ranged from 70.4 ± 1.23 to 3.24 ± 1.01% for CBQ-EA-2 and CBQ-ESFe-11, respectively. The most effective strains against M. phaseolina were CBQ-EA-2, -Plat-2 and -CD-24 with mean MGI values of 70.4 ± 1.23, 66.6 ± 0.78 and 64.6 ± 1.48%, respectively. On the other hand, 25 out of the 60 actinobacterial strains tested showed antagonistic effect against R. solani isolate CCIBP-Rh1. For this group of 25 strains, the MGI ranged from 78.3 ± 0.37 to 5.6 ± 0.47% for CBQ-EA-12 and CBQ-C-5, respectively. The most effective strains against R. solani were CBQ-EA-12, -EA-2 and -CD-24 with mean MGI values of 78.3 ± 0.37, 77.4 ± 1.20 and 75.4 ± 1.22%, respectively. In addition, 19 out of the 60 actinobacteria strains evaluated showed a MGI efficacy higher than 50% for both phytopathogenic fungi, with the strains CBQ-EA-2 (MGI = 70.4 ± 1.23 and 77.4 ± 1.20% for M. phaseolina and R. solani, respectively) and CBQ-CD-24 (MGI = 64.6 ± 1.48 and 75.4 ± 1.22% for M. phaseolina and R. solani, respectively) showing the highest common effectiveness for the two pathogens. At the same time, 5% of the total actinobacteria tested did not show any effect on MGI for any of the two pathogens evaluated (Table 1; Figure 1).

Table 1.
Antagonistic effect of the 60 actinobacterial strains on mycelial growth of Macrophomina phaseolina and Rhizoctonia solani in dual cultures.
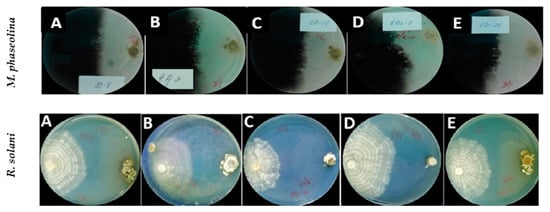
Figure 1.
Antagonistic effect of Streptomyces strains against Macrophomina phaseolina isolate CCIBP-Mp1 (top row photos) and Rhizoctonia solani isolate CCIBP-Rh1 (bottom row photos) growing in dual culture on PDA at 7 days after inoculation and incubated at 28 °C in the dark. Streptomyces strains evaluated were: (A) CBQ-B-8, (B) CBQ-EA-2, (C) CBQ-CB-14, (D) CBQ-EBa-5, and (E) CBQ-CD-24 (top row photos); and (A) CBQ-B-8, (B) CBQ-CB-14, (C) CBQ-EA-12, (D) CBQ-EBa-21, and (E) CBQ-EA-2 (bottom row photos).
2.2. Qualitative Evaluation of Enzyme Activities of Actinobacterial Strains
There were significant differences between the 31 actinobacterial strains evaluated for their chitinolytic, cellulolytic or proteolytic activity (p < 0.0001) (Table 2). Twenty out of the 31 strains evaluated showed chitinolytic halo, which ranged from 25.3 ± 0.96 to 33.5 ± 1.91 mm for CBQ-CD-24 to CBQ-EBa-5, respectively. Concerning the cellulolytic activity, the cellulolytic halo ranged between 90.0 ± 0.41 (CBQ-B-8; -CB-14; -ECa-24; -ESFe-12; -Ni-32; -Plat-2; -Plat-3; -Plat-4; and -WP-14) and 36.3 ± 0.75 mm (CBQ-EA-3). Only three out of the 30 strains evaluated did not show cellulolytic halo (CBQ-EB-27; -EC-18; -OSS-4). Finally, 21 out of the 31 strains evaluated showed proteolytic halo, which ranged from 51.5 ± 1.50 to 27.0 ± 0.71 mm for CBQ-EA-12 to CBQ-ECa-24, respectively (Table 2).

Table 2.
Chitinolytic, cellulolytic and proteolytic activity of the 31 actinobacterial strains selected for these experiments.
2.3. Phenotypic Characterization
The macroscopic features of the 11 representative actinobacterial strains selected for this experiment are show in Table 3. In general, the colonies were mostly white in color, circular in shape, convex in elevation, with an entire edge, hard consistency and variable pigment production (Figure 2). Microscopic observation of Gram-stained bacterial cells showed stable branched mycelium bearing aerial hyphae, which differentiate into short or long spore chains. Microscopic characterization using the microculture technique revealed details of aerial and vegetative mycelium, mycelial fragmentation and clustering of spores. A spiral arrangement of spores was observed on most of the microculture slides of each sample. In addition, all the strains were characterized as Gram-positive suggesting that they belong to the genus Streptomyces.

Table 3.
Macroscopic characteristics of colonies of 11 actinobacterial strains (Streptomyces spp.) grown on Casein Starch Agar at 28 °C in darkness for 10 days *.
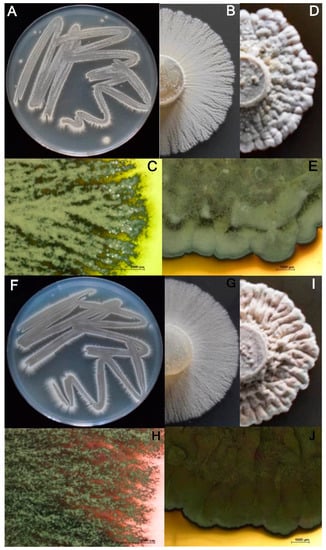
Figure 2.
Two-weeks-old colonies of Streptomyces strains CBQ-B-8 (A–E), and CBQ-EA-2 (F–J) growing on ACA medium (A–C,F–H) and on PDA medium (D,E,I,J) at 28 °C in the dark.
2.4. Biochemical Characterization and Assimilation of Carbon Sources
None of the eleven strains under study were positive for indole production and the Voges Proskauer test. Strains CBQ-J-4, -OSS-3, -EA-2 and -EBa-5, were positive for casein hydrolysis; and the latter two strains were also able to be positive for the methyl red test, in addition to strains CBQ-B-8, -CB-14, -EBa-21 and -Plat-2. Only the strains CBQ-EA-12 and -ESFe-4 did not hydrolyse gelatine. The strains CBQ-OSS-3 and -Plat-2 did not hydrolyse starch (Table 4).

Table 4.
Biochemical test results of the 11 actinobacterial strains selected for this experiment.
On the other hand, all the evaluated strains were positive for catalase citrate utilization, nitrate reduction and urea hydrolysis. Variability between strains was also observed for the assimilation and utilization of carbohydrates (Table 4).
2.5. Molecular Characterization
BLASTn searches on GenBank showed that the 16S rDNA sequences of the strains CBQ-EA-2 and CBQ-B-8 had 99.71 and 99.93% identity with strains of Streptomyces sp. HBUM206419 (MT540570) and MP47-91 (EU263063), respectively. The sequences logged in GenBank and Blast results of the two representative actinobacterial strains selected for their highest effectiveness in vitro in this study are shown in Table 5.

Table 5.
Identification by sequencing the 16S rDNA gene of the two actinobacterial strains selected for molecular characterization with their corresponding GenBank accession numbers and data of Blast results obtained from GenBank.
2.6. Effect of Actinobacterial Strains against Macrophomina phaseolina and Rhizoctonia solani Infections in Planta
Because significant differences between sterilized and non-sterilized soils, treatments, and their interaction (p ≤ 0.0001 in all cases) were observed on their effect on total Disease Severity (DS) (for seedlings inoculated with M. phaseolina) and on DSstem and DSroot (for seedlings inoculated with R. solani), individual ANOVA per each type of soil was conducted to evaluate the effect of treatment on DS of each tissue.
