Abstract
Jacaranones are a small group of specific plant metabolites with promising biological activities. The occurrence of jacaranones is limited to only a few plant families, with Asteraceae being the most abundant source of these compounds. Therefore, jacaranones can also serve as chemotaxonomic markers. Our phytochemical investigation of Crepis pulchra L. (Asteraceae) resulted in three jacaranone derivatives (jacaranone, 2,3-dihydro-2-hydroxyjacaranone, 2,3-dihydro-2-methoxyjacaranone), and (6R,9S)-3-oxo-α-ionol-β-d-glucopyranoside, fulgidic acid, 12,15-octadecadienoic acid methyl ester, scopoletin and apigenin-7-O-β-d-glucoside. This is the first report on the isolation of jacaranones from a species belonging to the Cichorioideae subfamily of Asteraceae. Jacaranone derivatives were subjected to an in vitro antiproliferative assay against a panel of human cancer cell lines (MCF-7, MDA-MB-231, HeLa, and C33A), revealing high or moderate activities, with IC50 values ranging from 6.3 to 26.5 μM.
1. Introduction
Jacaranone derivatives, bearing an unsaturated cyclohexanone skeleton, occur rarely in the plant kingdom. Jacaranone [methyl 2-(1-hydroxy-4-oxocyclohexa-2,5-dien-1-yl)acetate] (2), the methyl ester derivative of quinolacetic acid (1), was first isolated from Jacaranda caucana (Bignoniaceae) by Ogura et al. in 1976 [1]. Since then, jacaranone derivatives (n = 35) have been isolated from other plant species as well, most of them belonging to the family Asteraceae. Almost all of the isolated compounds have been studied for their biological activities (e.g., cytotoxic, antimicrobial, anti-inflammatory, antioxidant, sedative, antiprotozoal, and antifeedant effects), and many of them showed multiple activities [2,3,4,5,6,7,8].
The genus Crepis (family Asteraceae) includes about 200 annual, biennial, or perennial plant species occurring widely in Eurasia, Africa, and North America [9]. In folk medicine, decoctions prepared from aerial parts of members of the genus Crepis are used for the treatment of various diseases, e.g., cough (Uganda) [10]; hepatitis, jaundice, and gallstones (Yemen) [11]; tumors (USA and China) [12]; and cardiovascular diseases, diabetes, cold, catarrh, and eye diseases (Turkey) [13,14,15,16]. Some species are traditionally used for their diuretic or laxative properties (Italy) [17,18] or externally for healing wounds, bruises, or inflammation (Spain and Bangladesh) [19,20].
Crepis species are rich sources of guaianolide- and eudesmane-type sesquiterpenes [21,22,23] and flavonoids [24,25]. In vitro pharmacological assessments revealed that Crepis extracts possess hepatoprotective [11], antimicrobial [21], antiviral [26], antioxidant [27], anti-inflammatory [28], and antiproliferative [26,27] activities.
Smallflower hawksbeard (Crepis pulchra L.) occurs in different ruderal stands. The species can be found in dry, open habitats, grasslands, pastures, abandoned fields, waste areas, alongside railroads, and roadsides [29,30]. According to the literature data, previously, guaianolide-type sesquiterpenes (8-epi-isoamberboin, vernoflexuoside, macrocliniside A, and diaspanoside A) were isolated from the plant by Kisiel et al. [31].
In continuation of our research aiming at the discovery of new bioactive specific metabolites from medicinal plants, the methanol extract of Crepis pulchra L. was investigated. The isolated special metabolites are discussed in comparison with the literature dealing with naturally occurring jacaranones.
2. Results
2.1. Isolation of Compounds from C. pulchra
The dried and ground whole plant material (1.25 kg) was extracted with methanol at room temperature. After evaporation, the extract was dissolved in 50% aqueous methanol, and solvent–solvent partition was performed with n-hexane, chloroform, and ethyl acetate. The chloroform phase was purified by a combination of different methods, including column chromatography (CC), vacuum liquid chromatography (VLC), thin layer chromatography (TLC), and HPLC to afford eight compounds. The structural determination was carried out by extensive spectroscopic analysis using 1D (1H, JMOD) and 2D NMR (1H-1H COSY, HSQC, HMBC, NOESY) spectroscopy, HRESIMS measurements, and the comparison of the spectral data with the literature values.
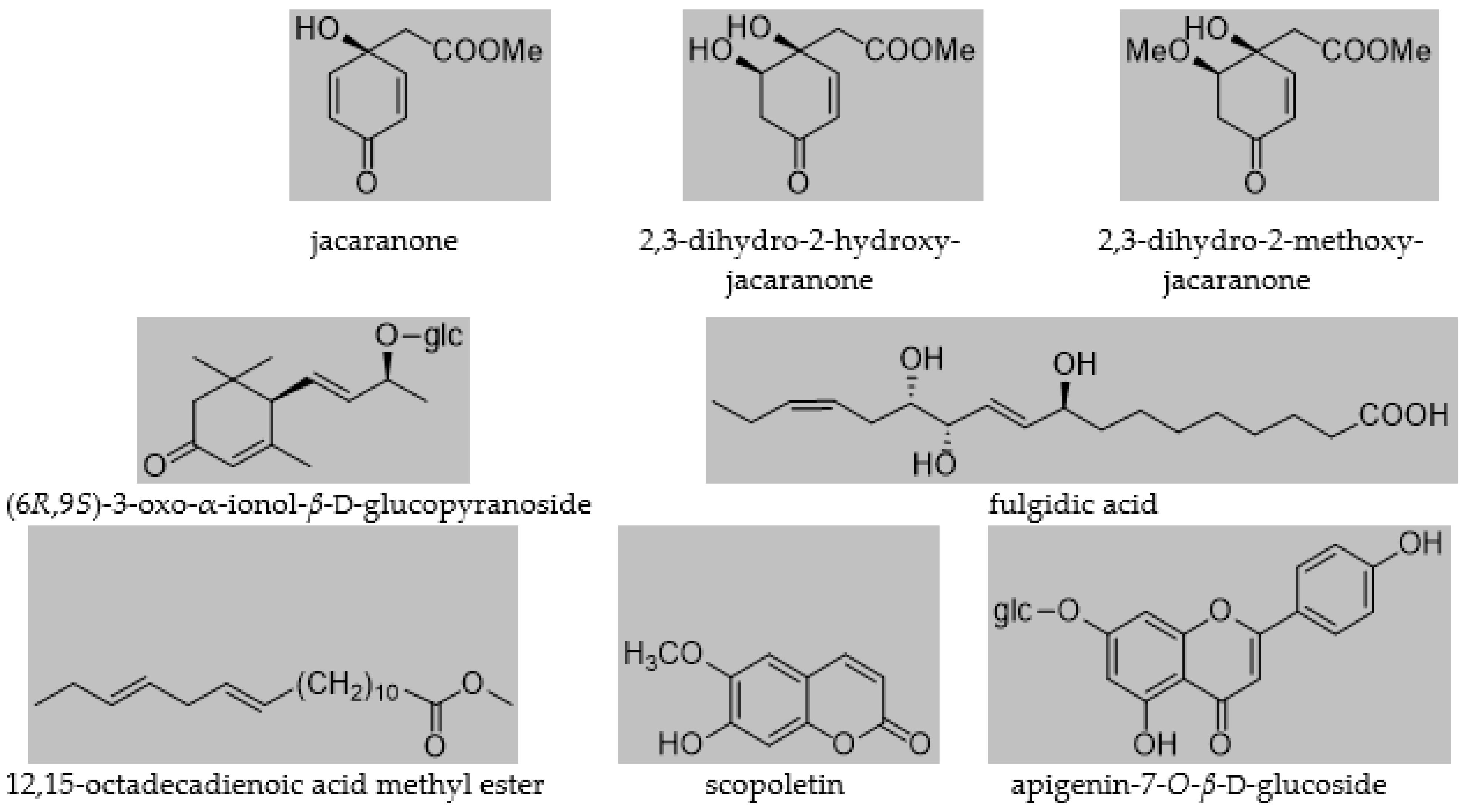
The isolated compounds were identified as jacaranone [5], 2,3-dihydro-2-hydroxyjacaranone [5]; 2,3-dihydro-2-methoxyjacaranone [5]; (6R,9S)-3-oxo-ionol-β-d-glucopyranoside [32]; fulgidic acid [33]; 12,15-octadecadienoic acid methyl ester, scopoletin [34]; and apigenin-7-O-β-d-glucoside [35] (Figure 1). All compounds were isolated for the first time from the plant. Moreover, this was the first time jacaranone derivatives were isolated from a Crepis species.
