Preliminary Phytochemical Profile and Bioactivity of Inga jinicuil Schltdl & Cham. ex G. Don
Abstract
:1. Introduction
2. Results and Discussion
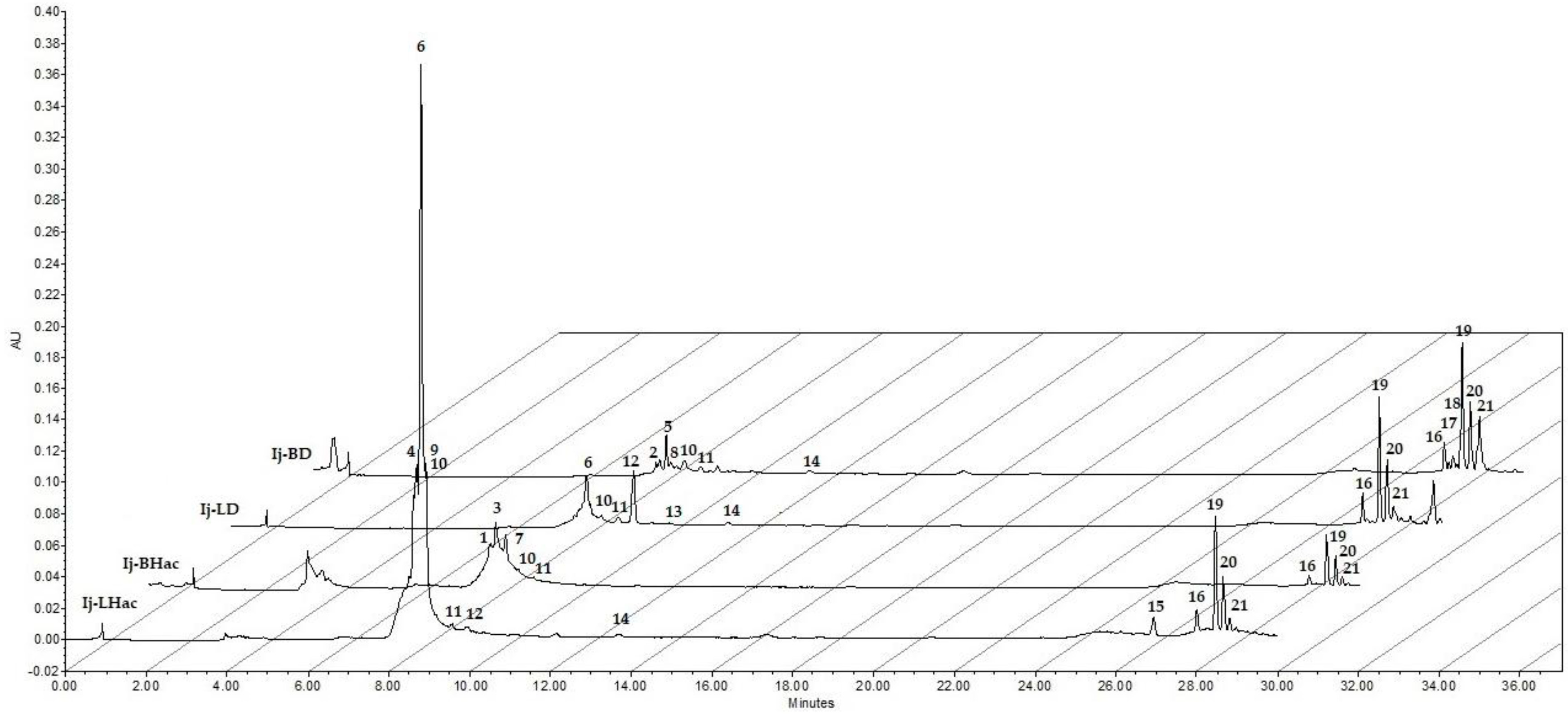
2.1. HPLC and UV-Vis Spectra Analysis of Polar Extracts from Inga jinicuil
2.2. Chemical Profile of Hexane Extracts from Bark and Leaves of I. jinicuil by GC-MS
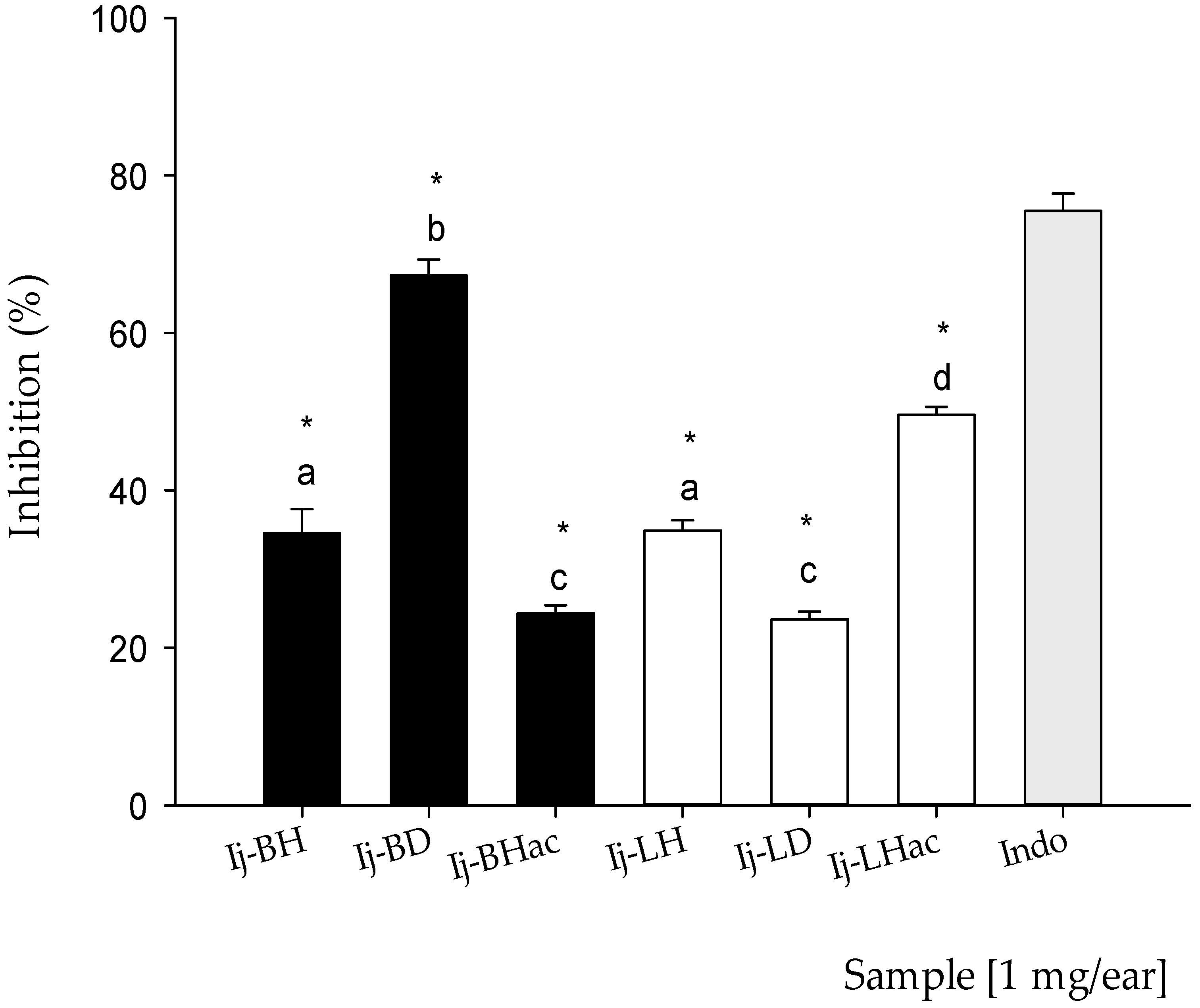
2.3. Anti-Inflammatory Activity of Organic Extracts from Inga jinicuil
2.4. Antibacterial Activity of Inga jinicuil Organic Extracts
3. Materials and Methods
3.1. Plant Material and Extraction of Inga jinicuil
3.2. HPLC Analysis
3.3. GC-MS Analysis of Hexane Extracts
3.4. Pharmacological Activity
3.4.1. Anti-Inflammatory Activity
3.4.2. Antibacterial Activity
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maione, F.; Russo, R.; Khan, H.; Mascolo, N. Medicinal plants with anti-inflammatory activities. Nat. Prod. Res. 2015, 30, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Heydari, H.; Saltan, I.G.; Eryilmaz, M.; Bahadir, A.Ö.; Yilmaz, S.S.; Tekin, M.; Çoban, T. Antimicrobial and Anti-Inflammatory Activity of Some Lathyrus L. (Fabaceae) Species Growing in Turkey. Turk. J. Pharm. Sci. 2019, 16, 240–245. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Traditional Medicine Strategy: 2014–2023; WHO Press, World Health Organization: Hong Kong, China, 2013; pp. 15–19. [Google Scholar]
- Ghasemian, M.; Owlia, S.; Owlia, M.B. Review of Anti-Inflammatory Herbal Medicines. Adv. Pharmacol. Sci. 2016, 2016, 9130979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf (accessed on 11 January 2022).
