Multi-Species Prediction of Physiological Traits with Hyperspectral Modeling
Abstract
:1. Introduction
2. Results
2.1. Diverse Responses of Sorghum and Corn to Water Deficit and Nitrogen Deficiency
2.2. Prediction of Relative Water Content Using One Species Model
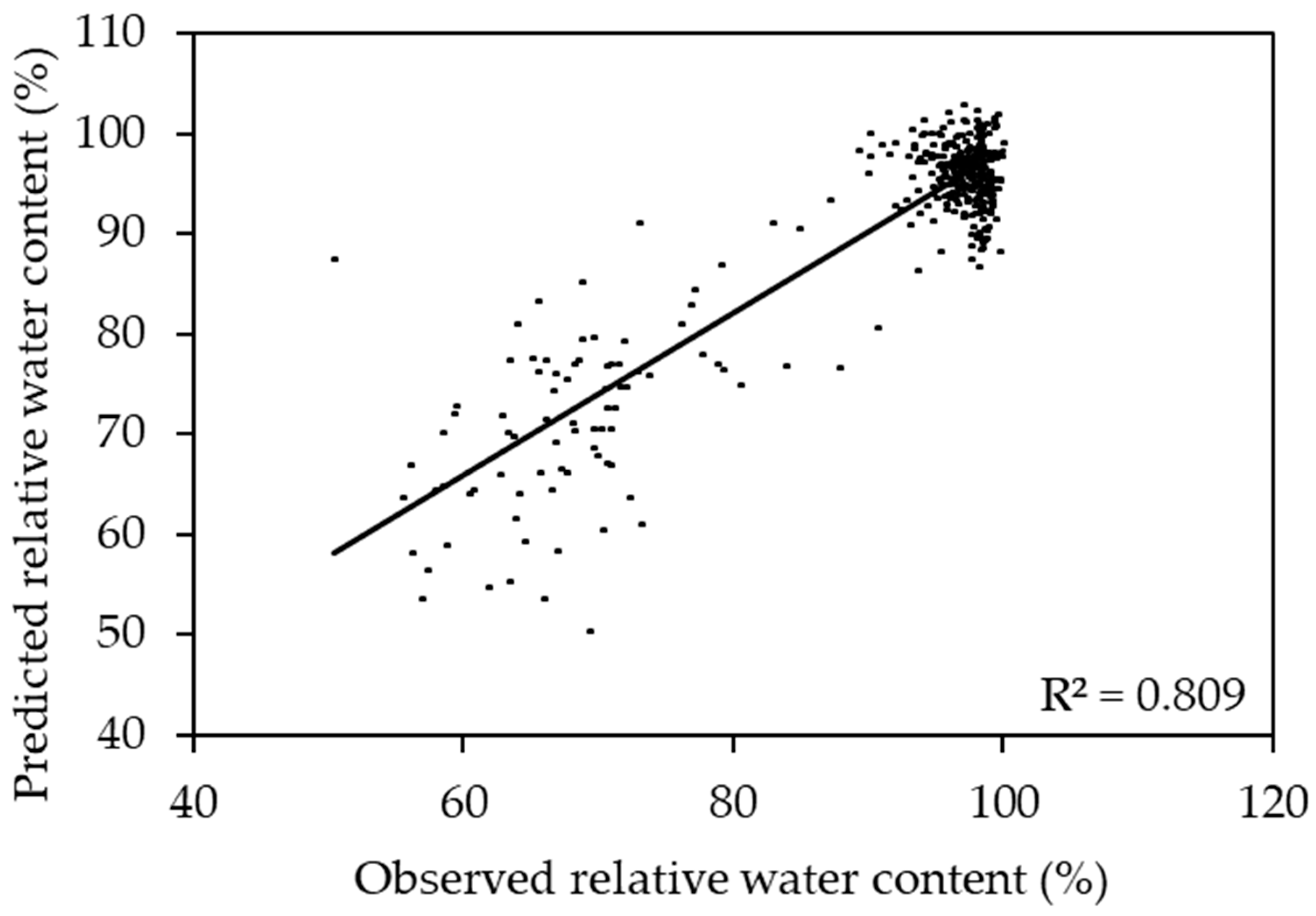
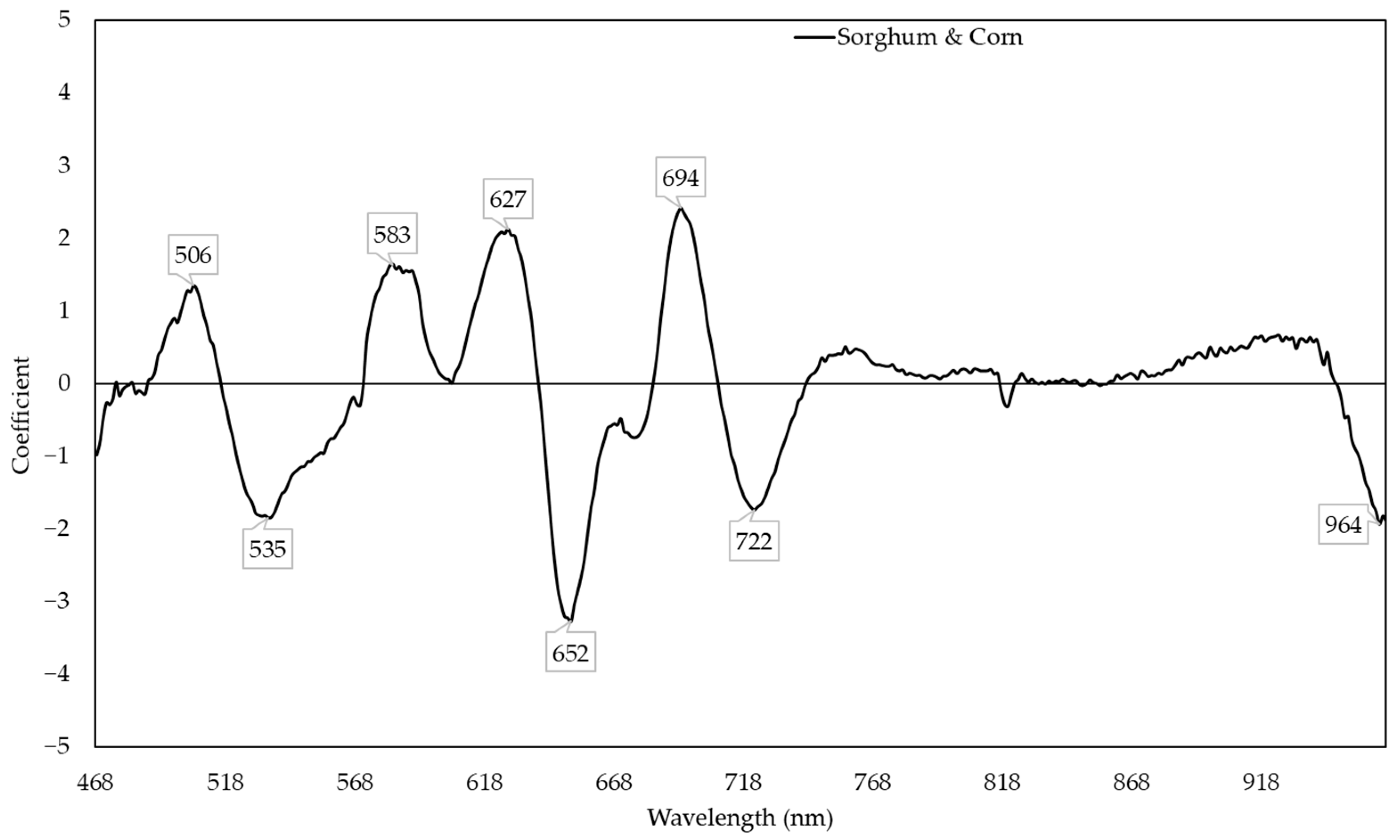
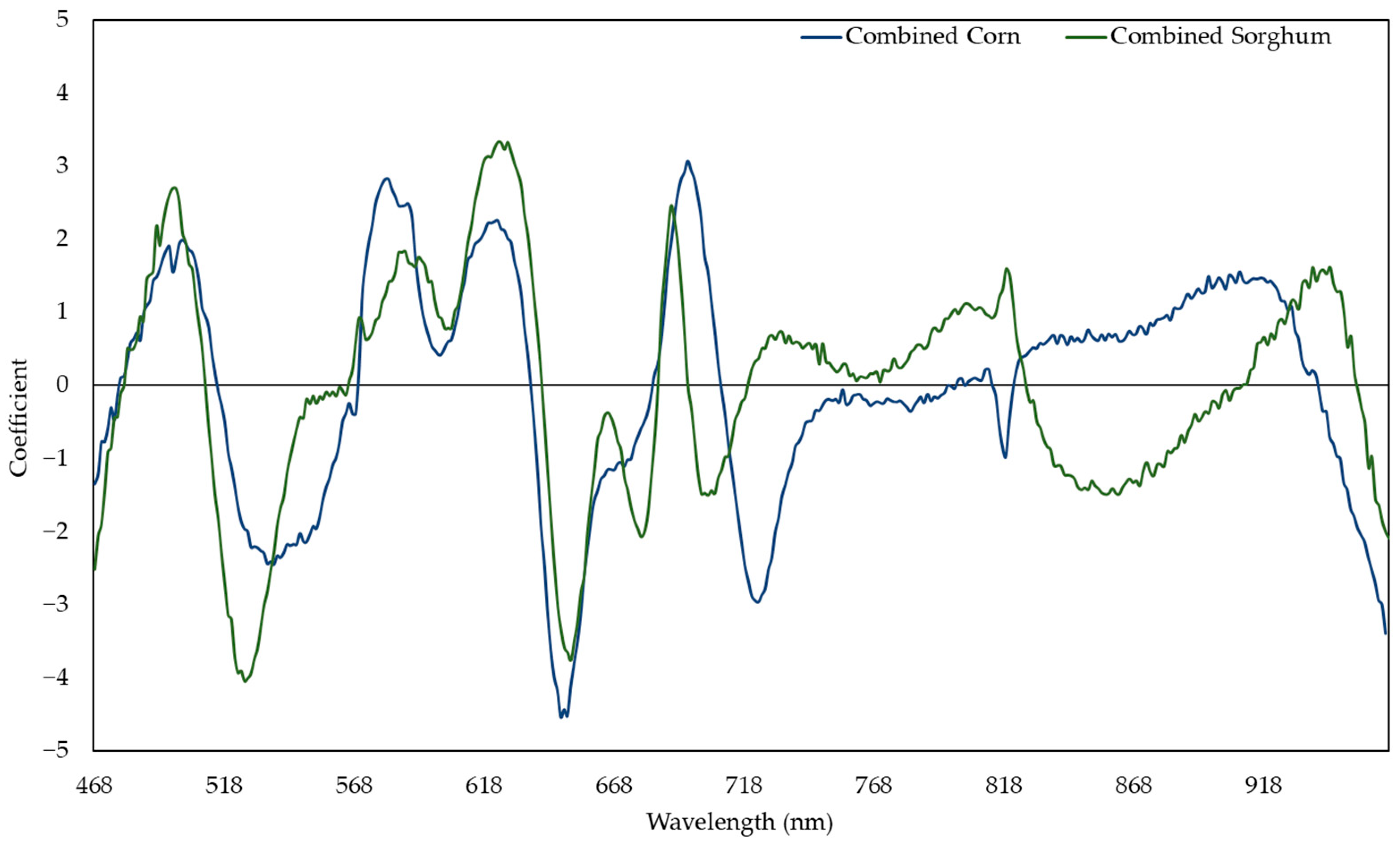
2.3. Prediction of Relative Water Content Using a Two Species Model
2.4. Prediction of Nitrogen Content Using One Species Models
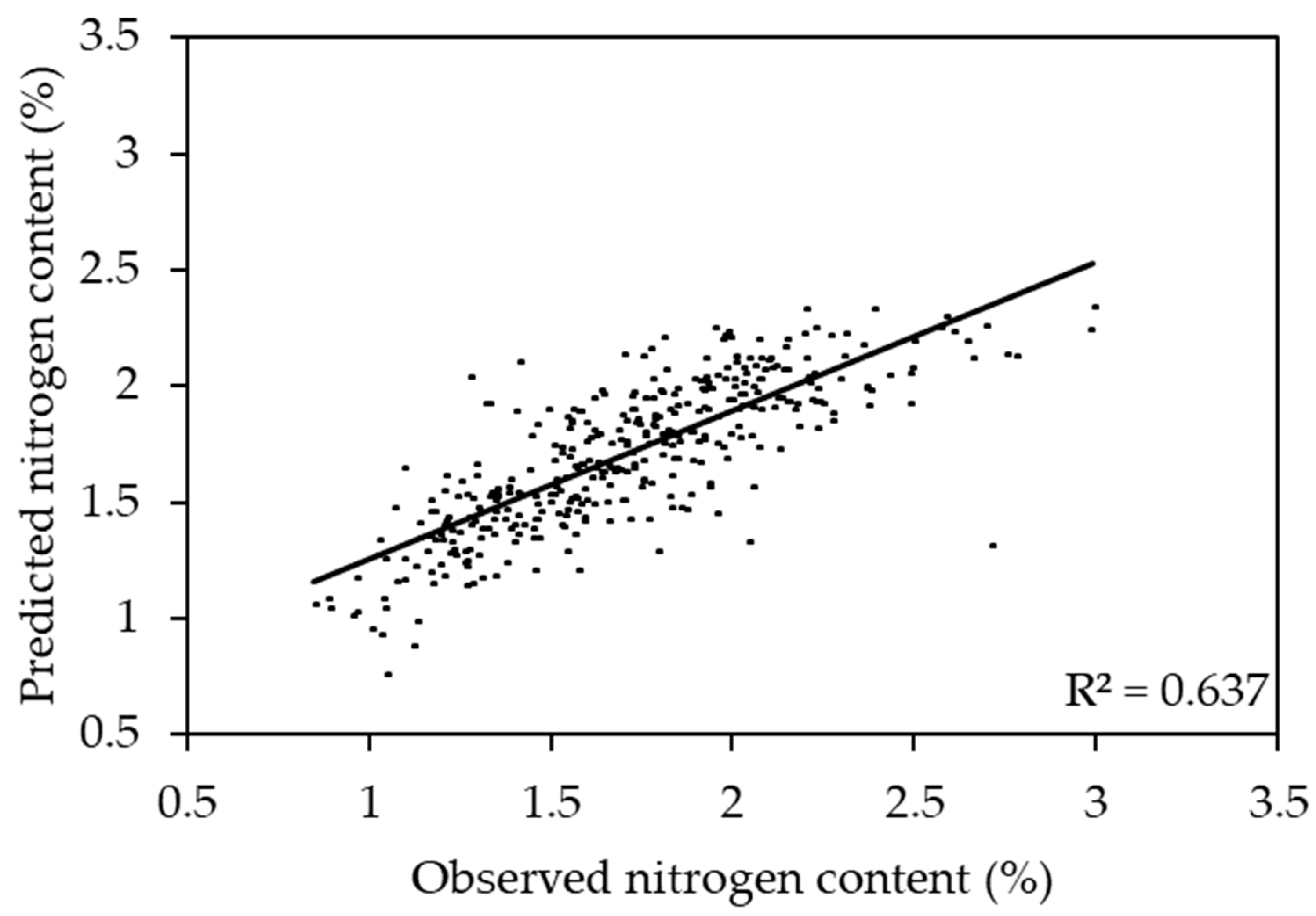
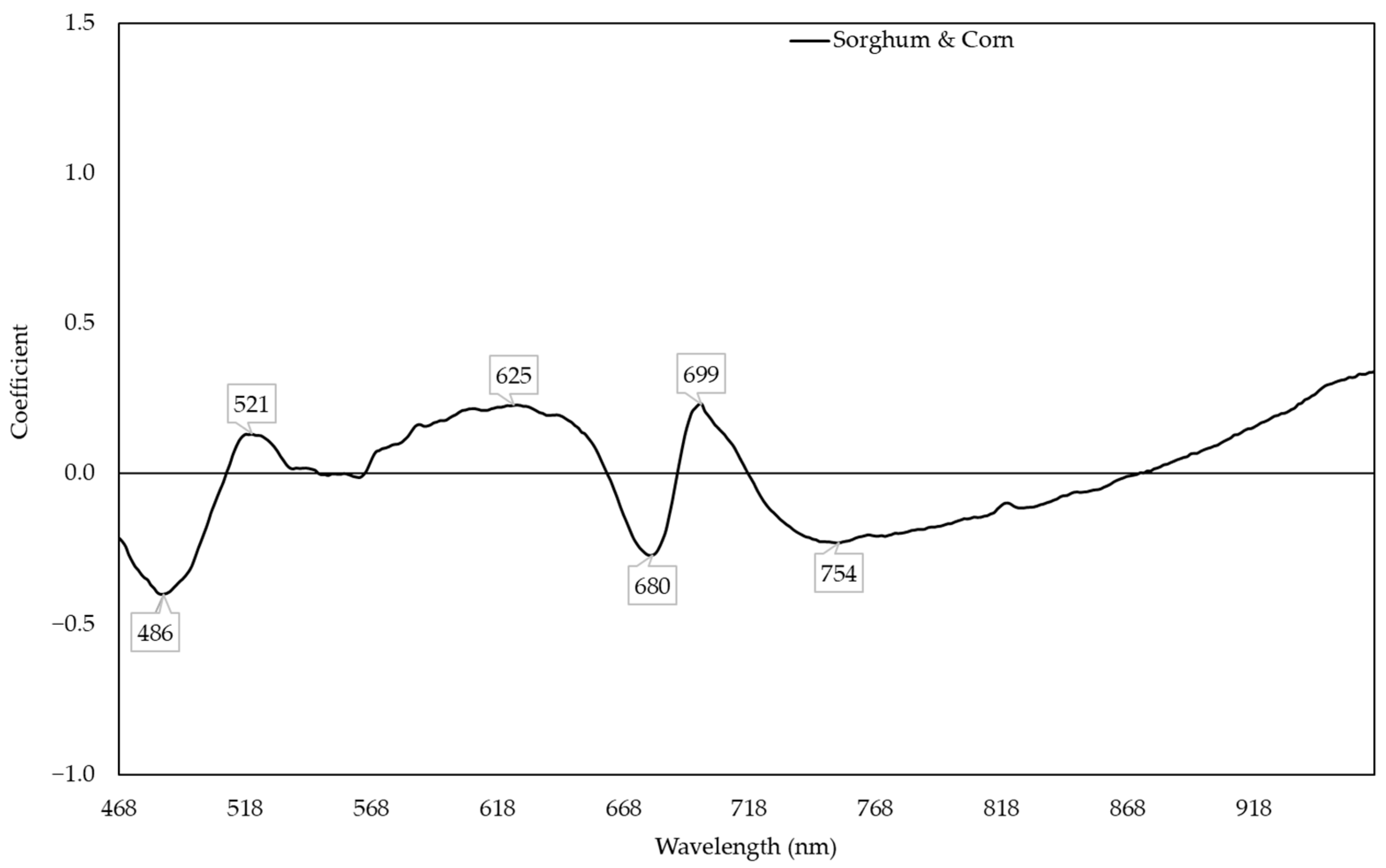
2.5. Prediction of Nitrogen Content Using Two Species Model
3. Discussion
3.1. Sorghum and Corn Models for Predicting the Relative Water Content
3.2. Sorghum and Corn Models for Predicting Nitrogen Content
3.3. Similarity in Adaptation and Genetics
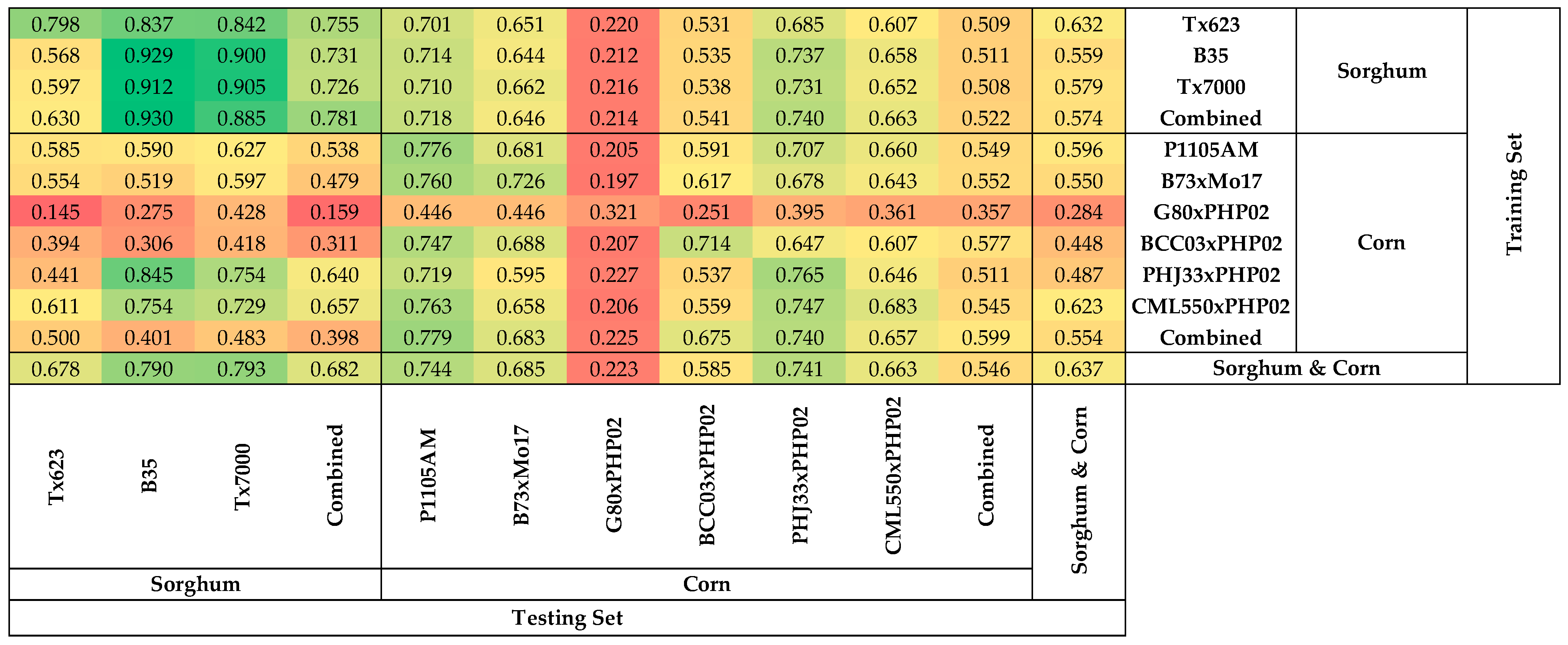
3.4. Asymmetric Heatmap of Coefficients of Determination of the Models
3.5. Poor Performance in Predicting Nitrogen Content of G80xPHP02
3.6. Non-Linear Models for Predicting the Relative Water Content and Nitrogen Content
4. Materials and Methods
4.1. Plant Material and Growing Condition
4.2. Phenotyping Facility
4.3. Ground Reference Data Collection
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minhas, P.S.; Rane, J.; Pasala, R.K. Abiotic Stresses in Agriculture: An Overview. In Abiotic Stress Management for Resilient Agriculture; Minhas, P.S., Rane, J., Pasala, R.K., Eds.; Springer: Singapore, 2017; pp. 3–8. ISBN 978-981-10-5744-1. [Google Scholar]
- Roberts, D.P.; Mattoo, A.K. Sustainable Agriculture—Enhancing Environmental Benefits, Food Nutritional Quality and Building Crop Resilience to Abiotic and Biotic Stresses. Agriculture 2018, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Großkinsky, D.K.; Svensgaard, J.; Christensen, S.; Roitsch, T. Plant Phenomics and the Need for Physiological Phenotyping across Scales to Narrow the Genotype-to-Phenotype Knowledge Gap. J. Exp. Bot. 2015, 66, 5429–5440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Han, B. Natural Variations and Genome-Wide Association Studies in Crop Plants. Annu. Rev. Plant Biol. 2014, 65, 531–551. [Google Scholar] [CrossRef]
- Yang, W.; Feng, H.; Zhang, X.; Zhang, J.; Doonan, J.H.; Batchelor, W.D.; Xiong, L.; Yan, J. Crop Phenomics and High-Throughput Phenotyping: Past Decades, Current Challenges, and Future Perspectives. Mol. Plant 2020, 13, 187–214. [Google Scholar] [CrossRef] [Green Version]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought Stress in Plants: A Review on Morphological Characteristics and Pigments Composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Younis, M.E.; El-Shahaby, O.A.; Abo-Hamed, S.A.; Ibrahim, A.H. Effects of Water Stress on Growth, Pigments and 14CO2 Assimilation in Three Sorghum Cultivars. J. Agron. Crop Sci. 2000, 185, 73–82. [Google Scholar] [CrossRef]
