Chemical Composition, Antioxidant, and Antimicrobial Activity of Dracocephalum moldavica L. Essential Oil and Hydrolate
Abstract
:1. Introduction
2. Results
2.1. Volatile Compounds of Essential Oil and Hydrolate
2.2. Antioxidant Activity
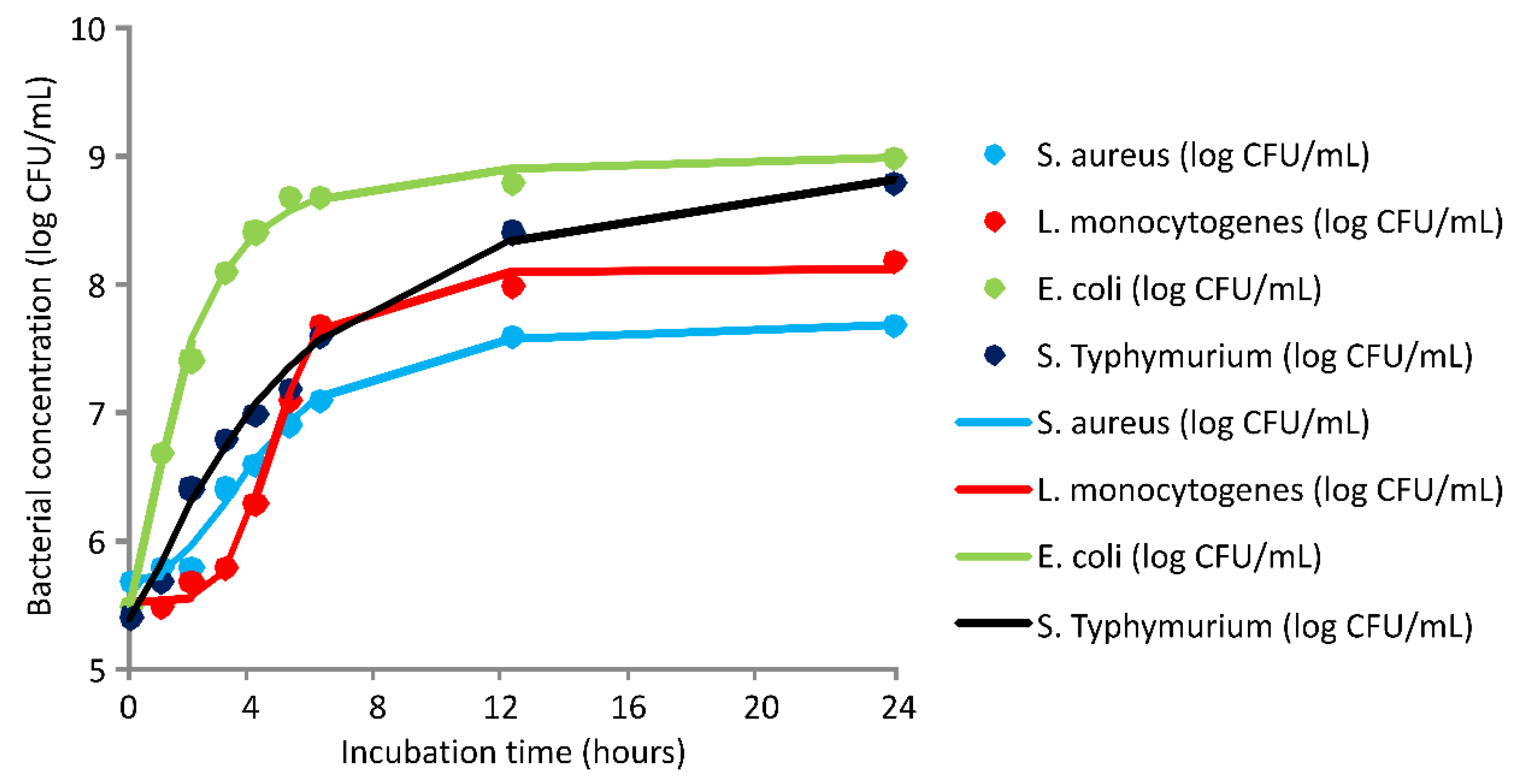
2.3. Antimicrobial Activity
3. Discussion
3.1. Volatile Compounds of Essential Oil and Hydrolate
3.2. Antioxidant Activity
3.3. Antimicrobial Activity
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oil Isolation
4.3. Analysis of Volatile Compounds
4.4. In vitro Assessment of Antioxidant Activity
4.4.1. DPPH• (2,2-diphenyl-1-picrylhydrazyl)
4.4.2. ABTS•+ (2,2′-azino-bis-3-ethylbenzothiazoline-6-sulphonic Acid)
4.4.3. SOA (Superoxide Anion)
4.4.4. RP (Reducing Power)
4.4.5. BCB (β-Carotene Bleaching)
4.5. In Vitro Assessment of the Antimicrobial Activity
4.5.1. Screening of Antimicrobial Effect of DMEO and DMH (Disk Diffusion Method)
4.5.2. Minimal Inhibitory Concentration (MIC)
4.5.3. Assessment of Antimicrobial Activity Using a Time-Kill Procedure
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Liang, D.; Wang, G.T.; Wen, J.; Wang, R.J. Nutritional and functional properties of wild food medicine plants from the coastal region of South China. J. Evid.-Based Integr. Med. 2020, 25, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aćimović, M.; Stanković, J.; Cvetković, M.; Todosijević, M.; Rat, M. The chemical composition of the essential oil of Dracocephalum moldavica L. from Vojvodina Province (Serbia). Biol. Nyssana. 2019, 10, 23–28. [Google Scholar] [CrossRef]
- Suschke, U.; Sporer, F.; Schneele, J.; Geiss, H.K.; Reichling, J. Antibacterial and cytotoxic activity of Nepeta cataria L., N. cataria var. citriodora (Beck.) Balb. and Melissa officinalis L. essential oils. Nat. Prod. Commun. 2007, 2, 1277–1286. [Google Scholar] [CrossRef] [Green Version]
- Aćimović, M.; Sikora, V.; Brdar-Jokanović, M.; Kiprovski, B.; Popović, V.; Koren, A.; Puvača, N. Dracocephalum moldavica: Cultivation, chemical composition and biological activity. J. Agron. Technol. Eng. Manag. 2019, 2, 153–167. [Google Scholar]
- Poviliate, V.; Cuvelier, M.E.; Berset, C. Antioxidant properties of Moldavian dragonhead (Dracocephalum moldavica L.). J. Food Lipids 2001, 8, 45–64. [Google Scholar] [CrossRef]
- Mohtashami, S.; Babalar, M.; Mirjalili, M.H. Phenological variation in medicinal traits of Dracocephalum moldavica L. (Lamiaceae) under different growing conditions. J. Herbs. Spices Med. Plants. 2013, 19, 377–390. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Mihai, C.T.; Vochita, G.; Rotinberg, P.; Trifan, A.; Luca, S.V.; Petreus, T.; Gille, E.; Miron, A. Antigentoxic and antioxidant activities of a polyphenolic extract from European Dracocephalum moldavica L. Ind. Crops. Prod. 2016, 79, 248–257. [Google Scholar] [CrossRef]
- Aslanipour, B.; Heidari, R.; Farnad, N. Phenolic combination and comparison ofantioxidant activity in three different alcoholic extracts of Dracocephalum moldavica L. Turk. J. Agric. Food Sci. Technol. 2017, 5, 199–206. [Google Scholar]
- Fallah, S.; Rostaei, M.; Lorigooini, Z.; Sukri, A.A. Chemical compositions of essential oil and antioxidant activity of dragonhead (Dracocephalum moldavica) in sole crop and dragonhead-soybean (Glycine max) intercropping system under organic manure and chemical fertilizers. Ind. Crops Prod. 2018, 115, 158–165. [Google Scholar] [CrossRef]
- Sonboli, A.; Mojarrad, M.; Gholipour, A.; Ebrahimi, S.N.; Arman, M. Biological activity and composition of the essential oil of Dracocephalum moldavica L. grown in Iran. Nat. Prod. Commun. 2008, 3, 1547–1550. [Google Scholar] [CrossRef] [Green Version]