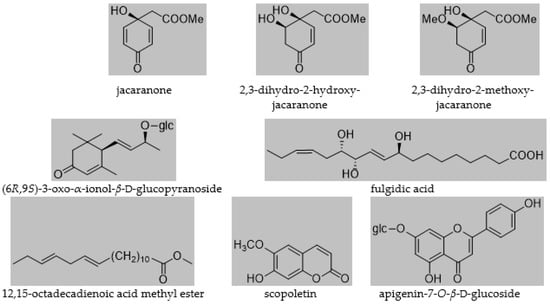
Figure 1.
Compounds isolated from C. pulchra.
2.2. Antiproliferative Investigation of the Isolated Jacaranones
The jacaranone derivatives, isolated from C. pulchra, were subjected to an in vitro cytotoxicity (MTT) assay against human cancer (breast cancer (MCF-7 and MDA-MB-231), and cervical cancer (HeLa and C33A) cell lines (Table 1). Jacaranone proved to be the most active against all four tested cell lines (IC50 6.27–14.61 µM). Its activity was comparable with that of the positive control, cisplatin. 2,3-Dihydro-2-hydroxyjacaranone and 2,3-dihydro-2-methoxyjacaranone differ from jacaranone only in the substitution of C-2 (a hydroxy group in the case of 2,3-dihydro-2-hydroxyjacaranone, and a methoxy group in 2,3-dihydro-2-methoxyjacaranone) and in the saturation of the double bond between C-2–C-3. These modifications resulted in the decrease of the antiproliferative activity in the case of the two jacaranone derivatives. Our results confirm that the presence of an α,β-unsaturated carbonyl group in the molecule is essential for the antiproliferative activity of jacaranones [36].

Table 1.
Antiproliferative activity of the isolated jacaranone derivatives.