2.6.1. Effect of Treatments against Macrophomina phaseolina in Planta
For the treatments conducted with seedlings grown in non-sterilized soil, significant differences between treatments were observed for their effect on DS (p ≤ 0.0001). DS ranged from 21.7 ± 2.1 to 6.4 ± 2.8% for seedlings treated with Streptomyces sp. CBQ-EA-2 and Celest® Top 312 FS, respectively, with all treatments showing a significant effect on the disease progress in comparison with the non-treated and inoculated seedlings (positive control; DS = 70.3 ± 3.1%) (Figure 3).
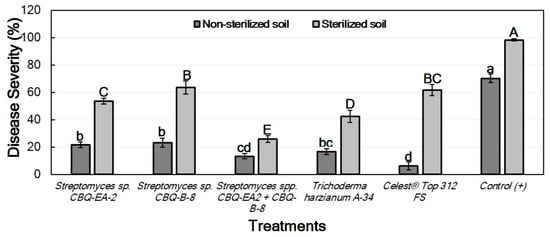
Figure 3.
Disease severity (%) in Phaseolus vulgaris cv. Quivicán seedlings treated with biological or chemical compounds and inoculated with Macrophomina phaseolina isolate CCIBP-Mp1 at 35 days growing on non-sterilized or sterilized soil. Each column represents the mean of 40 seedlings per soil and treatment combination. Columns with a common uppercase or lowercase letter do not differ significantly according to Fisher’s protected LSD test (p = 0.05) for treatments on non-sterilized or sterilized soil, respectively. Vertical bars are the standard errors of the means.
Concerning the treatments conducted with seedlings grown in sterilized soil, significant differences between treatments were also observed for their effect on DS (p ≤ 0.0001). In this case, all treatments also resulted in significant effectiveness compared to the positive control (DS = 98.3 ± 0.7%). DS among treated seedlings ranged from 63.6 ± 4.9 to 25.8 ± 2.5 for treatments with Streptomyces sp. CBQ-B-8 and Streptomyces sp. CBQ-EA-2+ CBQ-B-8, respectively (Figure 3).
The Disease Incidence (DI) was markedly lower in treated seedlings grown in non-sterile soil than those grown in sterile soil. In both cases, not only there were significant differences in DI between treatments, but also significant differences were observed between all the treatments and the positive control (p ≤ 0.0001 in all cases), the latter always showing the highest DI values. In all cases, the treatments with Streptomyces sp. CBQ-EA-2 + CBQ-B-8, T. harzianum A-34, or Celest® Top 312 FS showed the lowest DI values (Figure 4). No seedling mortality was not observed in any case, except for the positive control grown in sterile soil which presented 100% mortality.
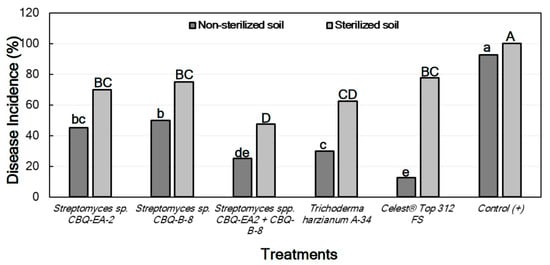
Figure 4.
Disease incidence (DI, %) in Phaseolus vulgaris cv. Quivicán seedlings treated with biological or chemical compounds and inoculated with Macrophomina phaseolina isolate CCIBP-Mp1 at 35 days growing on non-sterilized or sterilized soil. Each column represents the mean of 40 seedlings per soil and treatment combination. Columns with a common uppercase or lowercase letter do not differ significantly according to Dunn’s multiple comparisons for proportions test (p = 0.05) for treatments on non-sterilized or sterilized soil, respectively.
2.6.2. Effect of Treatments against Rhizoctonia solani in Planta
For treatments conducted with seedlings grown in non-sterilized soil, significant differences between treatments were observed for their effect on both DSstem (p = 0.0015) and DSroot (p ≤ 0.0001). In all cases, DSstem was lower than DSroot, ranging from 18.7 ± 2.21 to 4.4 ± 0.77% for seedlings treated with Streptomyces sp. CBQ-EA-2 and Celest® Top 312 FS, respectively. But no important differences were observed for their effect on DSstem compared to the positive control (DSstem = 10.6 ± 3.78%). However, all the treatments showed significantly higher effectiveness on DSroot compared to the positive control (DSroot = 86.8 ± 4.38%). Treatments with Streptomyces sp. CBQ-EA-2 (DSroot = 36.9 ± 1.53%) or CBQ-B-8 (DSroot = 40.6 ± 2.21%) were the least effective, while the treatment that combined the two strains was highly effective (DSroot = 21.2 ± 1.53%) showing results similar to those observed for T. harzianum A-34 (DSroot = 27.5 ± 2.07%) (Figure 5).
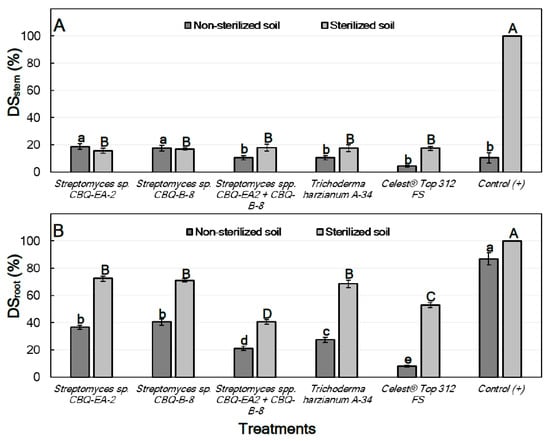
Figure 5.
Disease severity (%) in stem (A; DSstem) and roots (B; DSroot) of Phaseolus vulgaris of cv. Quivicán seedlings treated with biological or chemical compounds and inoculated with Rhizoctonia solani isolate CCIBP-Rh1 at 28 days growing on non-sterilized or sterilized soil. Each column represents the mean of 40 seedlings per soil and treatment combination. Columns with a common uppercase or lowercase letter do not differ significantly according to Fisher’s protected LSD test (p = 0.05) for treatments on non-sterilized or sterilized soil, respectively. Vertical bars are the standard errors of the means.
Regarding the treatments conducted with seedlings grown on sterilized soil, significant differences were also observed between treatments for their effect on both DSstem (p ≤ 0.0001) and DSroot (p ≤ 0.0001). In this case, all the treatments were highly effective compared to the positive control (DSstem = 100%), but no significant differences in effectiveness between treatments were observed. DSstem ranged from 18.1 ± 2.50 to 15.6 ± 1.71% for treatments with Streptomyces sp. CBQ-EA-2+ CBQ-B-8, and with Streptomyces sp. CBQ-EA-2, respectively. On the other hand, all the treatments also showed significantly lower DSroot values compared to the positive control (DSroot = 100%), but significant differences were also observed between treatments for their effect against the disease. The most effective treatment was Streptomyces sp. CBQ-EA-2+ CBQ-B-8 (DSroot = 40.6 ± 2.21%), and the least effective were Streptomyces sp. CBQ-EA-2 (DSroot = 72.5 ± 1.82%), Streptomyces sp. CBQ-B-8 (DSroot = 71.3 ± 1.17%) and T. harzianum A-34 (DSroot = 68.7 ± 2.62%) (Figure 5).
A pattern similar to that observed for seedlings inoculated with M. phaseolina was found for the effect of the treatments on the DI of seedlings inoculated with R. solani, with significant differences in DI being between all treatments and the positive control (p ≤ 0.0001 in all cases). In all cases, the treatment with Streptomyces sp. CBQ-EA-2 + CBQ-B-8 showed significantly less DI than the treatments with Streptomyces sp. CBQ-EA-2 or Streptomyces sp. CBQ-B-8, and also showed DI values similar to those observed for T. harzianum A-34 and Celest® Top 312 FS (Figure 6).
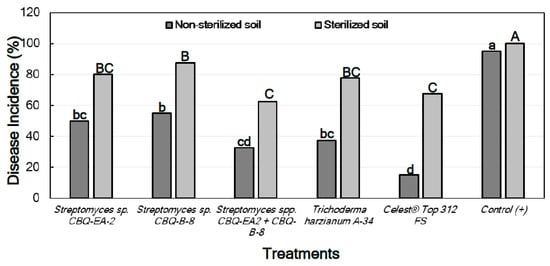
Figure 6.