- Nyachiro, J.M.; Briggs, K.G.; Hoddinott, J.; Johnson-Flanagan, A.M. Chlorophyll Content, Chlorophyll Fluorescence and Water Deficit in Spring Wheat. Cereal Res. Commun. 2001, 29, 135–142. [Google Scholar] [CrossRef]
- Mohammadkhani, N.; Heidari, R. Effects of Water Stress on Respiration, Photosynthetic Pigments and Water Content in Two Maize Cultivars. Pak. J. Biol. Sci. 2007, 10, 4022–4028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberte, R.S.; Thornber, J.P.; Fiscus, E.L. Water Stress Effects on the Content and Organization of Chlorophyll in Mesophyll and Bundle Sheath Chloroplasts of Maize. Plant Physiol. 1977, 59, 351–353. [Google Scholar] [CrossRef] [Green Version]
- Mueller-moule, P.; Li, X.; Niyogi, K. Non-Photochemical Quenching. A Response to Excess Light Energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [Green Version]
- Chool Boo, Y.; Jung, J. Water Deficit—Induced Oxidative Stress and Antioxidative Defenses in Rice Plants. J. Plant Physiol. 1999, 155, 255–261. [Google Scholar] [CrossRef]
- Spyropoulos, C.G.; Mavrommatis, M. Effect of Water Stress on Pigment Formation in Quercus Species. J. Exp. Bot. 1978, 29, 473–477. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Oxford University Press: Oxford, NY, USA, 2018; ISBN 978-1-60535-745-4. [Google Scholar]
- Chatterjee, A.; Kundu, S. Revisiting the Chlorophyll Biosynthesis Pathway Using Genome Scale Metabolic Model of Oryza Sativa Japonica. Sci. Rep. 2015, 5, 14975. [Google Scholar] [CrossRef] [Green Version]
- Shangguan, Z.; Shao, M.; Dyckmans, J. Effects of Nitrogen Nutrition and Water Deficit on Net Photosynthetic Rate and Chlorophyll Fluorescence in Winter Wheat. J. Plant Physiol. 2000, 156, 46–51. [Google Scholar] [CrossRef]
- Pietrzykowska, M.; Suorsa, M.; Semchonok, D.A.; Tikkanen, M.; Boekema, E.J.; Aro, E.-M.; Jansson, S. The Light-Harvesting Chlorophyll a/b Binding Proteins Lhcb1 and Lhcb2 Play Complementary Roles during State Transitions in Arabidopsis. Plant Cell 2014, 26, 3646–3660. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Li, W.; Hu, D.; Shi, X.; Zhang, X.; Zhang, F.; Fu, Z.; Ding, D.; Liu, Z.; Tang, J. Biological Responses and Proteomic Changes in Maize Seedlings under Nitrogen Deficiency. Plant Mol. Biol. Rep. 2015, 33, 490–504. [Google Scholar] [CrossRef]