3. Discussion
3.1. Occurrence of Jacaranones in Nature
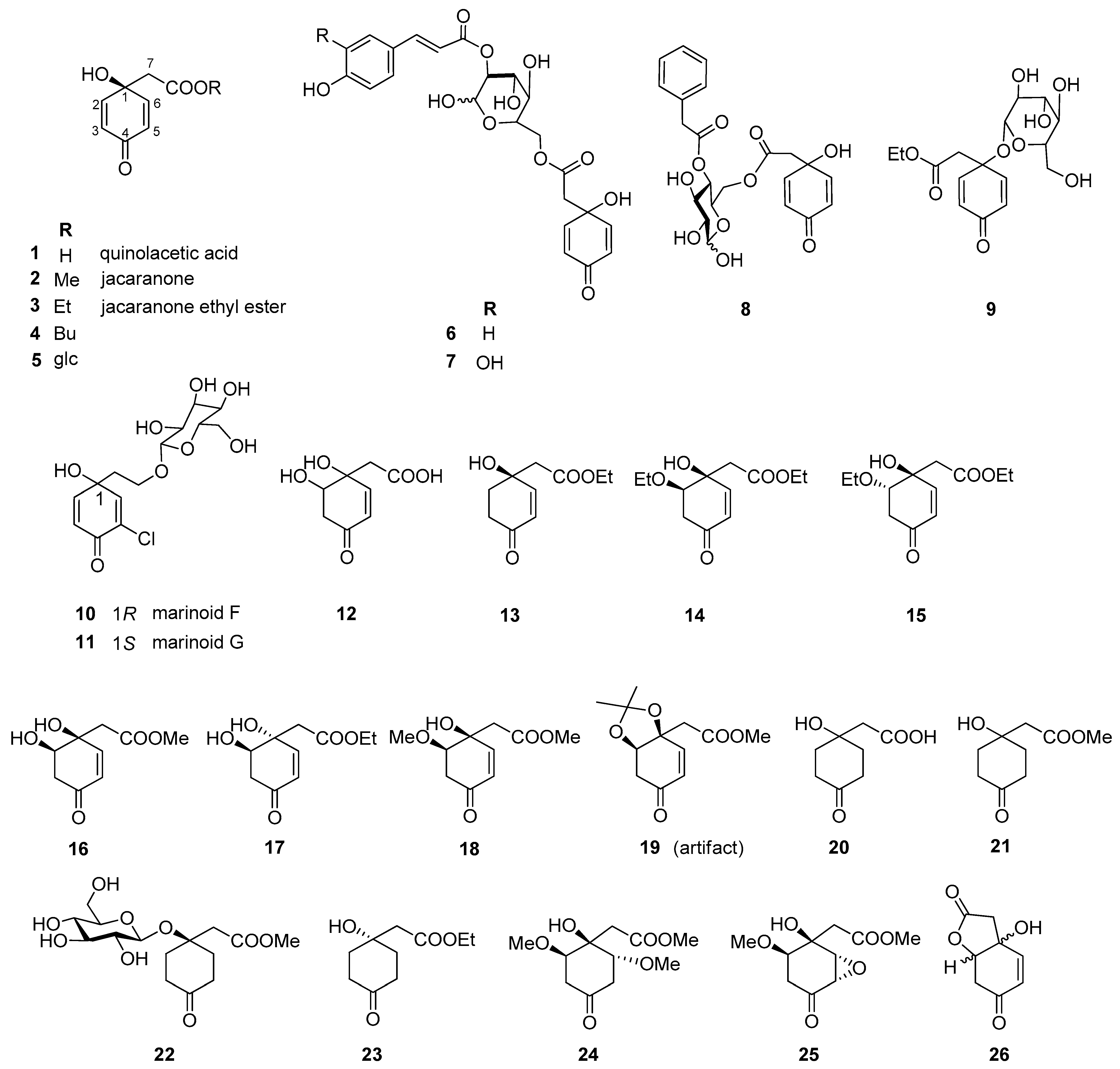
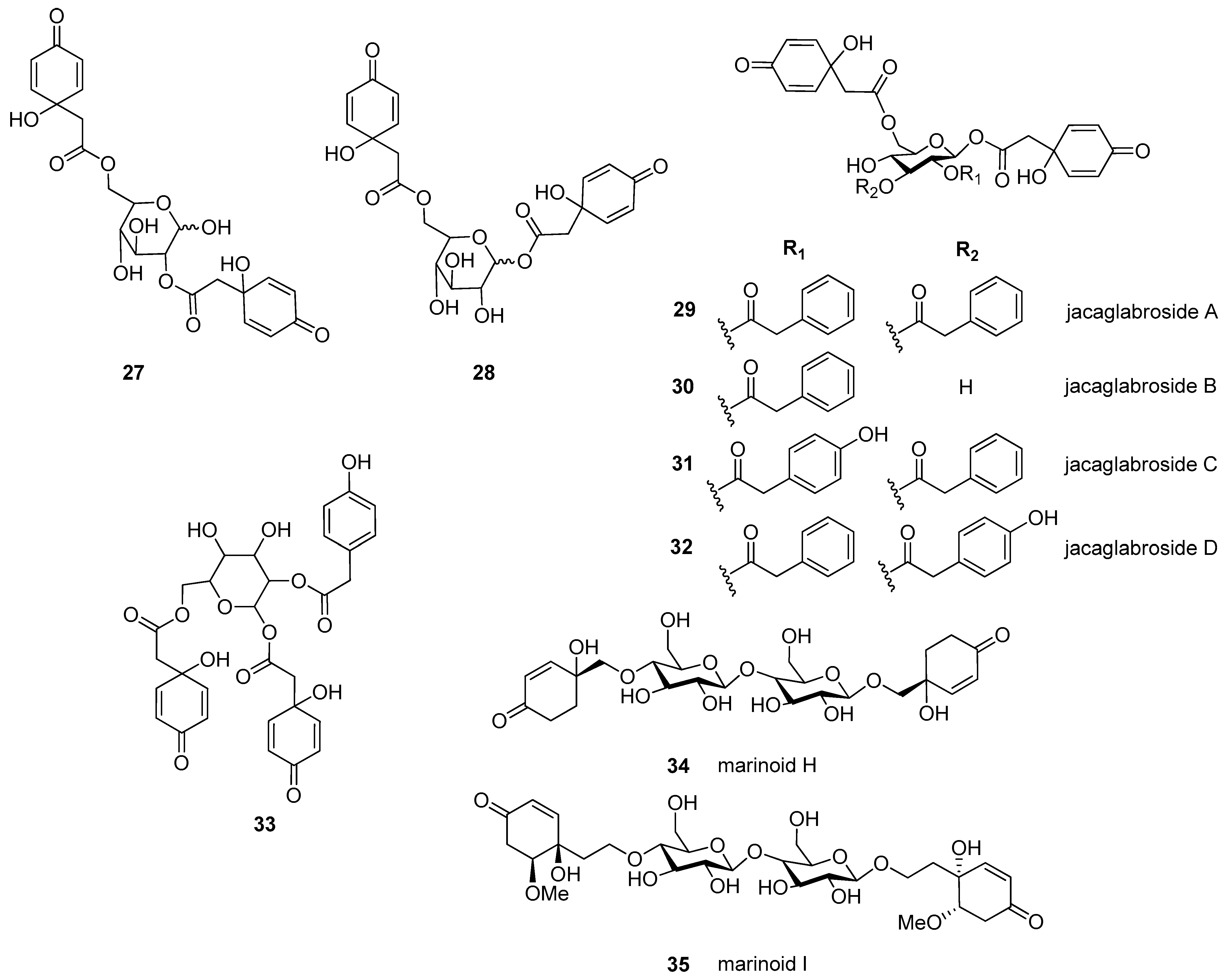
Altogether, 35 jacaranones were isolated from 37 plant species; three-quarters of the compounds are monomers (n = 26), and the others are dimers (n = 9) (Figure 2 and Figure 3). Although the best sources of jacaranones are Asteraceae species, certain species of the Acanthaceae [3], Bignoniaceae [1,2,37,38,39,40,41], Delesseriaceae [42], Gesneriaceae [43,44], Oleaceae [45], and Theaceae [4,5] families were also found to be sources of jacaranones (Table 2). Among Asteraceae species, the genus Senecio is represented by 20 jacaranone derivative-producing plant species [36,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]. Besides the Bethencourtia [8], Packera [63], and Pentacalia [6,7] genera, each is represented by one species. All species belong to the Asteroideae (Tubuliflorae) subfamily of Asteraceae. Crepis pulchra is the first representative of the Cichorioideae (Liguliflorae) subfamily. Jacaranone (2) is the most common compound, isolated from 28 plant species of the Asteraceae, Bignoniaceae, and Theaceae families.
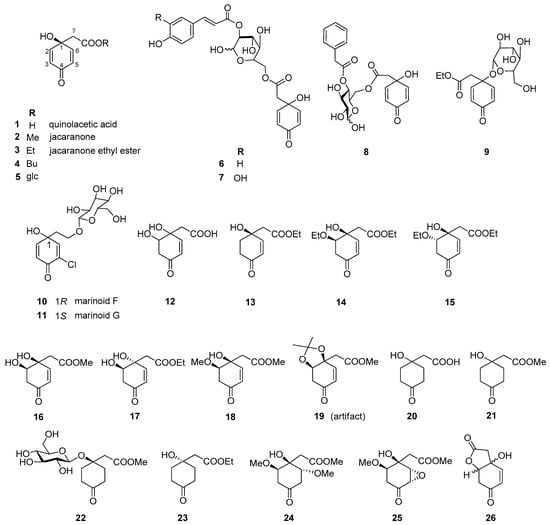
Figure 2.