Disease incidence (DI, %) in Phaseolus vulgaris cv. Quivicán seedlings treated with biological or chemical compounds and inoculated with Rhizoctonia solani isolate CCIBP-Rh1 at 28 days growing on non-sterilized or sterilized soil. Each column represents the mean of 40 seedlings per soil and treatment combination; and columns with a common uppercase or lowercase letter do not differ significantly according to Zar’s multiple comparisons for proportions test (p = 0.05) for treatments on non-sterilized or sterilized soil, respectively.
Finally, for both lots of plants growing in non-sterilized and sterilized soil, the linear correlation analysis showed that there was not significant linear correlation between DSstem and DSroot (non-sterilized soil: r = 0.2869; p = 0.5815; sterilized soil: r = 0.7739; p = 0.0709), and DSstem and DI (non-sterilized soil: r = 0.3826; p = 0.4541; sterilized soil: r = 0.7409; p = 0.0920). Nevertheless, a significant positive linear correlation was observed between DSroot and DI in both non-sterilized soil (r = 0.9944; p = 0.0001) and sterilized soil (r = 0.9710; p = 0.0013).
3. Discussion
Actinobacteria (Streptomyces spp. mainly) have been reported as potential BCAs against soil-borne pathogens of legumes during the last decade []. However, the use of actinobacteria as BCAs in the frame of the integrated management of the major diseases of common bean caused by soil-borne pathogens in Cuba has not been explored yet. Therefore, this study aimed to characterize a collection of 60 actinobacterial strains from Cuba based on their in vitro effectiveness against the two main soil-borne pathogens of common bean in Cuba, as well as on their phenotypic and biochemical characteristics.
All the selected actinobacteria formed a smooth surface colony in CAS, becoming white to beige, hard and compact with age, varying in pigmentation, powdery or velvety appearance as a result of the formation of short and long chains of spores, with typical smell of wet soil (Figure 2). In the totality of the microcultures a spiral arrangement of the spores was observed. Similar results were obtained by Ayuningrum and Jati [], whom reported that isolates of actinobacteria forming powdery colonies with well-developed aerial hyphae divided into spore chains were termed Streptomyces-like actinomycete bacteria. This fact together with the concordance of the morphological characters of our strains with those described by Bergey [] for the Streptomyces genus, indicate that all of our actinobacteria strains belong to this genus. In addition, our Streptomyces strains showed high levels of cellulolytic and proteolytic activity. Our results are also in concordance with those previously obtained by several authors, who reported the ability of Streptomyces strains to produce high levels cellulase and protease []. For instance, 62% of our Streptomyces strains revealed a high cellulolytic capacity with a halo between 80 to 90 mm in diameter, and 90% of them developed a halo with considerable extension around the colony, which denotes an important cellulolytic hydrolysis. Similar results were recently obtained by Rani et al. [], who reported that the 67.5 and 60.0% of the Streptomyces isolates of their collection showed cellulolytic and proteolytic activity, respectively. Furthermore, the 66.7% of our Streptomyces strains showed chitinolytic capacity, highlighting the CBQ-EBa-5 strain, with a 35.5 mm clearance halo surrounding the colony. These results are also in agreement with those obtained by Liu et al. [], who showed that S. hydrogenans (SSD60) and S. spororaveus (SDL15) had strong chitinolytic activity, and the 24% of the Streptomyces strains of their collection (n = 94) developed a clear halo surrounding the colony when evaluating their chitinolytic activity. Altogether, it not only confirms that our strains are well identified as Streptomyces, but also suggests that the actinobacteria form one of the most important microbial communities in soil rehabilitation and conservation, as they are largely responsible for their ability to produce extracellular cellulolytic, chitinolytic and proteolytic enzymes.
Actinobacteria represent a source of biologically active secondary metabolites, including enzymes []. In this study, we achieved specific qualitative metabolic characterization such as enzymatic, biochemical, morphological and antagonistic of at least 11 strains, which is the main criterion for determining their environmental role and their action in biogeochemical cycles. The challenge of our future research has its origins in this study, so evaluating the in vitro antagonistic activity of our strains showed that many of them disseminate secondary metabolites in the same culture medium in which they inhibit the growth of M. phaseolina and R. solani. After having evaluated the enzymatic activities, we could infer that the production of chitinases has a positive effect in this regard, since chitin is one of the major components of the fungal cell wall. In addition, actinobacteria combine with other soil microorganisms in their natural environment to decompose resistant plant debris, such as cellulose, as well as animal debris to maintain the biotic balance of the soil by cooperating with the nutrient cycle []. Although we were able to identify well all of our actinobacterial strains as Streptomyces spp. based on their phenotypic and biochemical characters, the identity of the two representative strains that showed that highest effectiveness on MGI in vitro in this study (CBQ-EA-2, and -B-8) was confirmed by sequencing the 16S rRNA gene using the universal primers 27f and 1492r for eubacteria. The consensus sequences obtained were blasted in GenBank and they match with more than 99% of maximum identity with reference sequences from Streptomyces spp. According to Law et al. [], the 16S rRNA gene has been extensively studied with proven sensitivity for taxonomic and phylogenetic identification of most bacteria including actinobacteria such as Streptomyces spp.
Regarding the in vitro efficacy of our 60 Streptomyces potential strains against M. phaseolina and R. solani, it varied depending on the soil-borne pathogen tested. It is worth mentioning that 40 and 25 out of the 60 actinobacterial strains inhibited the mycelial growth of M. phaseolina and R. solani, respectively. Among the most effective strains, 18 of them showed a common effect against both pathogens, with the CQB-EA2, and -CD-24 being among the strains that showed greater efficacy in inhibiting mycelial growth of the two pathogens. Our results are similar than those described by Dalal et al. [], who evaluated in vitro the antagonistic activity of 15 strains of actinobacteria against various soil-borne soybean pathogens. These authors reported that the 15 strains showed some effectiveness in inhibiting the mycelial growth of R. solani, and six of the 15 strains were also able to inhibit mycelial growth of M. phaseolina []. Similarly, Singh et al. [] evaluated the antifungal activity of 80 strains of actinobacteria against C. truncatum, F. oxysporum, M. phaseolina, and S. rolfsii, highlighting the greater efficacy of Streptomyces sp. strain ACITM-1 on inhibition of mycelial growth of all pathogens. In addition to these, several Streptomyces sp. strains has also been reported for their high efficacy in inhibiting the mycelial growth of soil-borne pathogenic fungi, such as R. solani [], R. bataticola [], M. phaseolina [], F. oxysporum, Alternaria sp., and Magnaporthe oryzae [].
Finally, seed treatments with Streptomyces sp. CBQ-EA-2 and -B-8 were evaluated separately and in combination against infections by M. phaseolina and R. solani in inoculated seedlings of common bean under semi-controlled conditions. In general, the treatments conducted using a mix of the two Streptomyces sp. strains (CBQ-EA-2 + -B-8) showed a significant greater effectiveness against both pathogens compared to treatments performed with the two strains alone. In addition, the effectiveness of the two combined Streptomyces strains in controlling the disease was similar to that observed for the other comparative treatments such as T. harzianum A-34 or the chemical (Celest® Top 312 FS). Interestingly, the DS was higher in seedlings grown in sterilized soils than in those grown in non-sterilized soils, also varying the effectiveness of the different treatments with the soil used. It suggests that the microbiota of the soil is in active and positive interaction with the plant and the pathogen, making difficult the pathogen infection and development. Further research to evaluate the effect of the microbiota of the soils used in this study on the biology of both M. phaseolina and R. solani should be conducted to determine the potential plant-soil-pathogen interactions.