- Vargas, S.G.; Pire, R. Inga jinicuil Schtdl; Multiuso, Á., Ed.; Universidad Juárez Autónoma de Tabasco: Villahermosa, Mexico, 2017; pp. 2–4. ISBN 978-607-606-393-4. [Google Scholar]
- Alejandro, M.A.M.; Campillo, L.M.G.; Méndez, R.M. El Uso de las Plantas Medicinales en las Comunidades Maya-Chontales de Nacajuca, Tabasco, México. Polibotánica 2010, 29, 213–2062. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-27682010000100011&lng=es&nrm=iso (accessed on 13 January 2022).
- Maldonado, M.F. Flora Medicinal del Estado de Tabasco: Uso, Manejo y Conservación, 2nd ed.; Instituto para el Desarrollo de Sistemas de Producción del Trópico Húmedo de Tabasco: Villahermosa, Tabasco, Mexico, 2005; p. 50. ISBN 968-7991-24-0. [Google Scholar]
- Maziero, M.; Lovato, M.O.; Lorenzoni, V.V.; Moraes, G.G.; Dornelles, R.C.; Sagrillo, M.R.; Horner, R.; Manfron, M.P. Phytochemical Study, an Evaluation of the Antioxidant Potential and the Antimicrobial Activity of Inga semialata (Vell.) C. Mart. Hydroalcohol Extract. Nat. Prod. Res. 2019, 34, 192–196. [Google Scholar] [CrossRef]
- Pompeu, D.R.; Rogez, H.; Monteiro, K.M.; Tinti, S.V.; Carvalho, J.E. Capacidad antioxidante e triagem farmacológica de extratos brutos de folhas de Byrsonima crassifolia e de Inga edulis. Acta Amaz. 2012, 42, 165–172. [Google Scholar] [CrossRef]
- Dib, H.X.; de Oliveira, D.G.L.; de Oliveira, C.F.R.; Taveira, G.B.; de Oliveira Mello, E.; Verbisk, N.V.; Chang, M.R.; Corrêa, D., Jr.; Gomes, V.M.; Macedo, M.L.R. Biochemical Characterization of a Kunitz Inhibitor from Inga edulis Seeds with Antifungal Activity against Candida spp. Arch. Microbiol. 2019, 201, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Alves do Nascimento, V.H.; Guimarães Sobrinho, A.C.; De Oliveira Souza, C.; Silva de Souza, J.N.; Sousa, C.L. Determination of Phenolic Compounds with Antimicrobial Activity of Byrsonima crassifolia and Inga edulis Leaves Extracts. Ens. Ciênc. 2021, 25, 21–28. [Google Scholar] [CrossRef]
- Lima, N.; Santos, V.; La Porta, F. Chemodiversity, Bioactivity and Chemosystematics of the Genus Inga (FABACEAE): A Brief Review. Rev. Virtual Quim. 2018, 10, 459–473. [Google Scholar] [CrossRef]
- Rugerio, M.O.; Hernández, M.H.H.; Suárez, R.A.; Vargas, D.M.E.; Daniel, A.B. Estudio químico y antimicrobiano de Inga jinicuil del estado de Tlaxcala. In Proceedings of the 7th Reunión Internacional de Investigación de Productos Naturales “Dr. Pedro Joseph-Nathan”, Revista Latinoamérica de Química, Morelia, Mexico, 18–20 May 2011; Navarete, A., Ed.; Mixim: Naucalpan de Juárez, Mexico, 2011; C-95, p. 149. Available online: https://www.yumpu.com/es/document/view/27637927/latinoamericana-de-quimica-revista-latinoamericana-de- (accessed on 13 January 2022).