- El-Baky, H.H.; El-Baroty, G.S. Chemical and biological evaluation of the essential oil of Egyptian Moldavian dragonhead (Dracocephalum moldavica L.). Int. J. Integr. Biol. 2008, 3, 202–208. [Google Scholar]
- Ehsani, A.; Alizadeh, O.; Hashemi, M.; Afshari, A.; Aminzare, M. Phytochemical, antioxidant and antibacterial properties of Melissa officinalis and Dracocephalum moldavica essential oils. Vet. Res. Forum 2017, 8, 223–229. [Google Scholar] [PubMed]
- Keikhaie, R.K.; Jahantigh, H.R.; Bagheri, R.; Kehkhaie, R.A. The effect of the ethanol extract of Dracocephalum moldavica (Badrashbu) against strains of antibiotic resistant Escherichia coli and Klebsiella pneumoniae. Int. J. Infection. 2018, 5, e65295. [Google Scholar] [CrossRef]
- Martinez-Vazquez, M.; Estrada-Reyes, R.; Martinez-Laurrabaquio, A.; Lopez-Rubalcava, C.; Heinze, G. Neuropharmacological study of Dracocephalum moldavica L. (Lamiaceae) in mice: Sedative effect and chemical analysis of an aqueous extract. J. Ethnopharmacol. 2012, 441, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Olha, B.; Antonina, P.; Mariia, S. Chemical compositions and sedative activities of the Dracocephalum moldavica L. and Ocimum americanum L. essential oils. Pharmacol. Online 2021, 2, 179–187. [Google Scholar]
- Zuniga, M.I.J.; Mariles, A.J.H.; Flores, J.L.C.; Herrera, J.A.M.; Sotelo, M.G.R.; Montes, G.I.C.; Gomez, Y.I.M.G. Antidepressant-like effects of Dracocephalum moldavica L. in mouse models of immobility tests. Pharmacogn. J. 2019, 11, 976–983. [Google Scholar] [CrossRef] [Green Version]
- Maham, M.; Akbari, H.; Delazar, A. Chemical composition and antinococeptive effect of the essential oil of Dracocephalum moldavica L. Pharm. Sci. 2013, 18, 187–192. [Google Scholar]
- Jiang, H.; Zeng, L.; Guo, S.; Xing, J.; Li, Z.; Liu, R. Tilianin extracted from Dracocephalum moldavica L. induces intrinsic apoptosis and drives inflammatory microenvironment response on pharyngeal squamous carcinoma cells via regulating TLR4 signaling pathways. Front. Pharmacol. 2020, 11, 205. [Google Scholar] [CrossRef]
- Nie, L.; Li, R.; Huang, J.; Wang, L.; Ma, M.; Huang, C.; Wu, T.; Yan, R.; Hu, X. Abietane diterpenoids from Dracocephalum moldavica L. and their anti-inflammatory activities in vitro. Phytochemistry 2021, 184, 112680. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, T.; Jiang, Z.; Gao, Z.; Zhang, M.; Wang, D.; Zheng, Q. Neuroprotective effect of Dracocephalum moldavica L. total flavonoids in transient cerebral ischemia in rats. Annu. Res. Rev. Biol. 2014, 4, 1915–1926. [Google Scholar] [CrossRef]
- Li, J.; Xu, S. Tilianin attenuates MPP+-induced oxidative stress and apoptosis of dopaminergic neurons in a cellular model of Parkinson’s disease. Exp. Ther. Med. 2022, 23, 293. [Google Scholar] [CrossRef]
- Najafi, M.; Ghasemian, E.; Fatizad, F.; Garjani, A. Effects of total extract of Dracocephalum moldavica on ischemia/reperfusion induced arrhythmias and infarct size in the isolated rat heart. Iran. J. Basic Med. Sci. 2009, 11, 229–235. [Google Scholar] [CrossRef]
- Zeng, C.; Jiang, W.; Yang, X.; He, C.; Wang, W.; Xing, J. Pretreatment with total flavonoid extract from Dracocephalum moldavica L. attenuates ischemia reperfusion-induced apoptosis. Sci. Rep. 2018, 8, 17491. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Yuan, Y.; Wang, Y.; Tian, L.; Wang, X.; Xin, J.; Wang, Y.; Guo, X.; Qin, D. The mechanism study of Dracocephalum moldavica L. total flavonoids on apoptosis induced by myocardial ischemia/reperfusion injury in vivo and in vitro. Biomed. J. Sci. Tech. Research. 2019, 20, 14985–14996. [Google Scholar] [CrossRef]