Our results are in concordance with those reported by Yadav et al. [], who showed that Streptomyces sp. S160 reduced the incidence of charcoal rot caused by M. phaseolina under greenhouse conditions in chickpea by 33.3% relative to the control. Similarly, Alekhya et al. [] found that Streptomyces sp. (BCA-546 and CAI-8) significantly reduced charcoal rot in sorghum caused by M. phaseolina under semi-controlled conditions. On the other hand, our results are also in correspondence with those reported by Korayem et al. [] who evaluated the biological activity of S. parvulus strain 10d against R. solani on green beans in a semi-controlled trial with sterilized and non-sterilized soil. These authors showed that seedlings plants treated with a spore suspension of S. parvulus strain 10d showed the highest survival rate (88%) and the lowest DSroot (28%) in the whole of the experiment, showing much better results than those observed for seedlings treated with specific chemicals such as Rhizolex® []. Similarly, Fatmawati et al. [] evaluated 10 strains of actinobacteria against R. solani on soybean seeds under controlled conditions, with Streptomyces spp. strain ASR53 showing the best results in suppressing damping-off disease by 68% and 91% in sterile soil and non-sterile soil, respectively.
This study represents the first report evaluating the effect actinobacteria against the main soil-borne pathogenic fungi of common bean in Cuba. It also shows that Streptomyces spp. should be considered as possible biocontrol alternatives against soil-borne pathogens, not only for their effectiveness in disease control, but also for their role in soil preservation which is highly recommended in the frame of sustainable agriculture. Due to the conclusions of this study are based on experiments under controlled conditions, the most effective Streptomyces strains of this study may be evaluated against the disease under natural field conditions in the future. Altogether will help us to develop potential BCAs for the control of M. phaseolina and R. solani associated with stem and root-rot diseases of common bean in Cuba.
4. Materials and Methods
4.1. Actinobacterial Strains and Growth Conditions
A total of 60 actinobacterial strains isolated from different substrates or geographical areas of west-central Cuba were included in this study. They were recovered from rhizosphere (21), stem (15) or root (9) samples from a wide diversity of hosts, among other sources (Table 6), and stored in the laboratory at 4 °C for no more than 72 h until processing. For isolation of actinobacteria from rhizosphere samples, 1 g of each sample was suspended in 9 mL of sterile distilled water (SDW) by vortexing and incubated in water bath at 55 °C for 6 min. Subsequently, serial dilutions (up to 10–5) were performed. The same procedure was carried out with stem or root samples, but they were previously macerated in a mortar with sterile sand. In all cases, 100 µL aliquots of each dilution were spread in 9.0 cm diameter Petri dishes containing casein-starch agar (CSA) supplemented with filtered cycloheximide (100 µg/mL) and nalidixic acid (30 µg/mL) []. The inoculated Petri dishes were incubated at 28 °C for 28 days in darkness. Based on macroscopic characters i.e., texture, appearance, surface with or without aerial mycelium, colonies of actinobacteria were selected, transferred to CSA, and incubated as described before. Subsequently, spore suspensions were obtained from the pure cultures of each selected strain, and they were kept in 2 mL translucent screw-capped microtubes (Zhejiang Runlab Technology Co., Taizhou, China) at −20 °C in 20% glycerol for further studies []. The collection belongs to the Microbiology Laboratory of the CBQ of the Universidad Central “Marta Abreu” de Las Villas (Cuba).

Table 6.
Origen of actinobacterial strains used in this study.
4.2. In Vitro Effect of Actinobacterial Strains against Macrophomina phaseolina and Rhizoctonia solani: Dual Culture Assays
All the 60 actinobacterial strains (Table 6) were evaluated for their effectiveness inhibiting mycelial growth of M. phaseolina isolate CCIBP-Mp1 and R. solani isolate CCIBP-Rh1 by means in vitro dual culture assays. The two pathogenic fungi were obtained from the collection of plant pathogenic fungi of the Instituto de Biotecnología de las Plantas (IBP) of the Universidad Central Marta Abreu de Las Villas (Cuba), where are maintained growing on PDA at 5 °C in darkness. These isolates were selected due to their high aggressiveness previously tested in the common bean crop [].
Prior to conduct the dual culture assay, the 60 actinobacterial strains were grown on CSA (pH = 7) at 30 °C for seven days in darkness. The inoculum of M. phaseolina and R. solani was prepared by seeding suspensions of mycelial fragments of each isolate on Potato Dextrose Agar (PDA; BioCen, Bejucal, Mayabeque, Cuba) at 28 °C for three days in darkness. In vitro dual culture assays were conducted in 9.0 cm in diameter Petri dishes with PDA []. To this end, a 7.0 mm in diameter mycelial plug of the pathogen was placed at one end of the plate, and another 7.0 mm in diameter mycelial plug of the actinobacterial strain was plated at 50.0 mm apart at the opposite end. Additionally, 7.0 mm in diameter mycelial plugs of M. phaseolina or R. solani isolates were seeded in the center of PDA plates without actinobacteria as a positive growth control. All Petri dishes were incubated at 28 °C in total darkness, and the radial mycelial growth of the two plant pathogens was assessed every 24 h, until seven days of incubation [,]. There were three replicated Petri dishes per actinobacterial strain (n = 60) and plant pathogen (n = 2) or control (n = 2) combination in a completely randomized design [(60 actinobacterial strains × 2 fungal pathogens × 3 Petri dishes) + (2 control × 3 Petri dishes) = 366 Petri dishes in total]. The experiment was performed three times under similar conditions.
For each fungal pathogen, the percentage of the inhibition of mycelial growth was calculated using the following formula:
where ‘rgr’ is the radial growth of M. phaseolina or R. solani in dual culture with each actinobacterial strain, and ‘RGR’ is the radial growth rate of the control treatment (fungal pathogen isolates growing on PDA without actinobacterial strains).
Mycelial growth inhibition (MGI) (%) = [(RGR-rgr)/RGR] × 100
4.3. Qualitative Evaluation of Enzyme Activities of Actinobacterial Strains
Of the 60 strains analyzed in vitro (4.2), the 31 most effective were selected to determine their chitinolytic, cellulolytic, proteolytic activity (Table 6). For chitinolytic activity, all the strains were grown on Colloidal Chitin Agar culture medium (pH = 7) at 28 °C for seven days in darkness []. For cellulolytic activity, the strains were grown on ISP2 (International Streptomyces Project) [] with cellulose (1%, w/v) (pH = 7.2) also at 28 °C for seven days in darkness; then a congo red solution (1%) was added as developer for 15 min; and finally, the congo red solution was removed and NaCl solution (1 M) was added for 15 min []. For proteolytic activity, the strains were grown on ISP2 with 1% skimmed milk at 30 °C for seven days in darkness []. For each parameter evaluated, there were three replicated Petri dishes per strain in a completely randomized design (93 Petri dishes in total), and the experiment was performed three times under similar conditions.
In all cases, after seven days of incubation, the halo surrounding the colonies of the actinobacterial strains was measured (mm) from the center of the inoculated mycelial disc.
4.4. Phenotypic Characterization
Taking into account the macroscopic appearance of the 60 actinobacterial strains evaluated for their effectiveness on MGI of the two pathogens in this study, a total of 11 strains (Table 6) were selected as representative of the main groups with slightly differences on the colony morphology to complete their macro- and microscopic morphological characterization. These strains were grown on CSA as described above, and then, macroscopic colony characters such as presence and color of aerial mycelium, as well as substrate color, shape, elevation, edges and consistency of colonies were recorded [,]. Subsequently, microscopic observations were conducted under optical microscope (LABOMED®, Fremont, CA, USA). Bacterial cell observations were carried out on fresh and stained preparations (simple and Gram staining) to define the shape, clustering and response to Gram stain []. Additional microscopic features such as aerial and vegetative mycelium, mycelial fragmentation, or clumping of spores were recorded by microcultures with lactophenol blue as a contrast stain [], and they were compared with those described in Bergey’s Manual of Bacteriological Determination []. There were three replicated Petri dishes per strain in a completely randomized design (33 Petri dishes in total), and the experiment was performed three times under similar conditions.