Naturally occurring jacaranone derivatives (monomers) *. * Stereochemistry of the compounds is indicated according to the source literature; Me = methyl, Et = ethyl, Bu = butyl, glc = glucose.
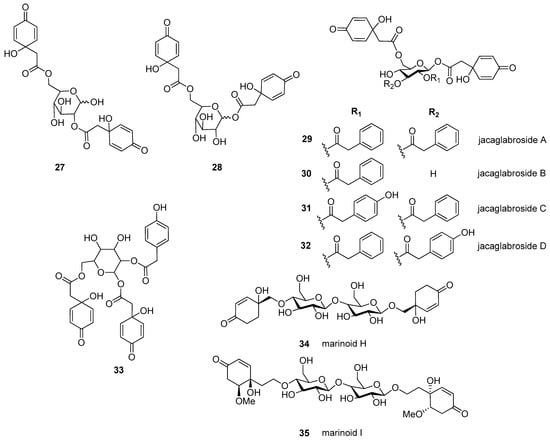
Figure 3.
Naturally occurring jacaranone derivatives (dimers) *. * Stereochemistry of each compound is given as it was published.

Table 2.
List of plant families and species containing jacaranones.
The basic monomer structure of jacaranones can be modified by different substituents. Regarding the cyclohexanone ring, substitution occurs mainly at C-2, where hydroxy- (12), methoxy- (24, 35), or ethoxy- (14, 15) groups are linked to the ring, or even an epoxide ring can be formed, as in the case of compound 25. A rare substituent can be found in cases of marinoid F (10) and marinoid G (11), where a chlorine atom is joined to the cyclohexanone ring at C-3. Most frequently, an ester is formed via the carboxyl group with aliphatic alcohols, e.g., methanol (2, 16, 18, 21, 22, 24, 25), ethanol (3, 13–15, 17, 23), or butanol (4), or with a sugar molecule (5–8). A sugar molecule can also be attached to the hydroxy group forming an acetal (9, 22). Compound 26, isolated from Senecio giganteus, is the only compound containing a lactone ring. Jacaranone dimers are formed from two monomers linked through one or two sugar molecules.
3.2. Antiproliferative Activity of Jacaranones
According to the relevant literature, the most promising biological effect of jacaranones is their anticancer activity. The methanolic extract of Jacaranda caucana and jacaranone (2) were tested against P-388 lymphocytic leukemia and Eagle’s 9KB carcinoma cells, and substantial antiproliferative activity was detected [1,64]. The cytotoxicity of 2 was investigated against six tumors (lung large cell carcinoma (COR-L23), colorectal adenocarcinoma (Caco-2), amelanotic melanoma (C32), hepatocellular carcinoma (HepG-2), renal cell adenocarcinoma (ACHN), and hormone dependent prostate carcinoma (LNCaP) and one normal (human fetal lung (MRC-5) cell lines [47,55]. Jacaranone (2) showed outstanding action against all tested cell lines (IC50 values ranging from 11.31 to 40.57 µM), which was comparable with that of the positive control vinblastine, while 2 did not adversely affect MRC-5 cells. Furthermore, 2 exerted antiproliferative and proapoptotic effects in eight human (A2058, SK-MEL-28, HCT-8, LS160, SiHa, HL-60, SK-BR-3) and one murine (B16F10-Nex2) tumor cell lines in vitro by downregulating Akt and activating p38 MAPK signaling pathways through the generation of reactive oxygen species (ROS). IC50 values varied from 9 to 145 µM for human cancer cells and was 17 µM for murine melanoma B16F10-Nex2 cells [65]. Moreover, the protective effect of this quinone (2) was also proved in a melanoma syngeneic model in vivo [65]. Jacaranone ethyl ester (3) was tested in KB screen and was found to be highly active (ED50 value 16.82 µM) [48]. Tian et al. investigated the cytotoxic effect of seven jacaranone analogs against various tumorous cell lines (HCT8, CaEa-17, A2780, HeLa, BEL-7402, KB, PC-3M, A549, BGC-823, and MCF-7) [59]. Jacaranone ethyl ester (3) possessed the most potent cytotoxic effect with IC50 values at a range of 2.85–4.33 µM. Fluorouracil was applied as a positive control (IC50s 4.15–6.84 µM). Apart from jacaranone ethyl ester (3), quinolacetic acid (1), jacaranone (2), and a new jacaranone-derivative (17) also showed relatively high cytotoxic activity. Based on the results of their cytotoxicity assay, the authors found that all the active compounds share the same structural moiety, the α,β-unsaturated carbonyl group, a segment that is known to be of crucial importance for the cytotoxic effect of other compounds as well [59].
Presser et al. synthesized 13 nitrogen-containing jacaranone derivatives from the natural-product-derived cyclohexadienone scaffold and investigated their antiproliferative activity against four human tumorous cell lines (MDA-MB-231 breast cancer, CCRF-CEM leukemia, HCT-116 colon cancer, and U251 glioblastoma), and one non-tumorigenic cell line (MRC-5 lung fibroblasts) at 5 µg/mL and 50 µg/mL concentrations [66]. The positive control vinblastine was applied at 0.01 µg/mL. At 50 µg/mL concentration, almost all derivatives were found to have cytotoxic effect against MDA-MB-231 and CCRF-CEM cells. During their investigations, the authors managed to reveal some structure–activity relationships of jacaranone-based nitrogenous cyclohexadienones as well. It was observed that the most potent compounds shared an α,β-unsaturated imide structural element. In the absence of this structural moiety, no cytotoxic effect could be detected [66].
4. Materials and Methods
4.1. General Experimental Procedures
NMR spectra were recorded in methanol d4 on a Bruker Avance DRX 500 spectrometer at 500 MHz (1H) and 125 MHz (13C). The signals of the deuterated solvents were chosen as references. The chemical shift values (δ) were given in ppm, and the coupling constants (J) are in Hz. Two-dimensional (2D) experiments were performed with standard Bruker software. In the 1H–1H COSY, HSQC, and HMBC experiments, gradient-enhanced versions were used. Column chromatography (CC) was performed on polyamide (MP Biomedicals Germany GmbH, Eschwege, Germany). Normal and reversed-phase vacuum liquid chromatography (VLC) was carried out on silica gel (Kieselgel 60 GF254, 15 µm, Merck, Darmstadt, Germany) and on reversed phase silica gel [RediSep C-18, 40–60 µm, Teledyne Isco, Lincoln, NE, USA]. Thin-layer chromatography was performed on a Kieselgel 60 RP-18 F254 and a Kieselgel 60 F254 (Merck, Darmstadt, Germany). Spots on UV active silica gel were detected under UV light (245 nm and 336 nm) and made visible with vanillin sulfuric acid and heating at 105 °C for 2 min. The high-performance liquid chromatographic (HPLC) separation was carried out on a Waters HPLC (Waters 600 controller, Waters 600 pump, and Waters 2998 photodiode array detector), using a normal phase (LiChrospher Si 100 (250 × 4 mm, 5 μm, Merck)) column. The flow rate was 1 mL/min, and the injection volume was 25 μL. The data were acquired and processed with Empower software. All solvents used for CC were of at least analytical grade (VWR Ltd., Szeged, Hungary). Ultrapure water was prepared with a Milli-Q water purification system (Millipore, Molsheim, France).