- Jin, M.; Yu, H.; Jin, X.; Yan, L.; Wang, J.; Wang, Z. Dracocephalum moldavica L. extract protect H9c2 cardiomyocytes against H2O2-induced apoptosis and oxidative stress. Hindawi BioMed Res. Int. 2020, 2020, 8379358. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.G. Nutraceutical potential of Apiaceae. In Bioactive Molecules in Food, Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Germany, 2017; pp. 1311–1341. [Google Scholar] [CrossRef]
- Oniszczuk, T.; Kasprzak-Drozd, K.; Olech, M.; Wojtowicz, A.; Nowak, R.; Rusinek, R.; Szponar, J.; Combrzynski, M.; Oniszczuk, A. The impact of formulation on the content of phenolic compounds in snakes enriched with Dracocephalum moldavica L. seed: Introduction to receiving a new functional food product. Molecules 2021, 26, 1245. [Google Scholar] [CrossRef]
- Nieto, G. How are medicinal plants useful when added to foods? Medicines 2020, 7, 58. [Google Scholar] [CrossRef]
- Amjadi, S.; Nouri, S.; Yorghanlou, R.A.; Roufegarinejad, L. Development of hydroxypropyl methylcellulose/sodium alginate blend active film incorporated with Dracocephalum moldavica L. essential oil for food preservation. J. Thermoplast. Compos. Mater. 2020, 1–7. [Google Scholar] [CrossRef]
- Napoli, E.; Di Vito, M. Toward a new future for essential oils. Antibiotics. 2021, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Rao, R. Hydrosols and water-soluble essential oils: Medicinal and biological properties. In Recent Progress in Medicinal Plants, Essential oils I; Govil, J.N., Bhattacharya, S., Eds.; Studium Press LLC: Houston, TX, USA, 2013; Volume 36, pp. 119–140. [Google Scholar]
- Aćimović, M.; Tešević, V.; Smiljanić, K.; Cvetković, M.; Stanković, J.; Kiprovski, B.; Sikora, V. Hydrolates–by-products of essential oil distillation: Chemical composition, biological activity and potential uses. Adv. Technol. 2020, 9, 54–70. [Google Scholar] [CrossRef]
- Sim, J.H.; Jamaludin, N.S.; Khoo, C.H.; Cheah, Y.K.; Halim, S.N.B.A.; Seng, H.L.; Tiekink, E.R.T. In vitro antibacterial and time-kill evaluation of phosphanegold(I) dithiocarbamates, R3PAu[S2CN(iPr)CH2CH2OH] for R = Ph, Cy and Et, against a broad range of Gram-positive and Gram negative bacteria. Gold Bull. 2014, 47, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Nasiri, Y. Crop productivity and chemical compositions of dragonhead (Dracocephalum moldavica L.) essential oil under different cropping patterns and fertilization. Ind. Crops Prod. 2021, 171, 113920. [Google Scholar] [CrossRef]
- Faridvand, S.; Rezaei-Chiyaneh, E.; Battaglia, M.L.; Gitari, H.I.; Raza, M.A.; Siddique, K.H.M. Application of bio and chemical fertilizers improves yield, and essential oil quantity and quality of Moldavian balm (Dracocephalum moldavica L.) intercropped with mung bean (Vigna radiate L.). Food Energy Secur. 2021, e319. [Google Scholar] [CrossRef]
- Fattahi, A.; Shakeri, A.; Tayarani-Najaran, Z.; Kharbach, M.; Segers, K.; Heyden, V.Y.; Taghizadeh, S.F.; Rahmani, H.; Asili, J. UPLC– PDA- ESI– QTOF– MS/MS and GC- MS analysis of Iranian Dracocephalum moldavica L. Food Sci. Nutr. 2021, 9, 4278–4286. [Google Scholar] [CrossRef] [PubMed]
- Rudy, S.; Dziki, D.; Biernacka, B.; Krykowski, A.; Rudy, M.; Gawlik-Dziki, U.; Kachel, M. Drying characteristics of Dracocephalum moldavica leaves: Drying kinetics and physiochemical properties. Processes 2020, 8, 509. [Google Scholar] [CrossRef]