- Sun, J.; Liang, F.; Bin, Y.; Li, P.; Duan, C. Screening Non-Colored Phenolics in Red Wines Using Liquid Chromatography/Ultraviolet and Mass Spectrometry/Mass Spectrometry Libraries. Molecules 2007, 12, 679–693. [Google Scholar] [CrossRef] [Green Version]
- Robbins, R.J. Phenolic Acids in Foods: An Overview of Analytical Methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef] [PubMed]
- Gabe, V.; Kacergius, T.; Abu-Lafi, S.; Kalesinskas, P.; Masalha, M.; Falah, M.; Abu-Farich, B.; Melninkaitis, A.; Zeidan, M.; Rayan, A. Inhibitory Effects of Ethyl Gallate on Streptococcus mutans Biofilm Formation by Optical Profilometry and Gene Expression Analysis. Molecules 2019, 24, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, C.M.; de Morais, S.A.L.; Martins, M.M.; Cunha, L.C.S.; da Silva, C.V.; Martins, C.H.G.; Leandro, L.F.; de Oliveira, A.; de Aquino, F.J.T.; do Nascimento, E.A.; et al. Chemical Composition, Antifungal, and Cytotoxicity Activities of Inga laurina (Sw.) Willd Leaves. Sci. World J. 2019, 2019, 9423658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcoski, T.O.R.; Lima, N.M.; Navegante, G.; Serafim, R.B.; Sorbo, J.M.; Valente, V.; Santos, V.N.C.; Santos, R.A.; Silva, D.H.S.; Soares, C.P. Genotoxicity, Cytotoxicity and Chemical Profile from Inga laurina (Fabaceae). Nat. Prod. Res. 2021, 35, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zeng, Q.-H.; Lu, H.-Q.; Meng, F.-C.; Shen, Y.; Zeng, W.-Y.; Chi, H.; Zhou, Y.-Q.; Chen, M. Two New Lignans from Zanthoxylum armatum. Nat. Prod. Res. 2020, 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Tong, C.; Fu, Q.; Xu, J.; Shi, S.; Xiao, Y. Identification of Minor Lignans, Alkaloids, and Phenylpropanoid Glycosides in Magnolia officinalis by HPLC-DAD-QTOF-MS/MS. J. Pharm. Biomed. Anal. 2019, 170, 153–160. [Google Scholar] [CrossRef]
- Holser, R.A. Principal Component Analysis of Phenolic Acid Spectra. ISRN Spectrosc. 2012, 2012, 493203. [Google Scholar] [CrossRef]
- Renuka, B.; Sanjeev, B.; Ranganathan, D. Evaluation of Phytoconstituents of Caralluma nilagiriana by FTIR and UV-VIS Spectroscopic Analysis. J. Pharmacogn. Phytochem. 2016, 5, 105–108. Available online: https://www.phytojournal.com/archives?year=2016&vol=5&issue=2&ArticleId=813 (accessed on 18 January 2022).