- Han, Y.; Vimolmangkang, S.; Soria-Guerra, R.E.; Rosales-Mendoza, S.; Zheng, D.; Lygin, A.V.; Korban, S.S. Ectopic Expression of Apple F3′H Genes Contributes to Anthocyanin Accumulation in the Arabidopsis Tt7 Mutant Grown Under Nitrogen Stress. Plant Physiol. 2010, 153, 806–820. [Google Scholar] [CrossRef] [Green Version]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Oxidative Stress and Antioxidant Responses in Young Leaves of Mulberry Plants Grown Under Nitrogen, Phosphorus or Potassium Deficiency. J. Integr. Plant Biol. 2007, 49, 313–322. [Google Scholar] [CrossRef]
- Krishna, G.; Sahoo, R.N.; Singh, P.; Bajpai, V.; Patra, H.; Kumar, S.; Dandapani, R.; Gupta, V.K.; Viswanathan, C.; Ahmad, T.; et al. Comparison of Various Modelling Approaches for Water Deficit Stress Monitoring in Rice Crop through Hyperspectral Remote Sensing. Agric. Water Manag. 2019, 213, 231–244. [Google Scholar] [CrossRef]
- Jin, X.; Xu, X.; Song, X.; Li, Z.; Wang, J.; Guo, W. Estimation of Leaf Water Content in Winter Wheat Using Grey Relational Analysis–Partial Least Squares Modeling with Hyperspectral Data. Agron. J. 2013, 105, 1385. [Google Scholar] [CrossRef]
- Ge, Y.; Bai, G.; Stoerger, V.; Schnable, J.C. Temporal Dynamics of Maize Plant Growth, Water Use, and Leaf Water Content Using Automated High Throughput RGB and Hyperspectral Imaging. Comput. Electron. Agric. 2016, 127, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Pôças, I.; Gonçalves, J.; Costa, P.M.; Gonçalves, I.; Pereira, L.S.; Cunha, M. Hyperspectral-Based Predictive Modelling of Grapevine Water Status in the Portuguese Douro Wine Region. Int. J. Appl. Earth Obs. Geoinf. 2017, 58, 177–190. [Google Scholar] [CrossRef]
- Neinavaz, E.; Skidmore, A.K.; Darvishzadeh, R.; Groen, T.A. Retrieving Vegetation Canopy Water Content from Hyperspectral Thermal Measurements. Agric. For. Meteorol. 2017, 247, 365–375. [Google Scholar] [CrossRef]
- Li, F.; Mistele, B.; Hu, Y.; Chen, X.; Schmidhalter, U. Reflectance Estimation of Canopy Nitrogen Content in Winter Wheat Using Optimised Hyperspectral Spectral Indices and Partial Least Squares Regression. Eur. J. Agron. 2014, 52, 198–209. [Google Scholar] [CrossRef]
- Yu, X.; Lu, H.; Liu, Q. Deep-Learning-Based Regression Model and Hyperspectral Imaging for Rapid Detection of Nitrogen Concentration in Oilseed Rape (Brassica Napus L.) Leaf. Chemom. Intell. Lab. Syst. 2018, 172, 188–193. [Google Scholar] [CrossRef]
- Abdel-Rahman, E.M.; Ahmed, F.B.; Ismail, R. Random Forest Regression and Spectral Band Selection for Estimating Sugarcane Leaf Nitrogen Concentration Using EO-1 Hyperion Hyperspectral Data. Int. J. Remote Sens. 2013, 34, 712–728. [Google Scholar] [CrossRef]