4.2. Plant Material
The whole plants of Crepis pulchra (1.25 kg dried plant material) were collected in the flowering period at Hegyeshalom (Hungary) in July 2019, and were identified by one of the authors, Gyula Pinke (Department of Water and Environmental Sciences, Széchenyi István University). A voucher specimen (No. 895) has been deposited at the Department of Pharmacognosy, University of Szeged, Szeged, Hungary.
4.3. Extraction and Isolation
The dried and ground whole plant of C. pulchra (1.25 kg) was percolated with methanol (12.5 L) at room temperature. The crude extract was concentrated in vacuo (152.5 g), redissolved in water, and subjected to solvent–solvent partition with n-hexane (5 × 500 mL), chloroform (5 × 500 mL), and ethyl acetate (5 × 400 mL), respectively. The concentrated chloroform-soluble fraction (35.7 g) was further separated by polyamide open-column chromatography with a gradient system of MeOH–H2O (2:3, 3:2, and 4:1 (600 mL/eluent); each eluent was collected as a fraction (Fractions I–III). Fraction I obtained with 40% MeOH (3.04 g) was subjected to vacuum liquid chromatography (VLC) on silica gel with a gradient system of CHCl3–MeOH (from 99:1 to 7:3 (300 mL/eluent) to yield 10 major fractions (I/1-10). Fraction I/3 (219 mg) was further purified by VLC on reversed phase silica gel using a MeOH–H2O gradient system (1:9 → 9:1, 100 mL/eluent) to afford 8 subfractions (I/3/1-8). Subfraction I/3/1 (159 mg) was subjected to normal phase VLC (n-hexane–isopropanol, gradient, 95:5 → 6:4, 25 mL/eluent), followed by reversed phase preparative TLC (using MeOH–H2O 1:1 as an eluent) to yield 4 (2 (27.3 mg), 16 (4.4 mg), 18 (3.6 mg), and scopoletine (12.9 mg) compounds. Fraction I/9 (1.0 g) was separated by normal phase VLC with a gradient system of CHCl3–MeOH (from 98:2 to 7:3 (50 mL/eluent) to afford 10 subfractions (I/9/1-10). Fraction I/9/6 (345.5 mg) was purified by reverse-phase VLC (gradient system of MeOH–H2O 1:9 → 8:2, 25 mL/eluent) and gel chromatography on Sephadex LH-20 (using CH2Cl2–MeOH 1:1 as an eluent) followed by normal-phase preparative TLC with EtOAc–MeOH–H2O (100:16:0,5) as a solvent system to yield fulgidic acid (5.19 mg). Fraction I/9/7 (185.0 mg) was separated by gel chromatography on Sephadex LH-20 (using CH2Cl2–MeOH 1:1 as an eluent) and then by preparative TLC on reverse-phase silica gel with MeOH–H2O (1:1) to yield (6R,9S)-3-oxo-ionol-β-d-glucopyranoside (3.34 mg). Fraction II obtained with 60% MeOH (2.08 g) was subjected to reverse-phase VLC, applying a gradient system of MeOH–H2O (from 1:9 to 1:0, 200 mL/eluent) to obtain nine main fractions (II/1-9). Separation of fraction II/1 (236.3 mg) by gel chromatography on Sephadex LH-20 afforded five subfractions (II/1/1-5). Subfraction II/1/4 (60.8 mg) was further purified by normal-phase VLC using CHCl3–MeOH as a mobile phase [from pure 1:0 to 6:4 (40 mL/eluent)] followed by preparative TLC on normal-phase silica gel with EtOAc–HCOOH–H2O (85:10:5) as a solvent system to obtain apigenin-7-O-glucoside (7.8 mg). Fraction II/9 (165.1 mg) was subjected to normal-phase VLC, applying a gradient system of cyclohexane–EtOAc–MeOH [from 9:1:0 to 6:3:1 (50 mL/eluent)] to afford ten subfractions (II/9/1-10). Subfraction II/9/2 (4.4 mg) was purified by normal-phase HPLC [isocratic, cyclohexane–EtOAc (8:2)] to yield 12,15-octadecadienoic acid methyl ester (1.5 mg).
4.4. Antiproliferative (MTT) Assay
The growth-inhibition properties of jacaranone derivatives were determined by standard MTT assays on four human malignant gynecological cell lines (breast cancer: MCF-7 and MDA-MB-231, and cervical cancer: HeLa and C33A). All cell lines were maintained in minimal essential medium (MEM) supplemented with 10% fetal bovine serum, 1% nonessential amino acids, and 1% penicillin–streptomycin–amphotericin B mixture in humidified air containing 5% CO2 at 37 °C. All cell types were seeded into 96-well plates at a density of 5000 with the exception of C33A, which was seeded at a density of 10,000 and treated by increasing concentrations (0.1–30 μM) of the compounds for 72 h under cell culturing conditions. After the incubation, 5 mg/mL MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] solution was added to samples for 4 h and precipitated blue formazan crystals were dissolved in DMSO. Absorbance values of the samples were measured at 545 nm using a microplate reader (Stat Fax-2100, Awareness Technologies Inc., Palm City, FL, USA), and untreated cells were used as a control. Normalized sigmoidal concentration−response curves were fitted to the determined data, and the IC50 values were calculated by GraphPad Prism 5.01 (GraphPad Software, San Diego, CA, USA). Cisplatin (Ebewe Pharma GmbH, Unterach, Austria) was used as a reference agent in the same concentration range.
5. Conclusions
In our experiment, a combination of different chromatographic techniques resulted in the isolation of eight compounds, among them three jacaranone derivatives from C. pulchra for the first time. Jacaranone (2) showed the highest antiproliferative activity against MDA-MB-231 (human breast cancer) and C33A (human cervical cancer) cells. Although jacaranones represent a small group of plant special metabolites, they can be interesting either for organic chemists or for pharmacologists because of their promising biological effects. Moreover, they can serve as chemotaxonomic markers. The importance of our results is that smallflower hawksbeard is the first representative of the Cichorioideae subfamily of Asteraceae in which jacaranones have been detected.