4.5. Biochemical Characterization and Assimilation of Carbon Sources
The biochemical characterization using traditional techniques of the same 11 actinobacterial analyzed in the Section 4.4 (Table 6) was evaluated by applying the following tests: catalase, acid production by using different carbohydrate sources (e.g., glucose, mannitol, dextrose, fructose, maltose, raffinose, sucrose and xylose), casein hydrolysis, citrate utilization, indole test, and gelatin hydrolysis []. The ability to produce hydrolytic enzymes for the utilization of polysaccharides such as starch was also determined. The hydrolysis of urea to reveal the activity of the enzyme urease [], methyl red (MR) and Voges Proskauer (VP) tests were carried out according to the ISP []. There were three replicated Petri dishes per strain in a completely randomized design (33 Petri dishes in total), and the experiment was performed three times under similar conditions.
4.6. Molecular Characterization
The actinobacterial strains CBQ-EA-2 and CBQ-B-8 were grown in tryptone-soya broth (BioCen) at 30 °C for three days, and centrifuged at 16,000 rpm. DNA was extracted from the resulting pellet using the PureLink™ Genomic DNA Mini Kit reagent (Invitrogen, Waltham, MA, USA), following the manufacturer’s instructions. The universal primers 27f and 1492r [] for eubacteria were used to amplify the 16S rRNA gene via Polymerase Chain Reaction (PCR). Each reaction mixture contained each primer at 20 µM, dNTPs at 10 µM, 5 µL of 10X MgSO4 and buffer, dimethyl sulfoxide (5%), 1 µg of genomic DNA and 1 unit of taq DNA polymerase, for a final volume of 50 µL. PCR steps included an initial denaturation at 94 °C for 3 min, followed by 30 cycles at 94 °C for 30 s, 47 °C for 33 s and 72 °C for 90 s and a final extension step at 72 °C for 7 min. PCR products were run through 1% agarose gel electrophoresis stained with RedSafe™ dye (iNtRONBiotechnology), followed by purification using the PureLink™ kit (Invitrogen, Waltham, MA, USA) and determination of amplicon quality by spectrophotometry (NanoDrop 2000, ThermoScientific; Waltham, MA, USA). Sequencing was carried out on the ABI310 Prism automated sequencer (Applied Biosystems; Waltham, MA, USA), and the resulting sequences were compared with those in the GenBank database using the BLAST (Basic Local Alignment Search Tool) algorithm to identify closely related sequences [,]. The consensus sequences were uploaded to GenBank data base (Table 6).
4.7. Effect of Actinobacterial Strains against Macrophomina phaseolina and Rhizoctonia solani Infections in Planta
4.7.1. Plant Material
Seedlings of the common bean (P. vulgaris L.) of cv. Quivicán (white testa) were used in this study. The seeds used are registered in the official list of commercial cultivars [] from the ‘UEB Semillas Villa Clara’. Prior to conduct the experiments, the viability of seeds was tested estimating the percentage of germination (%) using a humid chamber at 100% of relative humidity (RH). The seeds were previously disinfected in a serial wash by dipping them first in a 70% ethanol solution for 5 min, then in a 1.5% sodium hypochlorite solution for 15 min, and finally, three times in distilled water for 20 min.
4.7.2. Biological Control Agents and Inoculum Preparation
The actinobacterial strains CBQ-EA-2 and CBQ-B-8 were selected to conduct the experiments in planta because they were considered as representative of the strains showing high (CBQ-EA-2; MGI = 70.4 and 77.4% for M. phaseolina and R. solani, respectively) and moderate (CBQ-B-8; MGI = 63.1 and 69.0% for M. phaseolina and R. solani, respectively) effectiveness to both pathogens in the dual culture assays. In addition, their morphological, biochemical, and extracellular enzymatic characteristics together with their molecular characterization were also taken into account to ensure that they belong to Streptomyces genus together. To prepare the inoculum of the two strains for seed treatments (see below), 20 µL of the original spore suspension preserved at −20 °C in 20% glycerol were firstly added in a 5 mL sterile plastic tubes with tryptone soy broth (BioCen) and incubated at 28 °C for 48 h []. Then, they were transferred to 250 mL Erlenmeyer flasks with 100 mL of tryptone soy broth and shaken in a Gerhardt orbital shaker at 28 °C at a speed of 120 G for 3 days. Finally, the inoculum of each actinobacterial strain was adjusted at 1 × 108 spores mL−1 using a hemocytometer.
Additionally, Trichoderma harzianum strain A-34 belonging to the Plant Health Research Institute (INISAV, La Habana, Cuba) was also included in this experiment as a BCA for comparative purposes. The selected strain is the active ingredient of a bioproduct for the control of phytopathogenic soil fungi, foliar diseases and nematodes commonly used in Cuba []. To prepare the inoculum of T. harzianum A-34 for seed treatments (see below), sterile 250 mL Erlenmeyer flasks with 100 mL of Potato Dextrose Broth (PDB; BioCen) were inoculated by adding five 10-mm in diameter mycelial plugs of T. harzianum A-34 obtained from the active margin of colonies previously grown on PDA at 28 °C in darkness for 72 h. Then, the inoculated Erlenmeyer flask were shaken as described above, and the inoculum was adjusted at 1 × 108 spores mL−1.
4.7.3. Soil Inoculation with Macrophomina phaseolina and Rhizoctonia solani
The effectiveness of the selected BCAs was evaluated in planta against M. phaseolina isolate CCIBP-Mp 2, and R. solani isolate CCIBP-Rh1. To prepare the inoculum of both isolates, 1-L Erlenmeyer flasks were filled with 200 g of an artificial substrate (risk husk, part rice grain and distilled water; 3:1:0.5, weight:weight:volume) and sterilized at 120 °C for 1 h. Subsequently, the flasks were seeded with five 1.0-cm in diameter of mycelial plugs of M. phaseolina isolate CCIBP-Mp 2 or R. solani CCIBP-Rh1 anastomosis groups (AG-4_HGI), taken from the edge of the active growing colonies previously grown on PDA as described before. The inoculated flasks were incubated at 28 °C in darkness for 10 days, and they were manually shaken each 2 days to favor the homogeneous colonization of the substrate []. In this study, a medium washed fluffy brown soil [] non-sterilized and sterilized (120 °C for 20 min in cycles of three consecutive days, and subsequent sterility testing) was used in this study. In all cases, and for each pathogen, the inoculation was carried out at 2% by homogenizing the colonized substrate with the soil [].
Subsequently, plastic pots were filled with 1.5 Kg of this mix. After 48 h of mix preparation (soil + colonized substrate), four common bean seeds previously treated were sown per plastic pot, and soil moisture was kept at 80% of the field capacity (FC).
4.7.4. Seed Treatments, Growth Conditions and Experimental Design
Seed treatments were conducted by dipping the seeds for 30 min in the following suspensions: (i) actinobacterial strain CBQ-EA-2 at 1 × 108 spores mL−1; (ii) actinobacterial strain CBQ-B-8 at 1 × 108 spores mL−1; (iii) a mix of the actinobacterial strains CBQ-EA-2 and CBQ-B-8 at 1 × 108 spores mL−1 global concentration; (iv) T. harzianum strain A-34 at 1 × 108 spores mL−1; and (v) Celest® Top 312 FS (Syngenta®; Basilea, Switzerland) prepared in a water suspension of 192 mL of active ingredient per kg of seeds. The latter chemical compound was included for comparison purposes. Additionally, seeds dipped for 30 min in SDW were also included as non-treated control seeds, and lots of non-treated seeds were sowed in plastic pots with inoculated soil (treatment (vi): positive control) as well as in plastic pots with non-inoculated soil (treatment (vii): negative control).
After more than 50% of the seeds emerged, seedlings were treated every three days by wetting the substrate with 1 mL of the respective biological treatment (actinobacterial or T. harzianum) adjusted to 1 × 108 spores mL−1 until the end of the experiment [28 days after sowing (das)]. Both positive and negative controls and the chemical treatment were wetted every three days with 1 mL of SDW.