- Fatima, S.; Mansha, A.; Asim, S.; Shahzad, A. Absorption Spectra of Coumarin and Its Derivatives. Chem. Pap. 2022, 76, 627–638. [Google Scholar] [CrossRef]
- Hroboňová, K.; Sádecká, J. Coumarins Content in Wine: Application of HPLC, Fluorescence Spectrometry, and Chemometric Approach. J. Food Sci. Technol. 2020, 57, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Li, G.J.; Wu, H.J.; Wang, Y.; Hung, W.L.; Rouseff, R.L. Determination of Citrus Juice Coumarins, Furanocoumarins and Methoxylated Flavones Using Solid Phase Extraction and HPLC with Photodiode Array and Fluorescence Detection. Food Chem. 2019, 271, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; He, G.; Yu, H.; Yang, J.; Borthakur, D.; Zhang, L.; Shen, S.; Das, U.N. Free Zn2+ Enhances Inhibitory Effects of EGCG on the Growth of PC-3 Cells. Mol. Nutr. Food Res. 2008, 52, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Wodnicka, A.; Huzar, E. Synthesis and Photoprotective Properties of New Salicylic and Vanillic Acid Derivatives. Curr. Chem. Lett. 2017, 6, 125–134. [Google Scholar] [CrossRef]
- Lima, N.M.; de Marqui, S.R.; Silva, D.H.S. Phytochemical, Metabolic Profiling and Antiparasitic Potential from Inga semialata Leaves (Fabaceae). Nat. Prod. Res. 2020, 2020, 1–6. [Google Scholar] [CrossRef]
- Hussain, M.A.; Badshah, M.; Iqbal, M.S.; Tahir, M.N.; Tremel, W.; Bhosale, S.V.; Sher, M.; Haseeb, M.T. HPMC-Salicylate Conjugates as Macromolecular Prodrugs: Design, Characterization, and Nano-Rods Formation: Rapid Communication. J. Polym. Sci. A Polym. Chem. 2009, 47, 4202–4208. [Google Scholar] [CrossRef]
- Alves, G.A.D.; Fernandes, S.D.; Venteu, T.T.; de Souza, R.O.; Rogez, H.; Fonseca, M.J.V. Obtainment of an Enriched Fraction of Inga edulis: Identification Using UPLC-DAD-MS/MS and Photochemopreventive Screening. Prep. Biochem. Biotechnol. 2020, 50, 28–36. [Google Scholar] [CrossRef]
- Tchuenmogne, A.M.T.; Donfack, E.V.; Kongue, M.D.T.; Lenta, B.N.; Ngouela, S.; Tsamo, E.; Sidhu, N.; Dittrich, B.; Laatsch, H. Ingacamerounol, A New Flavonol and Other Chemical Constituents from Leaves and Stem Bark of Inga edulis Mart. Bull. Korean Chem. Soc. 2013, 34, 3859–3862. [Google Scholar] [CrossRef] [Green Version]
- Lima, N.M.; Andrade, T.J.A.S.; Silva, D.H.S. Dereplication of Terpenes and Phenolic Compounds from Inga edulis Extracts Using HPLC-SPE-TT, RP-HPLC-PDA and NMR Spectroscopy. Nat. Prod. Res. 2022, 36, 488–492. [Google Scholar] [CrossRef]
- Furtado, F.B.; de Aquino, F.J.T.; Nascimento, E.A.; de Martins, C.M.; de Morais, S.A.L.; Chang, R.; Cunha, L.C.S.; Leandro, L.F.; Martins, C.H.G.; Martins, M.M.; et al. Seasonal Variation of the Chemical Composition and Antimicrobial and Cytotoxic Activities of the Essential Oils from Inga laurina (Sw.) Willd. Molecules 2014, 19, 4560–4577. [Google Scholar] [CrossRef] [Green Version]
- Marqui, S.; Santos, L.; Young, M.; Torres, L.; Bolzani, V.; Moraes, M.; Soares, C.; Silva, D. Chemopreventive Potential of Rhamnosyl Depsides from Inga laurina. Planta Med. 2010, 76, 47. [Google Scholar] [CrossRef]