Author Contributions
Conceptualization, A.V.; methodology, A.V. and I.Z.; software, investigation, C.Z.D., Á.K. and N.K.; data curation, C.Z.D. and G.P.; writing—original draft preparation, C.Z.D. and A.V.; writing—review and editing, J.H.; project administration, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Economic Development and Innovation Operative Program GINOP-2.3.2-15-2016-00012, the UNKP-21-3-SZTE-265 New National Excellence Program, and TKP2021-EGA-32 of the Ministry of Innovation and Technology of Hungary from the source of the National Research, Development and Innovation Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ogura, M.; Cordell, G.A.; Farnsworth, N.R. Potential anticancer agents. III. Jacaranone, a novel phytoquinoid from Jacaranda caucana. J. Nat. Prod. 1976, 39, 255–257. [Google Scholar]
- Hirukawa, M.; Zhang, M.; Echenique-Díaz, L.M.; Mizota, K.; Ohdachi, S.; Begué-Quiala, G.; Delgado-Labañino, J.L.; Gámez-Díez, J.; Alvarez-Lemus, J.; Machado, L.G.; et al. Isolation and structure–activity relationship studies of jacaranones: Anti-inflammatory quinoids from the Cuban endemic plant Jacaranda arborea (Bignoniaceae). Tetrahedron Lett. 2020, 61, 152005. [Google Scholar] [CrossRef]
- Yi, X.X.; Chen, Y.; Xie, W.P.; Xu, M.B.; Chen, Y.N.; Gao, C.H.; Huang, R.M. Four new jacaranone analogs from the fruits of a Beibu Gulf mangrove Avicennia marina. Mar. Drugs 2014, 12, 2515–2525. [Google Scholar] [CrossRef] [Green Version]
- Lozada-Lechuga, J.; Villarreal, M.L.; Fliniaux, M.A.; Bensaddek, L.; Mesnard, F.; del Gutiérrez, M.C.; Cardoso-Taketa, A.T. Isolation of jacaranone, a sedative constituent extracted from the flowers of the Mexican tree Ternstroemia pringlei. J. Ethnopharmacol. 2010, 127, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Suh, J.; Shin, M.H.; Jung, J.H.; Im, K.S. Jacaranone and related compounds from the fresh fruits of Ternstroemia japonica and their antioxidative activity. Arch. Pharm. Res. 2005, 28, 885–888. [Google Scholar] [CrossRef]
- Gomes, K.S.; Tamayose, C.I.; Ferreira, M.J.; Murakami, C.; Young, M.C.; Antar, G.M.; Camilo, F.F.; Sartorelli, P.; Lago, J.H. Isolation of antifungal quinoid derivatives from leaves of Pentacalia desiderabilis (Vell.) Cuatre. (Asteraceae) using ionic liquid in the microwave assisted extraction. Quim. Nova 2019, 42, 156–158. [Google Scholar] [CrossRef]
- Morais, T.R.; Romoff, P.; Fávero, O.A.; Reimão, J.Q.; Lourenço, W.C.; Tempone, A.G.; Hristov, A.D.; Di Santi, S.M.; Lago, J.H.; Sartorelli, P.; et al. Anti-malarial, anti-trypanosomal, and anti-leishmanial activities of jacaranone isolated from Pentacalia desiderabilis (Vell.) Cuatrec. (Asteraceae). Parasitol. Res. 2011, 110, 95–101. [Google Scholar] [CrossRef]
- Fraga, B.M.; Díaz, C.E.; Amador, L.J.; Reina, M.; Santana, O.; González-Coloma, A. Bioactive compounds from transformed root cultures and aerial parts of Bethencourtia hermosae. Phytochemistry 2014, 108, 220–228. [Google Scholar] [CrossRef]
- Eliáš, P.; Turisová, I.; Ťavoda, O. Occurrence of small flower hawksbeard (Crepis pulchra L.) in Slovakia. Thaiszia J. Bot. 2010, 20, 127–135. [Google Scholar]
- Namukobe, J.; Kasenene, J.M.; Kiremire, B.T.; Byamukama, R.; Kamatenesi-Mugisha, M.; Krief, S.; Dumontet, V.; Kabasa, J.D. Traditional plants used for medicinal purposes by local communities around the Northern sector of Kibale National Park, Uganda. J. Ethnopharmacol. 2011, 136, 236–245. [Google Scholar] [CrossRef]
- Fleurentin, J.; Hoefler, C.; Lexa, A.; Mortier, F.; Pelt, J.M. Hepatoprotective properties of Crepis rueppellii and Anisotes trisulcus: Two traditional medicinal plants of Yemen. J. Ethnopharmacol. 1986, 16, 105–111. [Google Scholar] [CrossRef]
- Hartwell, J.L. Plants used against cancer. A survey. Lloydia 1968, 31, 71–170. [Google Scholar]
- Bakar, F.; Acikara, Ö.B.; Ergene, B.; Nebioğlu, S.; Çitoğlu, G.S. Antioxidant activity and phytochemical screening of some Asteraceae plants. Turk. J. Pharm. Sci. 2015, 12, 36–45. [Google Scholar] [CrossRef]
- Dalar, A. Plant taxa used in the treatment of diabetes in Van Province, Turkey. Int. J. Second. Metab. 2018, 5, 170–184. [Google Scholar] [CrossRef]
- Genç, G.E.; Özhatay, N. An ethnobotanical study in Çatalca (European part of İstanbul) II. Turk. J. Pharm. Sci. 2006, 3, 73–89. [Google Scholar]
- Kilic, O.; Bagci, E. An ethnobotanical survey of some medicinal plants in Keban (Elazığ-Turkey). J. Med. Plant Res. 2013, 7, 1675–1684. [Google Scholar] [CrossRef]
- Sansanelli, S.; Tassoni, A. Wild food plants traditionally consumed in the area of Bologna (Emilia Romagna region, Italy). J. Ethnobiol. Ethnomed. 2014, 10, 69. [Google Scholar] [CrossRef] [Green Version]
- Guarrera, P.M.; Savo, V. Perceived health properties of wild and cultivated food plants in local and popular traditions of Italy: A review. J. Ethnopharmacol. 2013, 146, 659–680. [Google Scholar] [CrossRef]
- González-Tejero, M.R.; Molero-Mesa, J.; Casares-Porcel, M.; Lirola, M.J.M. New contributions to the ethnopharmacology of Spain. J. Ethnopharmacol. 1995, 45, 157–165. [Google Scholar] [CrossRef]