For each pathogen, a split-plot design was used with soil (n = 2; sterilized and non-sterilized) as the main plot factor and treatments (n = 7) as sub-plot factor; with ten pots (replicates) per treatment, and 4 seeds per replicate (n = 40). They were maintained in a CBQ greenhouse at 28 °C, 70% RH and 1100 μmol m−2 s−1 light intensity.
4.7.5. Disease Severity Assessment
For treated seedlings inoculated with M. phaseolina, disease severity (DS) was assessed at 35 days after inoculation using the following DS rating scale: 1 = no visible disease symptoms; 3 = wilt restricted to cotyledons, lower stem tissues with small necrotic lesions; 5 = 10% of hypocotyl and lower stem tissues showing lesions, fungal fruiting structures starting the development in the affected tissues, 7 = 25% of hypocotyl and lower stem tissues showing lesions, with development of fungal fruiting structures in the affected tissues; and 9 = ≥50% of hypocotyl and lower stem tissues with lesions, with abundant development of fungal fruiting structures []. Subsequently, a DS index was estimated using the following formula:
in which ni = number of seedlings in the DS development stage i, sti = value of the DS stage (1–9), N = total number of plants assessed, and K = largest scale level (9) [].
Regarding treated seedlings inoculated with R. solani, DS was evaluated separately on stem and roots tissues at 28 days after inoculation by using the following DS rating scales: (i) DSstem: 1 = absence of lesions on hypocotyl, 2 = superficial lesions (yellow-brown discoloration) on hypocotyl, 3 = deep tissue lesions, and 4 = seedlings dead or wilted []; (ii) DSroot: 0 = healthy seedlings, 1 = yellowish-brown discoloration near hypocotyl, 2 = yellowish-brown discoloration plus lesions or brown spots near hypocotyl, 3 = entirely brown surface or lesions covering more than 75% of root surface, and 4 = pre-emergence damping off, seedlings dead or wilted []. Subsequently, a DS index was estimated for each tissue using the following formulas:
in which ni = number of stems or roots in the DS development stage i, sti = value of the DS stage (1–4 and 0–4 for stems and roots, respectively), N = total number of plants assessed, and K = largest scale level (4 in all cases) []. Furthermore, for each combination of soil and treatment, the incidence of disease (DI; % of affected plants) and mortality (% of dead plants) were estimated at 28 days after inoculation.
Ungerminated seeds or plants with lesions on the hypocotyl, roots and/or stem were subjected to wet chamber and microscope preparations to confirm the identity of the inoculated pathogens.
4.8. Data Analyses
Data from the repetitions of each experiment were combined after checking for homogeneity of the experimental error variances by the F test (p ≥ 0.05). Subsequently, data were tested for normality and homogeneity of variances prior to conduct analyses of variance (ANOVA). For the dual culture assay, factorial ANOVA was conducted with MGI as dependent variable, and actinobacterial strains, fungal pathogens and their interaction as independent variables. Significant differences were observed for the two independent variables as well as for their interaction (p < 0.0001 in any cases). Thus, independent ANOVA were conducted to determine differences between actinobacterial strains against each fungal pathogen. For each fungal pathogen, mean values were compared using Tukey’s honestly significant difference (HSD) tests at p = 0.05 []. For the enzymatic activity, data of the halo (mm) for each of the three parameters evaluated were analyzed separately by the non-parametric Kruskal-Wallys test due to the assumptions of normality and homogeneity of variances were not fulfilled even though logarithmically, arcsine or square root transformation of the data were conducted. Data from the actinobacterial strains that not develop halo (0.0 mm) were excluded from the statistical analysis in any cases. Mean values were compared using Dunn’s comparisons test at p = 0.05. In the in planta experiment, data of total DS (seedlings inoculated with M. phaseolina), and DSstem and DSroot (seedlings inoculated with R. solani) were tested for normality and homogeneity of variances prior to conduct analyses of variance (ANOVA). Data from negative control were omitted since no symptoms were observed in all cases. For each dependent variable, a split-plot ANOVA was conducted with soil (n = 2) as main-plot factor and treatments (n = 6) as the subplot factor. Due to significant differences were observed in all cases for the two independent variables as well as for their interaction (p < 0.005), independent ANOVA were conducted to determine differences between treatments for each disease. The treatment means of total DS, or DSstem and DSroot were compared according to Fisher’s protected LSD test at p = 0.05 []. For both inoculated plants with M. phaseolina and R. solani, data on the final DI (% of affected plants) and mortality (% of dead plants) were analyzed by multiple comparisons for proportions tests at p = 0.05 []. Additionally, for plants inoculated with R. solani, the Pearson correlation coefficients (r) between the DSstem and DSroot were calculated using the average values of the two variables for each of the treatment evaluated in sterilized or non-sterilized soil (n = 6 in each type of soil). All data analyses were conducted using Statistix 10 [].
5. Conclusions
The qualitative characterization of the extracellular enzyme activities, the antagonism of the Streptomyces spp. strains, as well as the in vivo studies against M. phaseolina and R. solani under semi-controlled conditions have allowed us to characterize promising strains as BCAs, and to have a biological alternative in the framework of the integrated management of the main common bean diseases caused by soil pathogens in Cuba. To confirm our laboratory results, the research should and will be evaluated under natural field conditions in further studies.
Author Contributions
Experimental and laboratory tasks, data analysis, wrote and edit the manuscript, M.D.-D.; supervising, review and edit the manuscript, A.B.-C.; review the manuscript, A.T.; sampling, isolation and collection of the actinobacterial strains used in this study, R.M.-M.; experimental design and data analysis, S.S.-R.; experimental and laboratory tasks, R.D.C.-S., isolation of actinobacterial strains, M.G.-B., data analyses, review and edit the manuscript, funding for Open Access publishing, C.A.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Institutional Project 9453: “Search for new strains of actinomycetes that produce antibiotics” of the Center of Chemical Bioactives (CBQ) of the Central University “Marta Abreu” de Las Villas (UCLV), Cuba. M.D-D is holder of scholarships from the Ibero-American University Association for Postgraduate Studies (AUIP) to carry out her pre-doctoral stages (3 stages of 3 months) at the Department of Agronomy at the University of Córdoba (UCO; Spain). We also acknowledge financial support from the Spanish Ministry of Science and Innovation, the Spanish State Research Agency, through the Severo Ochoa and María de Maeztu Program for Centres and Units of Excellence in R&D (Ref. CEX2019-000968-M).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank the Center of Chemical Bioactives (CBQ) of the Central University “Marta Abreu” de Las Villas (UCLV; Cuba) since all the Streptomyces strains used in this study were donated by the CBQ to develop the present study. The authors thank Edisleidy Águila Jiménez, Yoandry Arencibia, Eliannys Rodríguez Soris and Juan Pablo Mesa Marcillas and Technician Marlén Casanova González for their skillful technical assistance, as well as the colleagues of Department of Agronomy of the University of Córdoba (Spain) for host during the pre-doctoral stages.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Singh, G.; Dukariya, G.; Kumar, A. Distribution, Importance and Diseases of Soybean and Common Bean: A Review. Biotechnol. J. Int. 2020, 24, 86–98. [Google Scholar] [CrossRef]
- Food and Agricultural Organisation of the United Nations (FAOSTAT) Website. Available online: http://www.fao.org/faostat/es/#data/QCL/visualize (accessed on 11 August 2021).