- Alaei, S. Essential oil content and composition of Dracocephalum moldavica under different irrigation regimes. Int. J. Hortic. Sci. Technol. 2019, 6, 167–175. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Nouraein, M.; Sabaghnia, N. Influence of different weed management techniques on the growth and essential oils of dragonhead (Dracocephalum moldavica L.). Rom. Biotechnol. Lett. 2017, 22, 12950–12960. [Google Scholar]
- Hegazy, M.H.; Alzuibr, F.A.; Mahmoud, A.A.; Mohamed, H.F.Y.; Said-Al Ahl, H.A.H. The effects of zinc application and cutting on growth, herb, essential oil and flavonoids in three medicinal Lamiaceae plants. Eur. J. Med. Plants. 2016, 12, 1–12. [Google Scholar] [CrossRef]
- Said-Al Ahl, H.A.H.; Sabra, A.S.; Gendy, A.N.; Aziz, E.E.; Tkachenko, K.G. Changes in content and chemical composition of Dracocephalum moldavica L. essential oil at different harvest dates. J. Med. Plants Stud. 2015, 3, 61–64. [Google Scholar]
- Janmohammadi, M.; Sufi-Mahmoudi, Z.; Ahadnezhad, A.; Yousefzadeh, S.; Sabaghnia, N. Influence of chemical and organic fertilizer on growth, yield and essential oil of dragonhead (Dracocephalum moldavica L.) plant. Acta Agric. Slov. 2014, 103, 73–81. [Google Scholar] [CrossRef]
- Alaei, S.; Mahna, N. Comparison of essential oil composition in Dracocephalum moldavica in greenhouse and field. J. Essent. Oil-Bear. Plants 2013, 16, 346–351. [Google Scholar] [CrossRef]
- Aziz, E.E.; Hussein, M.S.; Wahba, H.E.; Razin, A.M. Essential oil constituents of Dracocephalum moldavica L. grown under salt stress and different sources of soil amendment. Middle-East J. Sci. Res. 2013, 16, 706–713. [Google Scholar] [CrossRef]
- Ganbarzadeh, Z.; Mohsenzadeh, S.; Rowshan, V.; Moradshahi, A. Evaluation of the growth, essential oil composition and antioxidant activity of Dracocephalum moldavica under water deficit stress and symbiosis with Claroideoglomus etunicatum and Micrococcus yunnanensis. Sci. Hortic. 2019, 256, 108652. [Google Scholar] [CrossRef]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium dioxide nanoparticles (TIO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef] [PubMed]
- Amini, R.; Ebrahimi, A.; Nasab, A.D.M. Moldavian balm (Dracocephalum moldavica L.) essential oil content and composition as affected by sustainable weed management treatments. Ind. Crops Prod. 2020, 150, 112416. [Google Scholar] [CrossRef]
- Golparvar, A.R.; Haidipanah, A.; Gheisari, M.M.; Khaliliazar, R. Chemical constituents of essential oil of Dracocephalum moldavica L. and Dracocephalum kotschyi Boiss. from Iran. Acta Agric. Slov. 2016, 107, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Amini, R.; Zafarani-Moattar, P.; Shakiba, M.R.; Sarikhani, M.R. Essential oil yield and composition of Moldavian balm (Dracocephalum moldavica L.) as affected by inoculation treatments under drought stress conditions. J. Essent. Oil-Bear. Plants 2020, 23, 728–742. [Google Scholar] [CrossRef]
- Davazdahemami, S.; Allahdadi, M. Essential oil yield and composition of four annual plants (ajowan, dill, Moldavian balm and black cumin) under saline irrigation. Food Ther. Health Care 2022, 4, 5. [Google Scholar] [CrossRef]
- Pouresmaeil, M.; Sabzi-Nojadeh, M.; Movafeghi, A.; Aghbash, B.N.; Kosari-Nasab, M.; Zengin, G.; Maggi, F. Phytotoxic activity of Moldavian dragonhead (Dracocephalum moldavica L.) essential oil and its possible use as bio-herbicide. Process. Biochem. 2022, 114, 86–92. [Google Scholar] [CrossRef]
- Ding, S.E.; Cixi, T.; Charles, P. Synthesis and toxicity of Chinese Dracocephalum moldavica (Labiatae) fundamental oil against two grain storage insect. Afr. J. Insects. 2015, 2, 077–081. [Google Scholar]