- Paterson, A.H.; Bowers, J.E.; Bruggmann, R.; Dubchak, I.; Grimwood, J.; Gundlach, H.; Haberer, G.; Hellsten, U.; Mitros, T.; Poliakov, A.; et al. The Sorghum Bicolor Genome and the Diversification of Grasses. Nature 2009, 457, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Tuinstra, M.R.; Grote, E.M.; Goldsbrough, P.B.; Ejeta, G. Identification of Quantitative Trait Loci Associated with Pre-Flowering Drought Tolerance in Sorghum. Crop Sci. 1996, 36, 1337–1344. [Google Scholar] [CrossRef]
- Kebede, H.; Subudhi, P.K.; Rosenow, D.T.; Nguyen, H.T. Quantitative Trait Loci Influencing Drought Tolerance in Grain Sorghum (Sorghum bicolor L. Moench). Appl. Genet. 2001, 103, 266–276. [Google Scholar] [CrossRef]
- Schoper, J.B.; Lambert, R.J.; Vasilas, B.L. Maize Pollen Viability and Ear Receptivity under Water and High Temperature Stress. Crop Sci. 1986, 26, 1029–1033. [Google Scholar] [CrossRef]
- Oluwaranti, A.; Edema, R.; Ajayi, S.A.; Atkinson, C.J.; Asea, G.; Makumbi, D.; Kwemoi, D.B. Characterization of Elite Maize Inbred Lines for Drought Tolerance Using Simple Sequence Repeats Markers. Int. J. Agric. Environ. Res. 2018, 4, 552–566. [Google Scholar]
- Mahama, G.Y.; Prasad, P.V.V.; Mengel, D.B.; Tesso, T.T. Influence of Nitrogen Fertilizer on Growth and Yield of Grain Sorghum Hybrids and Inbred Lines. Agron. J. 2014, 106, 1623–1630. [Google Scholar] [CrossRef] [Green Version]
- Trachsel, S.; Leyva, M.; Lopez, M.; Suarez, E.A.; Mendoza, A.; Montiel, N.G.; Macias, M.S.; Burgueno, J.; San Vicente, F. Identification of Tropical Maize Germplasm with Tolerance to Drought, Nitrogen Deficiency, and Combined Heat and Drought Stresses. Crop Sci. 2016, 56, 3031–3045. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Dweikat, I.; Huber, D.M.; Warren, H.L. Interrelationship of Nitrogen Nutrition with Maize (Zea Mays) Grain Yield, Nitrogen Use Efficiency and Grain Quality. J. Sci. Food Agric. 1992, 58, 1–8. [Google Scholar] [CrossRef]
- Maranville, J.W.; Madhavan, S. Physiological Adaptations for Nitrogen Use Efficiency in Sorghum. In Food Security in Nutrient-Stressed Environments: Exploiting Plants’ Genetic Capabilities; Adu-Gyamfi, J.J., Ed.; Developments in Plant and Soil Sciences; Springer: Dordrecht, The Netherlands, 2002; pp. 81–90. ISBN 978-94-017-1570-6. [Google Scholar]
- Mahama, G.Y. Variation among Grain Sorghum Genotypes in Response to Nitrogen Fertilizer. Master’s Thesis, Kansas State University, Manhattan, KS, USA, 2012. [Google Scholar]
- Asen, S.; Stewart, R.N.; Norris, K.H. Co-Pigmentation of Anthocyanins in Plant Tissues and Its Effect on Color. Phytochemistry 1972, 11, 1139–1144. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Miehé, J.A. Fluorescence Imaging as a Diagnostic Tool for Plant Stress. Trends Plant Sci. 1997, 2, 316–320. [Google Scholar] [CrossRef]
