Abstract
Puccinia triticina Erikss. is a causative agent of wheat leaf rust spread worldwide. Wheat rust is a major disease on wheat in southern regions of Russia, which are leaders in grain production and have favorable conditions for pathogen development. In this paper we studied the effectiveness of 52 NILs of cv. Thatcher with Lr genes in field trials and 41 NILs—in the juvenile phase in a greenhouse during 2011–2020. We conclude that the lines with Lr9, Lr42 and Lr43+24 genes remained immune in the adult phase during ten years of research. Lines with Lr genes: 19, 24, 29, 36, 37, 38, 43, 45, 47, 50 showed efficiency in field tests (1–5 R on the CIMMYT scale). No immune lines to Puccinia triticina were registered in the juvenile phase during 2011–2020. The line with the Lr9 gene remained immune up to 2020; Lr19 and Lr41—up to 2015; Lr42—up to 2018, and Lr50—up to 2019. In 2020, there was an increase of P. triticina isolates with virulence to Thatcher lines with Lr: 9, 14a, 16, 19, 21, 28, 30, 33, 40, 45, W, 50. Additionally, we registered a change in infection types towards more susceptible in isogenic Lr gene lines: 1, 2a, 12, 14b, 15, 18, 20, 23, 25, 28, 29, 32, 35, 36, 37, 38, 40, 44, 45 in the field. A sharp increase in the frequencies of virulent isolates was recorded in 2018–2020 due to unfavorable weather in the growing seasons. This indicates the ability of a dangerous pathogen to rapidly evolve in response to biotic and abiotic stresses. Therefore, annual monitoring of the reaction of isogenic lines, selected released varieties and the study of the virulence of the phytopathogen are important measures necessary to prevent and control leaf rust in grain-producing regions of the world.
Keywords:
leaf rust; Puccinia triticina; resistance genes; APR-genes; ASR-genes; population; effectiveness 1. Introduction
Puccinia triticina Erikss. is a causative agent of wheat leaf rust. It is an obligate biotrophic parasite spread worldwide. Statistics indicate that Russia is among the top five in world wheat production [1]. According to the Expert Analytical Center for Agribusiness, in 2020 the area under wheat in Russia amounted to 29.4 thousand hectares, which is 36.9% of the total area of all crops (https://ab-centre.ru/news/posevnye-ploschadi-i-sbory-osnovnyh-selskohozyaystvennyh-kultur-itogi-za2020-god (accessed on 16 July 2021)).
The main grain-producing geographical regions are located in Southern Russia: Rostov Region, Krasnodar region and Stavropol region. Their total gross harvest of grain is 35.4% of the total harvest in the country. Leaf rust is a major disease on wheat inSouthern Russia. From 1971 to 1998, leaf rust epiphytoties were periodically monitored in the region. This is due to an increase in the sown area under the leader varieties and overcoming the resistance of these varieties by the virulent pathotypes of P. triticina. The frequency of occurrence of leaf rust epidemics has reduced drastically from 1998 to the present. This is due to an increase in the genetic diversity of sown varieties and the absence of a leader variety [2]. However, despite the advances in modern breeding of rust-resistant wheat varieties, leaf rust is still a harmful disease that must be continuously monitored. To do so, it is necessary to track annual changes in the virulence of the pathogen population and evaluate the effectiveness of the known leaf rust resistance genes. In Russia, such studies are carried out in the main scientific centers located in different geographical regions. In the Northwest, it is All-Russian Institute of Plant Protection [3]; in the central part—All-Russian Institute of Phytopathology [4]; in the Volga region—Federal Center of Agriculture Research of the South—East Region [5]; in the south—Federal Scientific Center for Biological Plant Protection [6]; and in Siberia—Institute of Cytology and Genetics [7].
To date, about 80 leaf rust resistant genes are known [8]. Most of them are race specific resistance or all stage resistance (ASR) genes. Such resistance is not “durable” because it’s controlled by one or more genes [9,10]. The evolving nature of plant pathogens results in new virulent races that rapidly overcome ASR genes [11,12]. Race non-specific resistance genes (APR genes)—Lr34, Lr46, Lr67 and Lr68—are usually susceptible at the stage of seedlings. But at the stage of adult plants they exhibit slow rusting, prolonging plant resistance [13,14]. Combinations of several APR and ASR genes are especially effective [15,16]. Therefore, it is necessary to establish the effectiveness of Lr resistance genes in different phases of plant development in retrospect for successful wheat breeding programs.