- Hussein, M.S.; El-Sherbeny, S.E.; Khalil, M.Y.; Naguib, N.Y.; Aly, S.M. Growth characters and chemical constituents of Dracocephalum moldavica L. plants in relation to compost fertilizer and planting distance. Sci. Hortic. 2006, 108, 322–331. [Google Scholar] [CrossRef]
- Aćimović, M.; Ivanović, S.; Simić, K.; Pezo, L.; Zeremski, T.; Ovuka, J.; Sikora, V. Chemical characterization of Marrubium vulgare volatiles from Serbia. Plants 2021, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Ljujić, J.; Vulić, J.; Zheljazkov, V.D.; Pezo, L.; Varga, A.; Tumbas Šaponjac, V. Helichrysum italicum (Roth) G. Don essential oil from Serbia: Chemical composition, classification and biological activity—May it be a suitable new crop for Serbia? Agronomy 2021, 11, 1282. [Google Scholar] [CrossRef]
- Aćimović, M.; Pezo, L.; Zeremski, T.; Lončar, B.; Marjanović Jeromela, A.; Stanković Jeremić, J.; Cvetković, M.; Sikora, V.; Ignjatov, M. Weather conditions influence on hyssop essential oil quality. Processes 2021, 9, 1152. [Google Scholar] [CrossRef]
- Aćimović, M.; Lončar, B.; Pezo, M.; Stanković Jeremić, J.; Cvetković, M.; Rat, M.; Pezo, L. Volatile compounds of Nepeta nuda L. from Rtanj Mountain (Serbia). Horticulturae 2022, 8, 85. [Google Scholar] [CrossRef]
- Aćimović, M.; Stanković Jeremić, J.; Todosijević, M.; Kiprovski, B.; Vidović, S.; Vladić, J.; Pezo, L. Comparative study of the essential oil and hydrosol composition of sweet wormwood (Artemisia annua L.) from Serbia. Chem. Biodivers. 2022, 19, e202100954. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Aćimović, M.; Šeregelj, V.; Šovljanski, O.; Tumbas Šaponjac, V.; Švarc Gajić, J.; Brezo-Borjan, T.; Pezo, L. In vitro antioxidant, antihyperglycemic, anti-inflammatory and antimicrobial activity of Satureja kitaibelii Wierzb. ex Heuff. subcritical water extract. Ind. Crops Prod. 2021, 169, 113672. [Google Scholar] [CrossRef]
- Seema Farhath, M.S.; Vijaya, P.P.; Vimal, M. Antioxidant activity of geraniol, geranial acetate, gingerol and eugenol. Res. Pharm. 2013, 3, 1–6. [Google Scholar]
- de Lira, M.H.P.; de Andrade, F.P., Jr.; Moraes, G.F.Q.; Macena, G.S.; Pereira, F.O.; Lima, I.O. Antimicrobial activity of geraniol: An integrative review. J. Essent. Oil Res. 2020, 32, 187–197. [Google Scholar] [CrossRef]
- Celuppi, L.C.M.; Capelezzo, A.P.; Cima, L.B.; Zeferino, R.C.F.; Zanetti, M.; Riella, H.G.; Fiori, M.A. Antimicrobial cellulose acetate films by incorporation of geranyl acetate for active food packaging application. Res. Soc. Dev. 2022, 11, e40111125141. [Google Scholar] [CrossRef]
- Seidel, V.; Peyfoon, E.; Watson, D.G.; Fearnley, J. Comparative study of the antibacterial activity of propolis from different geographical and climatic zones. Phytother. Res. 2008, 22, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Antimicrobial activities of European propolis collected from various geographic origins alone and in combination with antibiotics. Medicines 2018, 5, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riabov, P.A.; Micić, D.; Božović, R.B.; Jovanović, D.V.; Tomić, A.; Šovljanski, O.; Đurović, S. The chemical, biological and thermal characteristics and gastronomical perspectives of Laurus nobilis essential oil from different geographical origin. Ind. Crops Prod. 2020, 151, 112498. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, C.; Zhang, C.; Han, C.; Kuchkarova, N.; Shao, H. Chemical Composition, Phytotoxic, Antimicrobial and Insecticidal Activity of the Essential Oils of Dracocephalum integrifolium. Toxins 2019, 11, 598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, N.; Fattahi, M.; Youbert, I.; Icon, G.; Sefidkon, F. Volatile Compounds and Antifungal Activity of Dracocephalum moldavica L. at different phenological stages. J. Essent. Oil Res. 2020, 34, 87–95. [Google Scholar] [CrossRef]