- Garbulsky, M.F.; Peñuelas, J.; Gamon, J.; Inoue, Y.; Filella, I. The Photochemical Reflectance Index (PRI) and the Remote Sensing of Leaf, Canopy and Ecosystem Radiation Use Efficiencies: A Review and Meta-Analysis. Remote Sens. Environ. 2011, 115, 281–297. [Google Scholar] [CrossRef]
- Khayatnezhad, M.; Gholamin, R. The Effect of Drought Stress on Leaf Chlorophyll Content and Stress Resistance in Maize Cultivars (Zea Mays). AJMR 2012, 6, 2844–2848. [Google Scholar] [CrossRef]
- Jagtap, V.; Bhargava, S.; Streb, P.; Feierabend, J. Comparative Effect of Water, Heat and Light Stresses on Photosynthetic Reactions in Sorghum bicolor (L.) Moench. J. Exp. Bot. 1998, 49, 1715–1721. [Google Scholar] [CrossRef] [Green Version]
- Siebke, K.; Ball, M.C. Non-Destructive Measurement of Chlorophyll B:A Ratios and Identification of Photosynthetic Pathways in Grasses by Reflectance Spectroscopy. Funct. Plant Biol. 2009, 36, 857–866. [Google Scholar] [CrossRef]
- Kebede, H.; Fisher, D.K.; Young, L.D. Determination of Moisture Deficit and Heat Stress Tolerance in Corn Using Physiological Measurements and a Low-Cost Microcontroller-Based Monitoring System. J. Agron. Crop Sci. 2012, 198, 118–129. [Google Scholar] [CrossRef]
- Ogbaga, C.C.; Stepien, P.; Johnson, G.N. Sorghum (Sorghum Bicolor) Varieties Adopt Strongly Contrasting Strategies in Response to Drought. Physiol. Plant. 2014, 152, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Xie, J.; Zhao, J.; Jiang, L. Temperature-Induced Decoupling of Phycobilisomes from Reaction Centers. Biochim. Biophys. Acta (BBA) Bioenerg. 2001, 1504, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Oukarroum, A.; Schansker, G.; Strasser, R.J. Drought Stress Effects on Photosystem I Content and Photosystem II Thermotolerance Analyzed Using Chl a Fluorescence Kinetics in Barley Varieties Differing in Their Drought Tolerance. Physiol. Plant. 2009, 137, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Gamon, J.A.; Fredeen, A.L.; Merino, J.; Field, C.B. Reflectance Indices Associated with Physiological Changes in Nitrogen- and Water-Limited Sunflower Leaves. Remote Sens. Environ. 1994, 48, 135–146. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Chivkunova, O.B.; Melø, T.B.; Naqvi, K.R. Does a Leaf Absorb Radiation in the near Infrared (780–900 Nm) Region? A New Approach to Quantifying Optical Reflection, Absorption and Transmission of Leaves. Photosynth. Res. 2002, 72, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Chishaki, N.; Horiguchi, T. Responses of Secondary Metabolism in Plants to Nutrient Deficiency. In Plant Nutrition for Sustainable Food Production and Environment: Proceedings of the XIII International Plant Nutrition Colloquium, 13–19 September 1997, Tokyo, Japan; Ando, T., Fujita, K., Mae, T., Matsumoto, H., Mori, S., Sekiya, J., Eds.; Developments in Plant and Soil Sciences; Springer: Dordrecht, The Netherlands, 1997; pp. 341–345. ISBN 978-94-009-0047-9. [Google Scholar]