The aim of this paper was to evaluate the effectiveness of Lr genes in the adult and juvenile phases of plants in Southern Russia in 2011–2020.
2. Results
2.1. Efficiency of Lr Genes in Adult Phase
Table 1 presents the evaluation results of a set of near isogenic lines of wheat cv. Thatcher. Lines with genes Lr9, Lr43 and Lr50 show absolute efficiency (no signs of disease on plants) in the field tests in 2011–2020. Lines with Lr42 and Lr43+24 genes had no signs of leaf rust in 2011–2019;but in 2020 they had minimal infection by infection type R (according to the CIMMYT scale) [17]. Effective (1R–5R) Lr genes are the following: 19, 24, 29, 36, 37, 38, 45, 47; moderately effective (10MR–20MR) Lr genes: 17, 21, 22a, 41, 52. Thatcher lines with known Lr resistance genes: 1, 2a, 2b, 2c, 3, 3bg, 3ka, 10, 11, 13, 14a, 14b, 15, 16, 20, 22b, 23, 26, 30, 33, 34, 40, B, Kanred were ineffective in the adult phase. Their prevalence varied within 20MS–90S (Table 1). Lines with Lr genes: 12, 18, 25, 28, 32, 35, 36, 37 were moderately effective from 2011 to 2019, but in 2020 they showed susceptibility to P. triticina.

Table 1.
Immunological assessment of near isogenic Thatcher lines for resistance to the North Caucasian P. triticina population (infectious site of FSCBPP, 2011–2020).
2.2. Efficiency of Lr Genes in Juvenile Phase
We assessed the resistance of Thatcher isogenic lines to P. triticina isolates in the greenhouse conditions of the Federal Scientific Center for Biological Plant Protection in 2011–2020. The results are described in Table 2. All lines with Lr genes were susceptible to P. triticina infection during the study period. Previously effective Lr9 became susceptible to virulent isolates of the fungus in 2020 (with a frequency of 12.8%). Isolates virulent to Lr24 were encountered in a small number years earlier [18], but in 2018 and 2020 their number increased to 20.0% and 14.8%, respectively.

Table 2.
The frequency of virulent and predominant infection types P. triticina isolates of the North Caucasian population to near isogenic Thatcher lines of wheat (FSCBPP greenhouse, 2011–2020).
The percentage of virulence frequency of P. triticina isolates to lines with the following Lr genes: 2c, 3, 3ka, 11, 14b, 23, 26, 28, 33, 40, B, Exch, Kanred was high (25–90%) during the entire study period. The frequency of P. triticina isolates virulence to Thatcher lines with Lr: 2a, 3bg, 10, 14a, 16, 17, 20, 21, 25, 30, 32 varied over the research years. The frequency of virulent isolates to Lr36, Lr38 increased significantly in 2018–2020. P. triticina isolates with virulence to Lr: 1, 18, 19, 44, 45, W increased during the study period. Isolates with virulence to Lr:15, 29, 42, 43+24 were rare (0 to 25%). At the same time, the line with the Lr42 gene was not affected by leaf rust from 2011 to 2017, and in 2018–2020, the frequency of virulence isolates was 1.7–7.5%. An increase in frequency P. triticina isolates with virulence to Lr 43+24 was observed from 2017 to 2019 (4.7–9.1%). The line with Lr47 was not affected until 2018, and the percentage of virulent isolates to Lr41 was low (2.2–8.8%), except for 2015.
3. Discussion
A ten-year study of the Lr genes effectiveness in different stages of plant development revealed that Lr9, Lr42, and Lr43 are immune to wheat rust both in seedling and adult phase in Southern Russia. At the same time, on the lines with genes Lr42, Lr43+24, disease symptoms first appeared in 2020 in adult phase. In a number of regions of Russia (Urals, Western Siberia), Lr9 (transferred to soft wheat from Aegilops umbellulata) has lost its effectiveness since 2007 due to the spread of varieties containing this gene [19,20]. In Moscow region, isolates virulent to Lr9 were encountered with a low frequency over the period from 2009 to 2017, but in 2013 their share was already 75% [21]. At the same time, in the Volga region, as in Dagestan, Lr9 is still effective [5,22]. Lr 42, which was transferred to soft wheat from Aegilops tauschii Coss [23], maintains absolute efficiency in all major grain-sowing regions of Russia [4]. But in Ukraine, Lr42 is effective only in field trials; and in the seedling stage, this line shows moderate susceptibility [24].