- Latha, C.; Anu, C.J.; Ajaykumar, V.J.; Sunil, B. Prevalence of Listeria monocytogenes, Yersinia enterocolitica, Staphylococcus aureus, and Salmonella enterica Typhimurium in meat and meat products using multiplex polymerase chain reaction. Vet. World 2017, 10, 927–931. [Google Scholar] [CrossRef] [Green Version]
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on major food-borne zoonotic bacterial pathogens. J. Trop. Med. 2020, 2020, 4674235. [Google Scholar] [CrossRef]
- Aćimović, M.; Cvetković, M.; Stanković Jeremić, J.; Pezo, L.; Varga, A.; Čabarkapa, I.; Kiprovski, B. Biological activity and profiling of Salvia sclarea essential oil obtained by steam and hydrodistillation extraction methods via chemometrics tools. Flavour Fragr. J. 2022, 37, 20–32. [Google Scholar] [CrossRef]
- Aborus, N.E.; Tumbas Šaponjac, V.; Čanadanović-Brunet, J.; Ćetković, G.; Hidalgo, A.; Vulić, J.; Šeregelj, V. Sprouted and freeze-dried wheat and oat seeds—Phytochemical profile and in vitro biological activities. Chem. Biodivers. 2018, 15, e1800119. [Google Scholar] [CrossRef]
- Girones-Vilaplana, A.; Valentão, P.; Moreno, D.A.; Ferreres, F.; García-Viguera, C.; Andrade, P.B. New beverages of lemon juice enriched with the exotic berries maqui, açaí, and blackthorn: Bioactive components and in vitro biological properties. J. Agric. Food Chem. 2012, 60, 6571–6580. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reaction–antioxidant activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Al-Saikhan, M.S.; Howard, L.R.; Miller, J.C., Jr. Antioxidant activity and total phenolics in different genotypes of potato (Solanum tuberosum, L.). J. Food Sci. 1995, 60, 341–343. [Google Scholar] [CrossRef]
- Micić, M.; Đurović, S.; Riabov, P.; Tomić, A.; Šovljanski, O.; Filip, S.; Tosti, T.; Dojčinović, B.; Božović, R.; Jovanović, D.; et al. Rosemary essential oils as a promising source of bioactive compounds: Chemical composition, thermal properties, biological activity, and gastronomical perspectives. Foods 2021, 10, 2734. [Google Scholar] [CrossRef] [PubMed]
- Pavlić, B.; Teslić, N.; Vidaković, A.; Vidović, S.; Velićanski, A.; Versari, A.; Radosavljević, R.; Zeković, Z. Sage processing from by-product to high-quality powder: I. Bioactive potential. Ind. Crops. Prod. 2017, 107, 81–89. [Google Scholar] [CrossRef]
- Ferro, B.E.; van Ingen, J.; Wattenberg, M.; van Soolingen, D.; Mouton, J.W. Time–kill kinetics of antibiotics active against rapidly growing mycobacteria. J. Antimicrob. Chemother. 2015, 70, 811–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, A.; Toraldo, G.; Cavella, S.; Masi, P. Description of leavening of bread dough with mathematical modelling. J. Food Eng. 2007, 83, 142–148. [Google Scholar] [CrossRef]



| No | Compound | RI | DMEO | DMH |
|---|---|---|---|---|
| 1 | 1-octen-3-ol | 974 | - | 0.5 |
| 2 | 6-methyl-5-hepten-2-one | 986 | 0.1 | 3.3 |
| 3 | dehydro-1,8-cineole | 988 | - | 1.3 |
| 4 | 3-octanol | 995 | - | 0.2 |
| 5 | 1,8-cineole | 1028 | - | 0.6 |
| 6 | Benzene acetaldehyde | 1041 | - | 0.6 |
| 7 | cis-linalool oxide (furanoid) | 1069 | - | 1.7 |
| 8 | trans-linalool oxide (furanoid) | 1086 | - | 0.7 |
| 9 | Linalool | 1096 | 1.6 | 8.7 |
| 10 | Camphor | 1141 | - | 0.5 |
| 11 | trans-chrysanthemal | 1147 | - | 0.3 |
| 12 | Nerol oxide | 1149 | 0.1 | 0.4 |
| 13 | Borneol | 1159 | 0.6 | 2.9 |
| 14 | Terpinen-4-ol | 1171 | 0.1 | 1.7 |
| 15 | Cryptone | 1185 | - | 0.1 |