- Ivanova, D.; Deneva, V.; Zheleva-Dimitrova, D.; Balabanova-Bozushka, V.; Nedeltcheva, D.; Gevrenova, R.; Antonov, L. Quantitative Characterization of Arnicae Flos by RP-HPLC-UV and NIR Spectroscopy. Foods 2018, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, T.R.; Schreiber, M.M.; Hoffer, R.M. Diffuse Reflectance Hypothesis for the Pathway of Solar Radiation through Leaves. Agron. J. 1973, 65, 276–283. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I. Visible and Near-Infrared Reflectance Techniques for Diagnosing Plant Physiological Status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Slaton, M.R.; Hunt, E.R.; Smith, W.K. Estimating Near-Infrared Leaf Reflectance from Leaf Structural Characteristics. Am. J. Bot. 2001, 88, 278–284. [Google Scholar] [CrossRef]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Read, J.J.; Koti, S. Selection of Optimum Reflectance Ratios for Estimating Leaf Nitrogen and Chlorophyll Concentrations of Field-Grown Cotton. Agron. J. 2005, 97, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Bajwa, S.G.; Mishra, A.R.; Norman, R.J. Canopy Reflectance Response to Plant Nitrogen Accumulation in Rice. Precis. Agric. 2010, 11, 488–506. [Google Scholar] [CrossRef]
- Yan, X.; Shi, W.; Zhao, W.; Luo, N. Estimation of Protein Content in Plant Leaves Using Spectral Reflectance: A Case Study in Euonymus Japonica. Anal. Lett. 2014, 47, 517–530. [Google Scholar] [CrossRef]
- Thomas, J.R.; Oerther, G.F. Estimating Nitrogen Content of Sweet Pepper Leaves by Reflectance Measurements. Agron. J. 1972, 64, 11–13. [Google Scholar] [CrossRef]
- Datt, B. A New Reflectance Index for Remote Sensing of Chlorophyll Content in Higher Plants: Tests Using Eucalyptus Leaves. J. Plant Physiol. 1999, 154, 30–36. [Google Scholar] [CrossRef]
- Girardin, P.; Tollenaar, M.; Muldoon, J.F. Effect of Temporary N Starvation on Leaf Photosynthetic Rate and Chlorophyll Content of Maize. Can. J. Plant Sci. 1985, 65, 491–500. [Google Scholar] [CrossRef]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Reddy, V.R. Nitrogen Deficiency Effects on Plant Growth, Leaf Photosynthesis, and Hyperspectral Reflectance Properties of Sorghum. Eur. J. Agron. 2005, 22, 391–403. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Solovchenko, A.E.; Pogosyan, S.I. Application of Reflectance Spectroscopy for Analysis of Higher Plant Pigments. Russ. J. Plant Physiol. 2003, 50, 704–710. [Google Scholar] [CrossRef]