- Rahman, M. An ethnobotanical investigation on Asteraceae family at Rajshahi, Bangladesh. Acad. J. Med. Plants 2013, 1, 92–100. [Google Scholar]
- Ndom, J.C.; Mbafor, J.T.; Wansi, J.D.; Kamdem, A.W.; Meva’a, L.M.; Vardamides, J.C.; Toukam, F.; Pegyemb, D.; Ngando, T.M.; Laatsch, H.; et al. Sesquiterpene lactones from Crepis cameroonica (Asteraceae). Nat. Prod. Res. 2006, 20, 435–442. [Google Scholar] [CrossRef]
- Michalska, K.; Kisiel, W.; Zidorn, C. Sesquiterpene lactones from Crepis aurea (Asteraceae, Cichorieae). Biochem. Syst. Ecol. 2013, 46, 1–3. [Google Scholar] [CrossRef]
- Ebada, S.S.; El-Kashef, D.H.; Müller, W.E.G.; Proksch, P. Cytotoxic eudesmane sesquiterpenes from Crepis sancta. Phytochem. Lett. 2019, 33, 46–48. [Google Scholar] [CrossRef]
- Mañez, S.; Recio, M.C.; Giner, R.M.; Sanz, M.J.; Terencio, M.C.; Peris, J.B.; Stübing, G.; Rios, J.L. A chematoxonomic review of the subtribe Crepidinase based on its phenol constituents. Biochem. Syst. Ecol. 1994, 22, 297–305. [Google Scholar] [CrossRef]
- Zidorn, C.; Schubert, B.; Stuppner, H. Phenolics as chemosystematic markers in and for the genus Crepis (Asteraceae, Cichorieae). Sci. Pharm. 2008, 76, 743–750. [Google Scholar] [CrossRef] [Green Version]
- Ooi, L.S.; Wang, H.; Luk, C.W.; Ooi, V.E. Anticancer and antiviral activities of Youngia japonica (L.) DC (Asteraceae, Compositae). J. Ethnopharmacol. 2004, 94, 117–122. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Uyar, P.; Aktumsek, A.; Uysal, S.; Kocak, M.S.; Ceylan, R. Crepis foetida L. subsp. rhoeadifolia (Bieb.) Celak. as a source of multifunctional agents: Cytotoxic and phytochemical evaluation. J. Funct. Foods 2015, 17, 698–708. [Google Scholar] [CrossRef]
- Barda, C.; Grafakou, M.E.; Kalpoutzakis, E.; Heilmann, J.; Skaltsa, H. Chemical composition of Crepis foetida L. and C. rubra L. volatile constituents and evaluation of the in vitro anti-inflammatory activity of salicylaldehyde rich volatile fraction. Biochem. Syst. Ecol. 2021, 96, 104256. [Google Scholar] [CrossRef]
- Holub, J. Crepis pulchra L. In Červená Kniha Ohrozených a Vzácnych Druhov Rastlín a Živočíchov SR a ČR; Čeřovský, J., Feráková, V., Holub, J., Maglocký, Š., Procházka, F., Eds.; Vyššie Rastliny; Príroda a.s.: Bratislava, Slovakia, 1999; Volume 5, p. 355. (In Czech) [Google Scholar]
- Bogler, D.J. Crepis L. In Flora of North America: North of Mexico; Oxford University Press: New York, NY, USA, 2006; Volume 19, pp. 222–239. [Google Scholar]
- Kisiel, W.; Gromek, D. Guaianolides from Crepis pulchra. Pol. J. Chem. 1994, 68, 535–538. [Google Scholar] [CrossRef]
- Lee, E.H.; Kim, H.J.; Song, Y.S.; Jin, C.; Lee, K.T.; Cho, J.; Lee, Y.S. Constituents of the stems and fruits of Opuntia ficus-indica var. saboten. Arch. Pharm. Res. 2003, 26, 1018–1023. [Google Scholar] [CrossRef]
- Kurashina, Y.; Miura, A.; Enomoto, M.; Kuwahara, S. Stereoselective synthesis of malyngic acid and fulgidic acid. Tetrahedron 2011, 67, 1649–1653. [Google Scholar] [CrossRef]
- Adfa, M.; Yoshimura, T.; Komura, K.; Koketsu, M. Antitermite activities of coumarin derivatives and scopoletin from Protium javanicum Burm. f. J. Chem. Ecol. 2010, 36, 720–726. [Google Scholar] [CrossRef]
- Ersöz, T.; Harput, Ü.Ş.; Saracoğlu, İ.; Ҫaliş, İ. Phenolic compounds from Scutellaria pontica. Turk. J. Chem. 2002, 26, 581–588. [Google Scholar]
- Tian, Y.Q.; Niu, Y.F.; Shen, T.; Weng, C.W.; Xie, W.D.; Row, K.H. Cyclohexanone derivatives from Senecio argunensis. J. Chem. Res. 2010, 34, 25–27. [Google Scholar] [CrossRef]
- Gachet, M.S.; Kunert, O.; Kaiser, M.; Brun, R.; Muñoz, R.A.; Bauer, R.; Schühly, W. Jacaranone-derived glucosidic esters from Jacaranda glabra and their activity against Plasmodium falciparum. J. Nat. Prod. 2010, 73, 553–556. [Google Scholar] [CrossRef]
- Santos, C.A.; Raslan, D.S.; Chiari, E.; Oliveira, A.B. Bioguided assay of Jacaranda Macrantha Cham. (Bignoniaceae). Acta Hortic. 1999, 501, 151–154. [Google Scholar] [CrossRef]
- Rana, A.; Bhangalia, S.; Singh, H.P. A new phenylethanoid glucoside from Jacaranda mimosifolia. Nat. Prod. Res. 2013, 27, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.V.; Duarte, L.P.; Silva, R.R.; Takahashi, J.A. New jacaranone glucoside from Jacaranda oxyphylla leaves. Nat. Prod. Res. 2016, 30, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- Zanotelli, P.; Locateli, G.; Vecchia, C.D.; Gomes, D.B.; Oliveira, B.M.M.; Lutinski, J.A.; Predebom, A.J.; Miorando, D.; Zanatta, M.E.C.; Steffler, A.M.; et al. Gastroprotective potential of the hydroalcoholic extract from Jacaranda puberula in mice. Rev. Bras. Farmacogn. 2020, 30, 838–843. [Google Scholar] [CrossRef]
- Yvin, J.C.; Chevolot, L.; Chevolot-Magueur, A.M.; Cochard, J.C. First isolation of jacaranone from an alga, Delesseria sanguinea. A metamorphosis inducer of Pecten larvae. J. Nat. Prod. 1985, 48, 814–816. [Google Scholar] [CrossRef]
- Winiewski, V.; Serain, A.F.; de Sa, E.S.; Salvador, M.J.; Stefanello, M.E.A. Chemical constituents of Sinningia mauroana and screening of its extracts for antimicrobial, antioxidant and cytotoxic activities. Quim. Nova 2020, 43, 181–187. [Google Scholar] [CrossRef]