- Ulloa, J.A.; Ulloa, P.R.; Ramírez, R.J.; Ulloa, R.B. El frijol (Phaseolus vulgaris): Su importancia nutricional y como fuente de fitoquímicos. Rev. Fuente. 2011, 3, 5–9. [Google Scholar]
- Were, S.A.; Narla, R.; Mutitu, E.W.; Muthomi, J.W.; Munyua, L.M.; Roobroeck, D.; Valauwe, B. Biochar and Vermicompost Soil Amendments Reduce Root Rot Disease of Common Bean (Phaseolous vulgaris L.). Afr. J. Biol. Sci. 2021, 3, 176–196. [Google Scholar] [CrossRef]
- González, M. Enfermedades Fungosas Del Frijol en Cuba; Técnica, C., Ed.; Científico-Técnica: La Habana, Cuba, 1988; pp. 39–60. [Google Scholar]
- Olaya, G.; Abawi, G.S. Effect of water potential on mycelial growth and on production and germination of sclerotia of Macrophomina phaseolina. Plant Dis. 1996, 80, 1347–1350. [Google Scholar] [CrossRef]
- Spedaletti, Y.; Cardenas Mercado, G.; Taboada, G.; Aban, C.; Aparicio, M.; Rodriguero, M.; Vizgarra, O.; Sühring, S.; Galíndez, G.; Galván, M. Molecular identification and pathogenicity of Rhizoctonia spp. recovered from seed and soil samples of the main bean growing area of Argentina. Aust. J. Crop Sci. 2017, 11, 952–959. [Google Scholar] [CrossRef]
- Zhao, X.; Ni, Y.; Liu, X.; Zhao, H.; Wang, J.; Chen, Y.C.; Liu, H. A simple and effective technique for production of pycnidia and pycnidiospores by Macrophomina phaseolina. Plant Dis. 2020, 104, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de la Agricultura (MINAG). Guía Técnica Para el Cultivo de Frijol en Cuba; Instituto de Investigaciones Hortícolas “Liliana Dimitrova”: Buenaventura, Cuba, 2008; 18p. [Google Scholar]
- González, D. Micoflora Patogénica en Semillas de Frijol (Phaseolus vulgaris) y Habichuela (Vigna Unguiculata Sesquipedalis), su Efecto en la Germinación y su Control; Trabajo de Diploma; Facultad de Ciencias Agropecuarias; UCLV: Santa Clara, Cuba, 2007; pp. 18–30. [Google Scholar]
- Abu-Tahon, M.A.; Mogazy, A.M.; Isaac, G.S. Resistance assessment and enzymatic responses of common bean (Phaseolus vulgaris L.) against Rhizoctonia solani damping-off in response to seed presoaking in Vitex agnus-castus L. oils and foliar spray with zinc oxide nanoparticles. S. Afr. J. Bot. 2022, 146, 77–89. [Google Scholar] [CrossRef]
- Muharrem, T.; Kılıçoğlu, M.Ç.; Ismail, E. Characterization and pathogenicity of Rhizoctonia isolates collected from Brassica oleracea var. acephala in Ordu, Turkey. Phytoparasitica 2020, 48, 273–286. [Google Scholar]
- Sabaté, D.C.; Petroselli, G.; Erra-Balsells, R.; Carina Audisio, M.; Brandan, C.P. Beneficial effect of Bacillus sp. P12 on soil biological activities and pathogen control in common bean. Biol. Control 2019, 141, 104–131. [Google Scholar] [CrossRef]
- Torres, M.J.; Pérez-Brandan, C.; Sabaté, D.C.; Petroselli, G.; Erra-Balsells, R.; Audisio, M.C. Biological activity of the lipopeptide-producing Bacillus amyloliquefaciens PGPBacCA1 on common bean Phaseolus vulgaris L. pathogens. Biol. Control 2016, 105, 93–99. [Google Scholar] [CrossRef]
- Mayo-Prieto, S.; Porteous-Álvarez, A.J.; Mezquita-García, S.; Rodríguez-González, Á.; Carro-Huerga, G.; del Ser-Herrero, S.; Casquero, P.A. Influence of Physicochemical Characteristics of Bean Crop Soil in Trichoderma spp. Development. Agronomy 2021, 11, 274. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Giovanadri, D.; Stefani, E. Growth Promotion and Biocontrol Activity of Endophytic Streptomyces spp. In Prime Archives in Molecular Sciences, 2nd ed.; Giampietro, L., Ed.; Vide Leaf: Hyderabad, India, 2021; 55p. [Google Scholar]
- Olanrewaju, O.S.; Babalola, O.O. Streptomyces: Implications an dinteractions in plant growth promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirling, E.T.; Gottlieb, D. Methods for characterization of Streptomyces species. Int. J. Syst. Evol. Microbiol. 1966, 16, 313–340. [Google Scholar] [CrossRef] [Green Version]
- Bergey, D.H.; Holt, J.G.; Hensyl, R. Bergey’s Manual of Determinative Bacteriology; Lippincott Williams and Wilkins: Baltimore, MD, USA, 2005; Volume 2, pp. 1208–1232. [Google Scholar]
- Ayuningrum, D.; Jati, O. Screening of actinobacteria-producing amylolytic enzyme in sediment from Litopenaeus vannamei (Boone, 1931) ponds in Rembang District, Central Java, Indonesia. Biodiversitas 2021, 22, 1819–1828. [Google Scholar] [CrossRef]
- Manigundan, K.; Joseph, J.; Ayswarya, S.; Vignesh, A.; Vijayalakshmi, G.; Soytong, K.; Radhakrishnan, M. Identification of biostimulant and microbicide compounds from Streptomyces sp. UC1A-3 for plant growth promotion and disease control. Int. J. Agric. Technol. 2020, 16, 1125–1144. [Google Scholar]
- Rani, K.; Parashar, A.; Wati, L. Estimation of hydrolyzing potential of chickpea actinomycetes for degradation of complex compounds through enzymes and acid production. Pharma. Innov. 2021, 10, 220–223. [Google Scholar]
- Liu, X.; Dou, G.; Ma, Y. Potential of endophytes from medicinal plants for biocontrol and plant growth promotion. J. Gen. Plant Pathol. 2016, 82, 165–173. [Google Scholar] [CrossRef]
- Selim, M.S.M.; Abdelhamid, S.A.; Mohamed, S.S. Secondary metabolites and biodiversity of actinomycetes. J. Genet. Eng. Biotechnol. 2021, 19, 72. [Google Scholar] [CrossRef]
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef]
- Law, J.W.F.; Tan, K.X.; Wong, S.H.; Ab Mutalib, N.S.; Lee, L.H. Taxonomic and characterization methods of Streptomyces: A review. Prog. Microb. Mol. Biol. 2018, 1, a0000009. [Google Scholar] [CrossRef] [Green Version]
- Dalal, J.M.; Kulkarni, N.S. Antagonistic and Plant Growth Promoting Potentials of Indigenous Endophytic Actinomycetes of Soybean (Glycine max (L.) Merril). CIBTech J. Microbiol. 2014, 3, 1–12. [Google Scholar]
- Singh, C.; Parmar, R.S.; Kumar, A.; Jadon, P. Characterization of actinomycetes against phytopathogenic fungi of Glycine max (L.). Asian J. Pharm. Clin. Res. 2016, 9, 216–219. [Google Scholar]
- Fatmawati, U.; Meryandini, A.; Nawangsih, A.A.; Wahyudi, A.T. Damping-off disease reduction using actinomycetes that produce antifungal compounds with beneficial traits. J. Plant Prot. Res. 2020, 60, 233–243. [Google Scholar]
- Khendkar, A.S.; Deshpande, A.R. Isolation and screening of Streptomyces for biocontrol potential against Rhizoctonia bataticola infection of soybean. Indian J. Appl. Microbio. 2018, 21, 78–86. [Google Scholar]
- Yadav, A.K.; Yandigeri, M.; Vardhan, S.; Sivakumar, G.; Rangeshwaran, R.; Tripathi, C.P.M. Streptomyces sp. S160: A potential antagonist against chickpea charcoal root rot caused by Macrophomina phaseolina (Tassi) Goid. Ann. Microbiol. 2014, 64, 1113–1122. [Google Scholar] [CrossRef]
- Alekhya, G.; Sharma, R.; Gopalakrishnan, S. Streptomyces spp., a potential biocontrol agent of charcoal rot of sorghum caused by Macrophomina phaseolina. Indian J. Plant Prot. 2016, 44, 222–228. [Google Scholar]