In 2004–2006, the list of ASR genes studied in Southern Russia was longer and included the following genes transferred to common wheat from wild forms: Lr9, Lr19, Lr24, Lr29, Lr38, Lr41, Lr42, Lr43, Lr45 [25]. Most of these Lr genes have now lost their effectiveness in the juvenile phase.
Thus, genes Lr: 19, 24, 29, 36, 37, 38, 45, 47, 17, 21, 22a, 41, W (52) that are effective and moderately effective under field trials show different reactions to infection with P. triticina isolates in the seedling phase. The frequency of virulent isolates to Lr19, Lr45, and W (52) gradually increased, reaching its peak in 2020 (Figure 1). The Lr17, Lr21, Lr36, and Lr38 genes were ineffective in the juvenile phase. The frequency of isolates with virulence to these lines varied from 0 to 96.5% in different years. Lr24 decreased its effectiveness over time, and moderate virulence to this line (up to 20%) was observed in 2018 and 2020. The line with the Lr29 gene was affected by fungus isolates in different years from 0 to 21.4%.
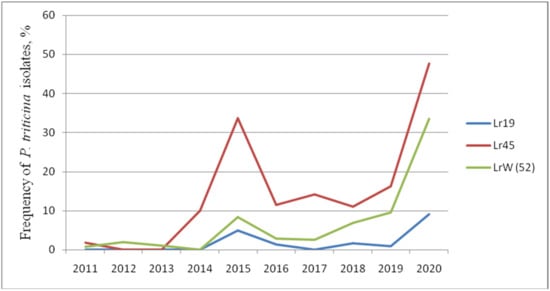
Figure 1.
Efficiency dynamics of Lr genes in the seedling phase against the North Caucasian population of P. triticina (2011–2020).
Lr19 and Lr24, introgressed into soft wheat from Aegilops elongatum [26], have a different history of their efficiency in Russia. Lr19, due to its active use in the composition of varieties and lines in breeding, lost its effectiveness in the juvenile phase in a number of regions of Russia: the Volga region [22], Western Siberia [27], and the Central part of Russia [21]. The effectiveness of Lr19 still contributes to wheat breeding in the Southern Urals [20], as well as in Ukraine [24] and Dagestan [22]. To prolong the genetic resistance of Lr19, breeders use its combination with various genes: Lr10, Lr26, Lr37, Lr39 [20]. The Lr24 gene remains effective in most regions of Russia in the adult phase [27] and in the seedling phase [4]. At the same time, in Saratov region, as well as in Ukraine, Lr24 is no longer effective in the germination phase [5,24]. The appearance and increase in the frequency of isolates virulent to Lr24 in Southern Russia may indicate a possible introduction of infection from Ukraine, since Lr24 is scarcely used in Russian breeding [20].
The efficiency of this and other Lr genes varies worldwide. For example, in South Africa, effective genes include Lr9, Lr19, Lr29, Lr34, Lr45, Lr47, Lr51 and Lr52. At the same time, varieties and lines containing the race-specific genes Lr1, Lr3a, Lr10, Lr13, Lr14a, Lr17b, Lr24, Lr26, Lr27, and Lr31 are no longer used, since they were overcome by new races of the pathogen [28]. In Egypt, Lr9 and Lr24 are ineffective [29], while molecular markers prove the presence of Lr13, Lr19, Lr24, Lr26, Lr34, Lr35 Lr36, Lr37, Lr39, and Lr46 genes in Egyptian varieties [30]. Lr24 is not effective in Iran either [31]. In Australia, Lr24 was effective in preventing wheat rust disease from 1983 until 2000, when a variety with this gene was first sown, and when a virulent isolate was found [32]. Australian commercial cultivars contain the following resistance genes: Lr1, Lr3a, Lr13, Lr13+, Lr14a, Lr17a, Lr17b, Lr20, Lr23, Lr24, Lr26, Lr27, Lr31, Lr34, Lr37 and Lr46. A significant number of cultivars possess Lr34 as well [33]. Recently, pathotypes virulent to Lr24 have also been found in New Zealand [12]. In Israel, Lr24 still retains its effectiveness [34].