- Nogueira, K.M.; de Souza, L.K.M.; Medeiros, J.V.R. Technological Prospection of Anti-Inflammatory Vanilic Acid Activity, with Emphasis on Its Semisynthetic Derivative Isopropyl Vanilate. Res. Soc. Dev. 2021, 10, e35910313451. [Google Scholar] [CrossRef]
- Enciso, E.; Arroyo, J. Efecto antiinflamatorio y antioxidante de los flavonoides de las hojas de Jungia rugosa Less (matico de puna) en un modelo experimental en ratas. An. Fac. Med. 2013, 72, 231. [Google Scholar] [CrossRef] [Green Version]
- Howes, M.J.R. Phytochemicals as Anti-Inflammatory Nutraceuticals and Phytopharmaceuticals. In Immunity and Inflammation in Health and Disease; Chatterjee, S., Jungraithmayr, W., Bagchi, W., Eds.; Academic Press: Cambridge, UK, 2018; pp. 363–388. [Google Scholar] [CrossRef]
- Molehin, O.R.; Adeyanju, A.A.; Adefegha, S.A.; Oyeyemi, A.O.; Idowu, K.A. Protective Mechanisms of Protocatechuic Acid against Doxorubicin-Induced Nephrotoxicity in Rat Model. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 20180191. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, L.; Bertoli, A.; Noccioli, C.; Mendez, J.; Musmanno, R.A.; Di Maggio, T.; Coratza, G. Antimicrobial Activity of Inga fendleriana Extracts and Isolated Flavonoids. Nat. Prod. Commun. 2009, 4, 1679–1683. [Google Scholar] [CrossRef] [Green Version]
- Favela-Hernández, J.M.J.; Clemente-Soto, A.F.; Balderas-Rentería, I.; Garza-González, E.; del Camacho-Corona, M.R. Potential Mechanism of Action of 3’-Demethoxy-6-O-Demethyl-Isoguaiacin on Methicillin Resistant Staphylococcus aureus. Molecules 2015, 20, 12450–12458. [Google Scholar] [CrossRef]
- Wang, D.; Jin, Q.; Xiang, H.; Wang, W.; Guo, N.; Zhang, K.; Tang, X.; Meng, R.; Feng, H.; Liu, L.; et al. Transcriptional and Functional Analysis of the Effects of Magnolol: Inhibition of Autolysis and Biofilms in Staphylococcus aureus. PLoS ONE 2011, 6, e26833. [Google Scholar] [CrossRef]
- Pisano, M.B.; Kumar, A.; Medda, R.; Gatto, G.; Pal, R.; Fais, A.; Era, B.; Cosentino, S.; Uriarte, E.; Santana, L.; et al. Antibacterial Activity and Molecular Docking Studies of a Selected Series of Hydroxy-3-Arylcoumarins. Molecules 2019, 24, 2815. [Google Scholar] [CrossRef] [Green Version]
- Kampranis, S.C.; Gormley, N.A.; Tranter, R.; Orphanides, G.; Maxwell, A. Probing the Binding of Coumarins and Cyclothialidines to DNA Gyrase. Biochemistry 1999, 38, 1967–1976. [Google Scholar] [CrossRef]
- De Araújo, R.S.A.; Barbosa, F.J.M.; Scotti, M.T.; Scotti, L.; da Cruz, R.M.D.; Falcão, S.V.D.S.; de Siqueira, J.J.P.; Mendonça, J.F.J.B. Modulation of Drug Resistance in Staphylococcus aureus with Coumarin Derivatives. Scientifica 2016, 2016, 6894758. [Google Scholar] [CrossRef] [Green Version]
- Adam, R.P. Identification of Essential Oils Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Business Media: Carol Stream, IL, USA, 2007; pp. 1–804. ISBN 978-193-263-32-14. [Google Scholar]
- SAGARPA. Norma Oficial Mexicana NOM-062-ZOO-1999. Especificaciones Técnicas para la Producción, Cuidado y Uso de los Animales de Laboratorio (Technical Specifications for the Production, Care, and Use of Laboratory Animals). México Diario Oficial de la Federación. 22 August 2001. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 2 February 2022).