| 16 | α-Terpineol | 1188 | - | 0.9 |
| 17 | trans-Isocitral | 1777 | 0.4 | - |
| 18 | Nerol | 1227 | - | 2.2 |
| 19 | Neral | 1234 | 10.7 | 22.4 |
| 20 | Linalool acetate | 1247 | 7.9 | - |
| 21 | Geraniol | 1254 | - | 21.3 |
| 22 | Geranial | 1266 | 16.8 | 23.4 |
| 23 | Lavandulyl acetate | 1285 | 0.1 | - |
| 24 | Methyl geranate | 1318 | 0.1 | - |
| 25 | Eugenol | 1356 | - | 0.5 |
| 26 | Neryl acetate | 1358 | 4.6 | 0.1 |
| 27 | α-Copaene | 1369 | 0.2 | - |
| 28 | Geranyl acetate | 1379 | 53.2 | 1.7 |
| 29 | trans-caryophyllene | 1412 | 0.6 | - |
| 30 | α-humulene | 1447 | 0.1 | - |
| 31 | trans-β-farnesene | 1451 | 0.1 | - |
| 32 | γ-muurolene | 1474 | 0.4 | - |
| 33 | E,E-α-farnesene | 1502 | 0.1 | - |
| 34 | Caryophyllene oxide | 1575 | 0.3 | - |
| Total | 98.1 | 96.0 |
| Antioxidant Activity (μmolTE/100 mL) | DMEO | DMH |
|---|---|---|
| DPPH• | 246.39 ± 1.17 b | 8.82 ± 0.36 a |
| ABTS•+ | 312.54 ± 11.63 b | 25.44 ± 1.98 a |
| SOA | 294.77 ± 13.29 b | 19.58 ± 0.11 a |
| BCB | 397.20 ± 36.12 b | 41.63 ± 2.17 a |
| RP | 171.46 ± 2.56 b | 9.50 ± 0.64 a |
| Test Organisms | The Inhibition Zone * (mm) | |||
|---|---|---|---|---|
| D. moldavica | Controls | |||
| DMEO | DMH | Antibiotic | Actidione | |
| B. cereus ATCC 11778 | 16.0 ± 0.0 | 11.0 ± 0.0 | 27.0 ± 0.0 | - |
| S. aureus ATCC 25923 | 40.0 ± 0.0 | 28.33 ± 0.0 | 28.0 ± 0.0 | - |
| L. monocytogenes ATCC 35152 | 40.0 ± 0.0 | 27.0 ± 0.0 | 26.3 ± 0.6 | - |
| E. coli ATCC 25922 | 40.0 ± 0.0 | 34.33 ± 0.58 | 27.0 ± 0.0 | - |
| P. aeruginosa ATCC 27853 | 21.0 ± 0.0 | nd | 21.0 ± 0.0 | - |
| S. Typhimurium ATCC 13311 | 40.0 ± 0.0 | 10.33 ± 0.58 | 29.33 ± 0.6 | - |
| S. cerevisiae ATCC 9763 | 11.33 ± 0.58 | nd | - | 34.0 ± 0.0 |
| C. albicans ATCC 10231 | nd | nd | - | 37.0 ± 0.0 |
| A. brasiliensis ATCC 16404 | nd | nd | - | 27.3 ± 0.6 |
| Test Organisms | DMEO | DMH |
|---|---|---|
| B. cereus ATCC 11778 | >100 * | >100 |
| S. aureus ATCC 25923 | 0.78 | 12.5 |
| L. monocytogenes ATCC 35152 | 1.56 | 6.25 |
| E. coli ATCC 25922 | 3.125 | 3.215 |
| P. aeruginosa ATCC 27853 | >100 | >100 |
| S. Typhimurium ATCC 13311 | 0.78 | >100 |
| S. cerevisiae ATCC 9763 | >100 | >100 |
| C. albicans ATCC 10231 | >100 | >100 |
| A. brasiliensis ATCC 16404 | >100 | >100 |
| Coefficient | Bacterial Concentration (Log CFU/mL) | |||
|---|---|---|---|---|
| S. aureus | L. monocytogenes | E. coli | S. Typhimurium | |
| d | 7.72 | 8.12 | 9.04 | 9.27 |
| a | 5.7 | 5.53 | 5.52 | 5.38 |
| c | 4.29 | 4.60 | 1.64 | 4.95 |
| b | 2.58 | 5.59 | 1.65 | 1.30 |
| Coefficients | DMEO Concentration | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | L. monocytogenes | E. coli | S. Typhimurium | |||||||||
| MIC | 2×MIC | 4×MIC | MIC | 2×MIC | 4×MIC | MIC | 2×MIC | 4×MIC | MIC | 2×MIC | 4×MIC | |
| d | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| a | 5.05 | 5.6 | 5.6 | 5.13 | 5.27 | 5.4 | 5.77 | 4.97 | 5.31 | 5.20 | 5.05 | 5.39 |
| c | 3.03 | 1.05 | 0.96 | 2.06 | 2.59 | 0.96 | 2.78 | 3.25 | 1.53 | 2.45 | 3.03 | 0.77 |
| b | 11.64 | 14.9 | 11.7 | 2.34 | 3.65 | 11.89 | 2.26 | 3.9 | 2.06 | 5.72 | 11.64 | 1.99 |
| Coefficients | DMH Concentration | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | L. monocytogenes | E. coli | S. aureus | |||||||||
| MIC | 2×MIC | 4×MIC | MIC | 2×MIC | 4×MIC | MIC | 2×MIC | 4×MIC | MIC | 2×MIC | 4×MIC | |
| d | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| a | 5.65 | 5.36 | 5.53 | 5.31 | 5.59 | 5.25 | 5.36 | 5.4 | 5.46 | 5.65 | 5.36 | 5.53 |
| c | 2.68 | 2.40 | 1.43 | 2.47 | 1.76 | 2.43 | 1.76 | 1.52 | 1.05 | 2.68 | 2.40 | 1.43 |