- Silva, A.S.; Amorim, M.S.; Fonseca, M.M.; Salvador, M.J.; de Sa, E.L.; Stefanello, M.E.A. A new cytotoxic naphthoquinone and other chemical constituents of Sinningia reitzii. J. Braz. Chem. Soc. 2019, 30, 2060–2065. [Google Scholar] [CrossRef]
- Yan, X.J.; Bai, X.Y.; Liu, Q.B.; Liu, S.; Gao, P.Y.; Li, L.Z.; Song, S.J. Two new glycosides from the fruits of Forsythia suspense. J. Asian Nat. Prod. Res. 2014, 16, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Mezache, N.; Derbré, S.; Akkal, S.; Laouer, H.; Séraphin, D.; Richomme, P. Fast counter current chromatography of n-butanolic fraction from Senecio giganteus (Asteraceae). Nat. Product Commun. 2009, 4, 1357–1362. [Google Scholar] [CrossRef] [Green Version]
- Loizzo, M.R.; Tundis, R.; Statti, G.A.; Menichini, F. Jacaranone: A cytotoxic constituent from Senecio ambiguus subsp. ambiguus (Biv.) DC. against renal adenocarcinoma ACHN and prostate carcinoma LNCaP cells. Arch. Pharm. Res. 2007, 30, 701–707. [Google Scholar] [CrossRef]
- Gelbaum, L.T.; Zalkow, L.H.; Hamilton, D. Cytotoxic agent from Senecio anonymus Wood. J. Nat. Prod. 1982, 45, 370–372. [Google Scholar] [CrossRef]
- Xie, W.D.; Weng, C.W.; Gao, X.; Zhao, H.; Row, K.H. A new farnesene derivative and other constituents from Senecio Cannabifolius. J. Chin. Chem. Soc. 2010, 57, 436–438. [Google Scholar] [CrossRef]
- Lajide, L.; Escoubas, P.; Mizutani, J. Cyclohexadienones-insect growth inhibitors from the foliar surface and tissue extracts of Senecio cannabifolius. Experientia 1996, 52, 259–263. [Google Scholar] [CrossRef]
- Ma, H.Y.; Yang, L.; Zhang, M.; Wang, C.H.; Wang, Z.T. A new compound from Senecio cannabifolius var. integrilifolius. Yao Xue Xue Bao 2008, 43, 626–629. [Google Scholar]
- Bohlmann, F.; Zdero, C.; King, R.M.; Robinson, H. The first acetylenic monoterpene and other constituents from Senecio clevelandii. Phytochemistry 1981, 20, 2425–2427. [Google Scholar] [CrossRef]
- Wu, C.H.; Zhang, L.; Zhou, P.P.; Sun, M.; Gao, K. Three new jacaranone derivatives from the aerial parts of Senecio chrysanoides DC. with their cytotoxic activity. Phytochem. Lett. 2015, 14, 245–248. [Google Scholar] [CrossRef]
- Pettit, G.R.; Einck, J.J.; Brown, P.; Harvey, T.B.; Ode, R.H.; Pase, C.P. Antineoplastic agents. 67. Senecio fendleri Gray. J. Nat. Prod. 1980, 43, 609–616. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Statti, G.A.; Menichini, F.; Houghton, P.J. In-vitro antiproliferative effects on human tumour cell lines of extracts and jacaranone from Senecio leucanthemifolius Poiret. J. Pharm. Pharmacol. 2005, 57, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.; Grande, C.; Anaya, J.; Grande, M. Secondary metabolites from Senecio minutus and Senecio boissieri: A new jacaranone derivative. Fitoterapia 2000, 71, 91–93. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, N.; Casida, J.E. Insecticides in Chinese medicinal plants: Survey leading to jacaranone, a neurotoxicant and glutathione-reactive quinol. J. Agric. Food Chem. 2003, 51, 2544–2547. [Google Scholar] [CrossRef]
- Yang, X.; Yang, L.; Xiong, A.; Li, D.; Wang, Z. Authentication of Senecio scandens and S. vulgaris based on the comprehensive secondary metabolic patterns gained by UPLC–DAD/ESI-MS. J. Pharm. Biomed. Anal. 2011, 56, 165–172. [Google Scholar] [CrossRef]
- Tian, X.Y.; Wang, Y.H.; Yang, Q.Y.; Yu, S.S.; Fang, W.S. Jacaranone analogs from Senecio scandens. J. Asian Nat. Prod. Res. 2009, 11, 63–68. [Google Scholar] [CrossRef]
- Tian, X.Y.; Wang, Y.H.; Yang, Q.Y.; Liu, X.; Fang, W.S.; Yu, S.S. Jacaranone glycosides from Senecio scandens. J. Asian Nat. Prod. Res. 2006, 8, 125–132. [Google Scholar] [CrossRef]
- Wang, W.S.; Lu, P.; Duan, C.H.; Feng, J.C. A new jacaranone derivative from Senecio scandens var. incisus. Nat. Prod. Res. 2010, 24, 370–374. [Google Scholar] [CrossRef]
- Mandić, B.; Gođevac, D.; Vujisić, L.; Trifunović, S.; Tesević, V.; Vajs, V.; Milosavljević, S. Semiquinol and phenol compounds from seven Senecio species. Chem. Pap. 2011, 65, 90–92. [Google Scholar] [CrossRef]
- Pérez-Castorena, A.L.; Arciniegas, A.; Martinez, F.; Marquez, C.; Villaseñor, J.L.; Romo de Vivar, A. Chemical constituents of Packera coahuilensis and Packera bellidifolia. Biochem. Syst. Ecol. 2001, 29, 203–206. [Google Scholar] [CrossRef]
- Ogura, M.; Cordell, G.A.; Farnsworth, N.R. Potential anticancer agents. IV. Constituents of Jacaranda caucana Pittier (Bignoniaceae). Lloydia 1977, 40, 157–168. [Google Scholar] [PubMed]
- Massaoka, M.H.; Matsuo, A.L.; Figueiredo, C.R.; Farias, C.F.; Girola, N.; Arruda, D.C.; Scutti, J.A.; Romoff, P.; Favero, O.A.; Ferreira, M.J.; et al. Jacaranone induces apoptosis in melanoma cells via ROS-mediated downregulation of Akt and p38 MAPK activation and displays antitumor activity in vivo. PLoS ONE 2012, 7, e38698. [Google Scholar] [CrossRef] [Green Version]
- Presser, A.; Lainer, G.; Kretschmer, N.; Schuehly, W.; Saf, R.; Kaiser, M.; Kalt, M.M. Synthesis of jacaranone-derived nitrogenous cyclohexadienones and their antiproliferative and antiprotozoal activities. Molecules 2018, 23, 2902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).