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Payá, M.; Ferrándiz, M.L.; Sanz, M.J.; Bustos, G.; Blasco, R.; Rios, J.L.; Alcaraz, M.J. Study of the Antioedema Activity of Some Seaweed and Sponge Extracts from the Mediterranean Coast in Mice. Phytother. Res. 1993, 7, 159–162. [Google Scholar] [CrossRef]
- Salazar, P.D.T.; Castro, A.N.; Moreno, G.M.E.; Nicasio, T.M.P.; Perez, H.J.; Alvarez, F.P. Antibacterial and Anti-Inflammatory Activity of Extracts and Fractions from Agave cupreata. Int. J. Pharmacol. 2017, 13, 1063–1070. [Google Scholar] [CrossRef]

| Extract | n-Hexane (% Yield) | Dichloromethane (% Yield) | Hydroalcoholic (% Yield) |
|---|---|---|---|
| Bark extract | 0.095 | 0.82 | 0.25 |
| Leaf extract | 0.95 | 1.02 | 4.65 |
| Peak | Retention Time (min) | Absorption Bands (nm) | Extract(s) * | Compound Affinity ** | Ref. |
|---|---|---|---|---|---|
| 1 | 8.46 | 220.4, 261.6, 294.7 | ■ | Protocatechuic acid | Standard [15,16] |
| 2 | 8.58 | 249.8, 273.6 | ● | Protocatechuic acid derivative | Standard [15,16] |
| 3 | 8.58 | 218.1, 276.9 | ■ | Gallic acid derivative | Standard [17,18] |
| 4 | 8.66 | 212.2, 251.5, 352.9 | □ | Glycosylated Flavone. Apigenin derivative | Standard [19,20] |
| 5 | 8.75 | 219.2, 249.8, 273.4 | ● | Lignane | Standard [21,22] |
| 6 | 8.81 | 215.7, 269.8, 337.4 | ○□ | Glycosylated Flavone. Apigenin derivative | Standard [19,20] |
| 7 | 8.85 | 219.2, 279.3 | ■ | Gallic acid derivative | Standard [17,18] |
| 8 | 8.86 | 215.7, 308.9 | ● | Coumaric acid derivative | Standard [23] |
| 9 | 8.91 | 207.5, 269.8, 335.1 | □ | Glycosylated Flavone. Apigenin derivative | [19,20] |
| 10 | 9.18 | 249.8 | ○□●■ | Terpene | [24] |
| 11 | 9.58 | 245.1 | ○□●■ | Terpene | [24] |
| 12 | 9.96 | 209.9, 294.7, 338.6 | ○□ | Coumarin derivative | [25,26,27] |
| 13 | 10.03 | 276.9 | ○ | Epigallocatechin Gallate derivative | Standard [28] |
| 14 | 12.30 | 235.7, 266.3 | ○□● | Terpene | [24] |
| 15 | 26.91 | 219.2, 273.4, 293.5 | □ | Vanillic acid derivative | [29,30] |
| 16 | 28.01 | 204, 248.6 | ○□●■ | Terpene | [24] |
| 17 | 28.11 | 278.1 | ● | Epigallocatechin Gallate derivative | [28] |
| 18 | 28.21 | 245.1, 278.1, 327.9 | ● | Coumarin derivative | [25,26,27] |
| 19 | 28.43 | 201.7, 261.6 | ○□●■ | Salicylate derivative | Standard [31] |
| 20 | 28.65 | 200.5, 263.9 | ○□●■ | Salicylate derivative | Standard [31] |
| 21 | 28.81 | 263.9 | ○□●■ | Salicylate derivative | Standard [31] |
| Peak | Retention Time (min) | Molecular Weight (amu) | Extract(s) (% in the Sample) * | Compound ** |
|---|---|---|---|---|
| 22 | 17.80, 17.75 | 268.5 | ▲ (1.07), △ (1.18) | 2-pentadecanone,6,10,14-trimethyl |
| 23 | 17.80 | 296.5 | ▲ (1.07) | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol |
| 24 | 18.55 | 270.5 | △ (1.04) | Hexadecanoic acid, methyl ester |
| 25 | 18.61 | 276.3 | △ (0.88) | 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione |
| 26 | 18.68, 18.61 | 270.5 | ▲ (3.83), △ (0.88) | Hexadecanoic acid, ethyl ester |
| 27 | 20.51 | 296.5 | ▲ (11.74) | Phytol |
| 28 | 20.60, 20.47 | 298.5 | ▲ (3.14), △ (0.44) | Octadecanoic acid, methyl ester |
| 29 | 22.53 | 324.5 | △ (1.35) | 4,8,12,16-tetramethylheptadecan-4-olide |