- Wharton, P.S.; Nicholson, R.L. Temporal Synthesis and Radiolabelling of the Sorghum 3-Deoxyanthocyanidin Phytoalexins and the Anthocyanin, Cyanidin 3-Dimalonyl Glucoside. New Phytol. 2000, 145, 457–469. [Google Scholar] [CrossRef]
- Shih, C.-H.; Chu, I.K.; Yip, W.K.; Lo, C. Differential Expression of Two Flavonoid 3′-Hydroxylase CDNAs Involved in Biosynthesis of Anthocyanin Pigments and 3-Deoxyanthocyanidin Phytoalexins in Sorghum. Plant Cell Physiol. 2006, 47, 1412–1419. [Google Scholar] [CrossRef]
- Grzesiak, M.T.; Grzesiak, S.; Skoczowski, A. Changes of Leaf Water Potential and Gas Exchange during and after Drought in Triticale and Maize Genotypes Differing in Drought Tolerance. Photosynthetica 2006, 44, 561–568. [Google Scholar] [CrossRef]
- Moussa, H.R.; Abdel-Aziz, S.M. Comparative Response of Drought Tolerant and Drought Sensitive Maize Genotypes to Water Stress. Aust. J. Crop Sci. 2008, 1, 31–36. [Google Scholar]
- Heckmann, D.; Schlüter, U.; Weber, A.P.M. Machine Learning Techniques for Predicting Crop Photosynthetic Capacity from Leaf Reflectance Spectra. Mol. Plant 2017, 10, 878–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walther, B.A.; Moore, J.L. The Concepts of Bias, Precision and Accuracy, and Their Use in Testing the Performance of Species Richness Estimators, with a Literature Review of Estimator Performance. Ecography 2005, 28, 815–829. [Google Scholar] [CrossRef]
- Pandey, P.; Ge, Y.; Stoerger, V.; Schnable, J.C. High Throughput In Vivo Analysis of Plant Leaf Chemical Properties Using Hyperspectral Imaging. Front. Plant Sci. 2017, 8, 1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.; Carpenter, N.; Maki, H.; Rehman, T.U.; Tuinstra, M.R.; Jin, J. Greenhouse Environment Modeling and Simulation for Microclimate Control. Comput. Electron. Agric. 2019, 162, 134–142. [Google Scholar] [CrossRef]
- Ma, D.; Carpenter, N.; Amatya, S.; Maki, H.; Wang, L.; Zhang, L.; Neeno, S.; Tuinstra, M.R.; Jin, J. Removal of Greenhouse Microclimate Heterogeneity with Conveyor System for Indoor Phenotyping. Comput. Electron. Agric. 2019, 166, 104979. [Google Scholar] [CrossRef]
- Turner, N.C. Techniques and Experimental Approaches for the Measurement of Plant Water Status. Plant Soil 1981, 58, 339–366. [Google Scholar] [CrossRef]
- de Jong, S. SIMPLS: An Alternative Approach to Partial Least Squares Regression. Chemom. Intell. Lab. Syst. 1993, 18, 251–263. [Google Scholar] [CrossRef]
- Wise, B.M.; Gallagher, N.B.; Bro, R.; Shaver, J.M.; Windig, W.; Koch, R.S. PLS_Toolbox 4.0; Eigenvector Research, Inc.: Wenatchee, WA, USA, 2006. [Google Scholar]