| b | 2.65 | 3.01 | 1.65 | 4.35 | 3.05 | 3.85 | 1.44 | 1.32 | 1.16 | 2.65 | 3.01 | 1.65 |
| No | Reference | 1,8-Cineole | 4-Terpineol | Fenchone | Geranial | Geraniol | Geranyl Acetate | Linalool | Methyl Chavicol | Neral | Nerol | Neryl Acetate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | [2] | 0.0 | 0.0 | 0.0 | 29.6 | 5.4 | 27.2 | 0.4 | 0.0 | 19.4 | 0.4 | 3.0 |
| 2 | [34] * | 0.3 | 0.0 | 0.1 | 16.3 | 22.3 | 35.6 | 0.3 | 0.0 | 11.9 | 1.0 | 2.6 |
| 3 | [35] * | 0.0 | 0.0 | 0.0 | 26.2 | 4.6 | 35.0 | 0.2 | 0.0 | 20.7 | 0.0 | 4.1 |
| 4 | [36] | 0.0 | 0.0 | 0.0 | 25.5 | 0.5 | 15.2 | 1.3 | 16.0 | 9.7 | 0.3 | 1.2 |
| 5 | [37] | 0.0 | 0.0 | 0.0 | 27.3 | 20.7 | 23.2 | 0.8 | 0.0 | 18.6 | 0.0 | 2.1 |
| 6 | [47] | 0.0 | 0.0 | 0.0 | 21.6 | 39.5 | 12.4 | 0.8 | 0.0 | 17.1 | 1.5 | 1.6 |
| 7 | [38] * | 0.0 | 0.0 | 0.0 | 9.3 | 16.0 | 52.7 | 0.6 | 0.0 | 5.1 | 0.3 | 2.9 |
| 8 | [9] * | 0.0 | 0.0 | 0.0 | 36.6 | 2.9 | 26.7 | 0.3 | 0.0 | 25.7 | 0.1 | 1.2 |
| 9 | [39] | 0.0 | 0.0 | 0.0 | 26.3 | 16.9 | 22.5 | 1.5 | 0.0 | 21.3 | 1.0 | 0.4 |
| 10 | [12] | 0.0 | 0.0 | 0.0 | 28.5 | 19.6 | 16.7 | 0.8 | 0.0 | 21.2 | 1.9 | 1.8 |
| 11 | [40] * | 0.0 | 0.0 | 0.0 | 23.6 | 16.8 | 29.2 | 2.0 | 0.0 | 20.2 | 1.9 | 0.0 |
| 12 | [48] | 0.0 | 0.0 | 0.0 | 11.2 | 24.3 | 36.6 | 0.8 | 0.0 | 16.3 | 0.4 | 0.9 |
| 13 | [52] | 31.3 | 22.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 14 | [41] | 0.0 | 0.0 | 0.0 | 19.8 | 15.1 | 27.9 | 2.4 | 0.0 | 18.0 | 2.2 | 4.2 |
| 15 | [42] | 0.0 | 0.0 | 0.0 | 30.9 | 34.2 | 25.4 | 0.6 | 0.0 | 0.3 | 0.0 | 1.5 |
| 16 | [42] | 0.0 | 0.0 | 0.0 | 27.8 | 36.0 | 27.5 | 1.0 | 0.0 | 0.4 | 0.0 | 1.8 |
| 17 | [43] | 0.0 | 0.0 | 0.0 | 8.4 | 15.9 | 46.7 | 0.5 | 0.0 | 5.8 | 0.3 | 2.6 |
| 18 | [17] | 0.0 | 0.0 | 0.0 | 31.1 | 0.0 | 0.0 | 1.5 | 0.0 | 31.1 | 17.1 | 4.8 |
| 19 | [44] | 0.0 | 0.0 | 0.0 | 19.1 | 9.3 | 30.4 | 2.7 | 0.0 | 17.8 | 2.9 | 2.5 |
| 20 | [10] | 0.0 | 0.0 | 0.0 | 21.6 | 17.6 | 19.9 | 1.1 | 0.0 | 32.1 | 0.0 | 1.6 |
| 21 | [53] * | 0.4 | 0.0 | 13.8 | 15.9 | 6.9 | 1.3 | 28.1 | 1.2 | 0.0 | 1.4 | 0.9 |
| 22 | [45] * | 0.01 | 0.0 | 0.0 | 50.7 | 3.4 | 10.0 | 0.1 | 0.0 | 26.8 | 0.0 | 0.0 |
| 23 | [49] | 0.0 | 0.0 | 0.0 | 44.0 | 28.0 | 14.0 | 0.4 | 0.0 | 6.3 | 0.2 | 2.6 |
| 24 | [46] | 0.3 | 0.0 | 0.1 | 41.9 | 5.3 | 19.0 | 0.5 | 0.0 | 25.3 | 0.3 | 0.6 |
| 25 | [50] | 0.0 | 0.0 | 0.0 | 18.3 | 19.5 | 34.9 | 0.4 | 0.0 | 14.8 | 0.0 | 2.9 |
| 26 | [51] | 0.0 | 0.0 | 0.0 | 24.5 | 8.8 | 32.6 | 0.8 | 0.0 | 22.7 | 2.4 | 3.4 |
| 27 | TS | 0.0 | 0.1 | 0.0 | 16.8 | 0.0 | 53.2 | 1.6 | 0.0 | 10.7 | 0.0 | 4.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aćimović, M.; Šovljanski, O.; Šeregelj, V.; Pezo, L.; Zheljazkov, V.D.; Ljujić, J.; Tomić, A.; Ćetković, G.; Čanadanović-Brunet, J.; Miljković, A.; et al. Chemical Composition, Antioxidant, and Antimicrobial Activity of Dracocephalum moldavica L. Essential Oil and Hydrolate. Plants 2022, 11, 941. https://doi.org/10.3390/plants11070941
Aćimović M, Šovljanski O, Šeregelj V, Pezo L, Zheljazkov VD, Ljujić J, Tomić A, Ćetković G, Čanadanović-Brunet J, Miljković A, et al. Chemical Composition, Antioxidant, and Antimicrobial Activity of Dracocephalum moldavica L. Essential Oil and Hydrolate. Plants. 2022; 11(7):941. https://doi.org/10.3390/plants11070941
Chicago/Turabian StyleAćimović, Milica, Olja Šovljanski, Vanja Šeregelj, Lato Pezo, Valtcho D. Zheljazkov, Jovana Ljujić, Ana Tomić, Gordana Ćetković, Jasna Čanadanović-Brunet, Ana Miljković, and et al. 2022. "Chemical Composition, Antioxidant, and Antimicrobial Activity of Dracocephalum moldavica L. Essential Oil and Hydrolate" Plants 11, no. 7: 941. https://doi.org/10.3390/plants11070941