| 30 | 24.63 | 390.6 | △ (0.80) | 1,2-benzenedicarboxylic acid diisooctyl ester |
| 31 | 27.76 | 380.6 | △ (1.62) | 15-Tetracosenoic acid, methyl ester |
| 32 | 29.19 | 518.7 | △ (1.02) | Tetradecanoic acid, 3,3a,4,6a,7,8,9,10,10a, 10b-decahydro-3a, 10a, dihydroxy-5-(hydroxymethyl)-2, 10-dimethyl-3-oxobenz [e] azulen-8-yl ester |
| 33 | 29.21 | 410.7 | ▲ (5.98) | Squalene |
| 34 | 30.05 | 408.8 | ▲ (12.55) | Nonacosane |
| 35 | 31.95 | 416.7 | ▲ (3.74) | β-Tocopherol |
| 36 | 32.44 | 436.8 | ▲ (16.66) | Hentriacontane |
| 37 | 33.07 | 430.7 | ▲ (40.49) | α-Tocopherol |
| 38 | 36.91 | 424.7 | △ (26.74) | Lup-20 (29)-en-3-one |
| 39 | 37.39 | 426.7 | △ (16.43) | Lupeol |
| 40 | 38.35 | 438.7 | △ (38.61) | 24-Methylenecycloartan-3-one |
| 41 | 38.64 | 412.7 | △ (2.27) | Stigmast-4-en-3-one |
| 42 | 41.85 | 440.7 | △ (4.99) | 9,19-Cyclolanostan-3-ol,24-methylene-, (3β)- |
| Bacterial | Strains | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gram- | Positive | Gram- | Negative | |||||||
| Extract | Sa1 | Sa2 | Se1 | Se2 | Sh | Ec1 | Ec2 | Ef | Kp | Pa |
| Ij-LH | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| Ij-LD | 50 | >200 | 200 | >200 | >200 | >200 | >200 | >200 | >200 | <3.12 |
| IjLHac | 50 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | <3.12 |
| Ij-BH | 50 | >200 | 50 | >200 | >200 | >200 | >200 | >200 | >200 | <3.12 |
| Ij-BD | 50 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | <3.12 |
| IjBHac | 50 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | <3.12 |
| C1 | * | * | * | * | * | * | * | * | * | * |
| C2 | * | * | * | * | * | * | * | * | * | * |
| C+ | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallegos-García, A.J.; Lobato-García, C.E.; González-Cortazar, M.; Herrera-Ruiz, M.; Zamilpa, A.; Álvarez-Fitz, P.; Pérez-García, M.D.; López-Rodríguez, R.; Ble-González, E.A.; Medrano-Sánchez, E.J.; et al. Preliminary Phytochemical Profile and Bioactivity of Inga jinicuil Schltdl & Cham. ex G. Don. Plants 2022, 11, 794. https://doi.org/10.3390/plants11060794
Gallegos-García AJ, Lobato-García CE, González-Cortazar M, Herrera-Ruiz M, Zamilpa A, Álvarez-Fitz P, Pérez-García MD, López-Rodríguez R, Ble-González EA, Medrano-Sánchez EJ, et al. Preliminary Phytochemical Profile and Bioactivity of Inga jinicuil Schltdl & Cham. ex G. Don. Plants. 2022; 11(6):794. https://doi.org/10.3390/plants11060794
Chicago/Turabian StyleGallegos-García, Ammy Joana, Carlos Ernesto Lobato-García, Manasés González-Cortazar, Maribel Herrera-Ruiz, Alejandro Zamilpa, Patricia Álvarez-Fitz, Ma Dolores Pérez-García, Ricardo López-Rodríguez, Ever A. Ble-González, Eric Jaziel Medrano-Sánchez, and et al. 2022. "Preliminary Phytochemical Profile and Bioactivity of Inga jinicuil Schltdl & Cham. ex G. Don" Plants 11, no. 6: 794. https://doi.org/10.3390/plants11060794
APA StyleGallegos-García, A. J., Lobato-García, C. E., González-Cortazar, M., Herrera-Ruiz, M., Zamilpa, A., Álvarez-Fitz, P., Pérez-García, M. D., López-Rodríguez, R., Ble-González, E. A., Medrano-Sánchez, E. J., Feldman, M. R., Bugarin, A., & Gómez-Rivera, A. (2022). Preliminary Phytochemical Profile and Bioactivity of Inga jinicuil Schltdl & Cham. ex G. Don. Plants, 11(6), 794. https://doi.org/10.3390/plants11060794