| Plant Species | Genotype | Adaption to Water Deficit | Nitrogen Use Efficiency |
|---|---|---|---|
| Sorghum | Tx623 | Susceptible | Low |
| B35 | Post-flowering tolerance | Low | |
| Tx7000 | Pre-flowering tolerance | High | |
| Corn | P1105AM | Unknown | Unknown |
| B73xMo17 | Susceptible | Low | |
| G80xPHP02 | Susceptible | Unknown | |
| BCC03xPHP02 | Susceptible | Unknown | |
| PHJ33xPHP02 | Tolerant | Unknown | |
| CML550xPHP02 | Tolerant | High |
| Relative Water Content (%) | Nitrogen Content (%) | |||||
|---|---|---|---|---|---|---|
| Treatment | Water Sufficient | Water Deficient | Significance | Nitrogen Sufficient | Nitrogen Deficient | Significance |
| Sorghum | 98.4 ± 0.54 a | 66.0 ± 5.29 b | *** | 1.81 ± 0.18 a | 1.16 ± 0.13 b | *** |
| Corn | 97.0 ± 1.60 a | 91.0 ± 11.10 b | *** | 1.99 ± 0.34 a | 1.59 ± 0.28 b | *** |
| Relative Water Content (%) | Nitrogen Content (%) | ||||
|---|---|---|---|---|---|
| Species | Genotypes | Water Sufficient | Water Deficient | Nitrogen Sufficient | Nitrogen Deficient |
| Sorghum | Tx623 | 98.4 ± 0.51 | 67.2 ± 5.43 | 1.82 ± 0.08 a | 1.19 ± 0.10 |
| B35 | 98.5 ± 0.59 | 66.1 ± 5.03 | 1.92 ± 0.22 a | 1.15 ± 0.13 | |
| Tx7000 | 98.5 ± 0.54 | 64.7 ± 5.37 | 1.69 ± 0.15 b | 1.13 ± 0.15 | |
| Significance | NS | NS | *** | NS | |
| Corn | P1105AM | 97.7 ± 1.05 a | 90.6 ± 11.6 | 2.16 ± 0.35 | 1.70 ± 0.27 a |
| B73xMo17 | 95.4 ± 1.42 c | 92.1 ± 6.2 | 2.00 ± 0.35 | 1.67 ± 0.29 a | |
| G80xPHP02 | 97.6 ± 1.27 ab | 89.0 ± 13.7 | 1.91 ± 0.28 | 1.65 ± 0.36 a | |
| BCC03xPHP02 | 96.8 ± 1.81 b | 90.2 ± 12.4 | 1.96 ± 0.28 | 1.57 ± 0.20 ab | |
| PHJ33xPHP02 | 97.5 ± 1.13 ab | 89.3 ± 13.6 | 2.02 ± 0.36 | 1.46 ± 0.19 b | |
| CML550xPHP02 | 97.2 ± 1.61 ab | 95.2 ± 6.2 | 1.89 ± 0.35 | 1.47 ± 0.25 b | |
| Significance | *** | NS | NS | * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.-Y.; Lynch, V.; Ma, D.; Maki, H.; Jin, J.; Tuinstra, M. Multi-Species Prediction of Physiological Traits with Hyperspectral Modeling. Plants 2022, 11, 676. https://doi.org/10.3390/plants11050676
Lin M-Y, Lynch V, Ma D, Maki H, Jin J, Tuinstra M. Multi-Species Prediction of Physiological Traits with Hyperspectral Modeling. Plants. 2022; 11(5):676. https://doi.org/10.3390/plants11050676
Chicago/Turabian StyleLin, Meng-Yang, Valerie Lynch, Dongdong Ma, Hideki Maki, Jian Jin, and Mitchell Tuinstra. 2022. "Multi-Species Prediction of Physiological Traits with Hyperspectral Modeling" Plants 11, no. 5: 676. https://doi.org/10.3390/plants11050676
APA StyleLin, M.-Y., Lynch, V., Ma, D., Maki, H., Jin, J., & Tuinstra, M. (2022). Multi-Species Prediction of Physiological Traits with Hyperspectral Modeling. Plants, 11(5), 676. https://doi.org/10.3390/plants11050676