- Korayem, A.S.; Abdelhafez, A.A.; Zaki, M.M.; Saleh, E.A. Biological control of green bean damping-off disease caused by Rhizoctonia solani by Streptomyces parvulus strain 10d. Egypt. J. Microbiol. 2020, 55, 87–94. [Google Scholar] [CrossRef]
- El Karkouri, A.; Assou, S.A.; El Hassouni, M. Isolation and screening of actinomycetes producing antimicrobial substances from an extreme Moroccan biotope. Pan Afr. Med. J. 2019, 33, 329. [Google Scholar] [CrossRef]
- Bernal, M.G.; Campa-Córdova, Á.I.; Saucedo, P.E.; González, M.C.; Marrero, R.M.; Mazón-Suástegui, J.M. Isolation and in vitro selection of actinomycetes strains as potential probiotics for aquaculture. Vet. World 2015, 8, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Díaz, C.M. Incidence of Rhizoctonia spp., Sclerotium rolfsii and Macrophomina phaseolina on common bean in Villa Clara. Ph.D. Thesis, Universidad Central “Marta Abreu” de Las Villas, Santa Clara, Cuba, 2011. [Google Scholar]
- Sellem, I.; Triki, M.; Elleuch, L.; Cheffi, M.; Chakchouk, A.; Smaoui, S.; Mellouli, L. The use of newly isolated Streptomyces strain TN258 as potential biocontrol agent of potato tubers leak caused by Pythium ultimum. J. Basic Microbiol. 2017, 57, 393–401. [Google Scholar] [CrossRef]
- Misk, A.; Franco, C. Biocontrol of chickpea root rot using endophytic Actinobacteria. BioControl 2011, 56, 811–822. [Google Scholar] [CrossRef]
- Cuesta, G.; García-de-la-Fuente, R.; Abad, M.; Fornes, F.; Torriani-Gorini, A.; Yagil, E.; Silver, S. Isolation and identification of actinomycetes from a compost-amended soil with potential as biocontrol agents. J. Environ. Manag. 2012, 95, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Kawase, T.; Saito, A.; Sato, T.; Kanai, R.; Fujii, T.; Nikaidou, N.; Miyashita, K.; Watanabe, T. Distribution and phylogenetic analysis of family 19 chitinases in Actinobacteria. Appl. Environ. Microbiol. 2004, 70, 1135–1144. [Google Scholar] [CrossRef] [Green Version]
- Meena, B.; Rajan, L.A.; Vinithkumar, N.V.; Kirubagaran, R. Novel marine actinobacteria from emerald Andaman & Nicobar Islands: A prospective source for industrial and pharmaceutical byproducts. BMC Microbiol. 2013, 13, 13–45. [Google Scholar]
- El-Sersy, N.A.; Abd-Elnaby, H.; Abou-Elela, G.M.; Ibrahim, H.A.; El-Toukhy, N.M. Optimization, economization and characterization of cellulase produced by marine Streptomyces ruber. Afr. J. Biotechnol. 2010, 9, 6355–6364. [Google Scholar]
- Ara, I.; Bukhari, N.A.; Wijayanti, D.R.; Bakir, M.A. Proteolytic activity of alkaliphilic, salt-tolerant actinomycetes from various regions in Saudi Arabia. Afr. J. Biotechnol. 2012, 11, 3849–3857. [Google Scholar]
- Franco-Correa, M. Utilización de los actinomicetos en procesos de biofertilización. Rev. Peru. Biol. 2009, 16, 239–242. [Google Scholar] [CrossRef]
- Oskay, A.M.; Üsame, T.; Cem, A. Antibacterial activity of some actinomycetes isolated from farming soils of Turkey. Afr. J. Biotechnol. 2004, 3, 441–446. [Google Scholar]
- Selvakumar, P.; Viveka, S.; Prakash, S.; Jasminebeaula, S.; Uloganathan, R. Antimicrobial activity of extracellularly synthesized silver nanoparticles from marine derived Streptomyces rochei. Int. J. Pharma Bio Sci. 2012, 3, 188–197. [Google Scholar]
- Saxena, A.; Upadhyay, R.; Kango, N. Isolation and identification of actinomycetes for production of novel extracellular glutaminase free L-asparaginase. Indian J. Exp. Biol. 2015, 53, 786–793. [Google Scholar]
- Coombs, J.T.; Franco, C.M. Isolation and identification of actinobacteria from surface-sterilized wheat roots. Appl. Environ. Microbiol. 2003, 69, 5603–5608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Cole, J.R.; Chai, B.; Farris, R.J.; Wang, Q.; Kulam, S.A.; Mcgarrell, D.M.; Garrity, G.M.; Tiedje, J.M. The Ribosomal Database Project (RDP-II): Sequences and tools for high-throughput rRNA analysis. Nucleic Acids Res. 2005, 33, 294–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministerio de la Agricultura (MINAG). Listado oficial de Variedades Comerciales; Dirección de Semillas y Recursos Filogenéticos; CENSA: La Habana, Cuba, 2016; 41p. [Google Scholar]
- Hamdali, H.; Hafidi, M.; Virolle, M.J.; Ouhdouch, Y. Growth promotion and protection against damping-off of wheat by two rock phosphate solubilizing actinomycetes in a P-deficient soil under greenhouse conditions. Appl. Soil Ecol. 2008, 40, 510–517. [Google Scholar] [CrossRef]
- Stefanova, M.; Díaz de Villegas, M.E.; Mena, C.J. Control biológico de enfermedades de plantas en Cuba. In Control Biológico de Enfermedades de Plantas en América Latina y el Caribe; Bettiol, W., Rivera, M.C., Mondino, P., Montealegre, J.R., Colmenarez, Y.C., Eds.; Faculdad de Agronomia, Universidad de la Republica: Montevideo, Uruguay, 2014; pp. 201–204. ISBN 978-9974-0-1091-8. [Google Scholar]
- Suryawanshi, P.P.; Krishnaraj, P.U.; Suryawanshi, M.P. Evaluation of actinobacteria for biocontrol of sheath blight in rice. J. Pharmacogn. Phytochem. 2020, 9, 371–376. [Google Scholar]
- Hernández, J.A.; Pérez, J.M.; Bosch, I.D.; Rivero, S.N. Clasificación de los Suelos de Cuba; Ediciones INCA: Mayabeque, Cuba, 2015; pp. 10–75. [Google Scholar]
- Hernández Pérez, D.; Díaz Castellanos, M.; Quiñones Ramos, R.; Santos Bermúdez, R.; Portal González, N.; Herrera Isla, L. Control de Rhizoctonia solani en frijol común con rizobacterias y productos naturales. Cent. Agríc. 2018, 45, 55–60. [Google Scholar]
- Van Schoonhoven, A.; Corrales, P. Sistema Estándar Para la Evaluación de Germoplasma de Frijol; CIAT: Cali, Colombia, 1987; 56p. [Google Scholar]
- Townsend, G.R.; Heuberger, J.W. Methods for estimating losses caused by diseases in fungicide experiments. Plant Dis. Rep. 1943, 27, 340–343. [Google Scholar]
- Bradley, C.A.; Hartman, G.L.; Nelson, R.L.; Müller, D.S.; Pedersen, W.L. Response of ancestral soybean lines and comercial cultivars to Rhizoctonia solani root and hypocotyls rot. Plant Dis. 2001, 85, 1091–1095. [Google Scholar] [CrossRef] [Green Version]
- Keijer, J.; Korman, M.G.; Dullemans, A.M.; Houterman, P.M.; De Bree, J.; Van Silfhout, C.H. In vitro analysis of host plant specificity in Rhizoctonia solani. Plant Pathol. 1997, 46, 659–669. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H. Bioestadística: Principios y Procedimientos, 2nd ed; McGraw Hill: Bogotá, Colombia, 1985; 613p. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pte: New Delhi, India; Pearson Education: Singapore, 2010. [Google Scholar]
- Analytical Software. StatistixUser’s Manual. Analytical Software, Tallahassee, FL, USA. 2013. Available online: https://www.scribd.com/document/381816978/Statistix-10-Manual (accessed on 18 October 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).