Our results suggest the need for both monitoring the virulence of the leaf rust pathogen population and studying the effectiveness of known resistance genes in different phases of plant development. This will help to find new diverse effective resistance genes that can be used for wheat breeding in Russia and worldwide.
4. Conclusions
We studied the effectiveness of Lr genes against the wheat leaf rust pathogen population in both juvenile and adult phases during 2011–2020 in Southern Russia. We found that the lines with Lr9, Lr42, and Lr43+24 genes remained immune throughout ten years of research in all phases of plant development. Lines with Lr: 19, 24, 29, 36, 37, 38, 43, 45, 47, 50 showed efficiency in field trials (1R–5R on the CIMMYT scale). In the juvenile phase, no immune lines to Puccinia triticina were found in 2011–2020. The line with Lr9 gene was highly resistant by 2020; Lr19 and Lr41—by 2015; Lr42—by 2018; and Lr50—by 2019.
Nevertheless, the Lr9, Lr42, Lr43+24 genes are able to resist leaf rust in juvenile and adult phases. In 2020, we noted both an increase in the frequencies of virulent isolates to lines with Lr: 9, 14a, 16, 19, 21, 28, 30, 33, 40, 45, W, 50, as well as changes in infection types towards more susceptible in Thatcher lines with Lr: 1, 2a, 12, 14b, 15, 18, 20, 23, 25, 28, 29, 32, 35, 36, 37, 38, 40, 44, 45 in field trials. In addition, sharp increases in the frequencies of virulent isolates for a number of Lr-lines were noted in 2013, 2018, and 2020. We hypothesize that this is due to the unfavorable weather (relatively low rainfall) in the growing seasons during these years (Figure 1).
Several literature sources mention that virulent isolates may predominate in unfavorable weather [35]. This indicates the ability of a dangerous pathogen to rapidly evolve in response to biotic and abiotic stresses. Of no small importance are the genotypes of varieties grown in the region, which contribute to the selection of virulent isolates of the fungus. The loss of efficiency of the Lr genes in the field trials and juvenile phase dictates the need for a more thorough study of resistance genetics of widely planted varieties, including the application of molecular markers. This should be considered for wheat variety selection and placement.
There are few data on the genetics of resistance of breeding varieties in the south of Russia, and such work is our task for the future. Annual monitoring of the effectiveness of isogenic lines, selected released varieties and the study of the pathogen virulence are important measures necessary to prevent and control leaf rust in grain-producing regions of the world.
5. Materials and Methods
5.1. Growing Seasons 2011–2020
Figure 2 shows the weather of the three most significant months for disease development (April, May, June) during 2011–2020. The data is provided by a weather station at the Federal Scientific Center for Biological Plant Protection, Krasnodar (FSCBPP). The combination of sufficient rainfall and elevated temperatures is typical for most spring months in Southern Russia. This is ideal for leaf rust development [36]. Therefore, most growing seasons were favorable for disease development. The growing seasons of 2012, 2018 and 2020 were an exception, for which a reduced amount of precipitation was noted. In 2012, the lack of precipitation was in June, and in 2020, in April. Lack of precipitation from April to June in 2018 did not contribute to disease development.
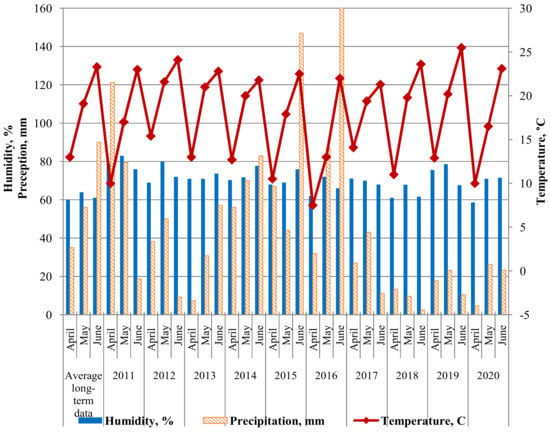
Figure 2.
Climatogram of weather conditions for the research period 2011–2020 (according to the FSCBPP meteorological station).
5.2. Obtaining Infectious Material of P. triticina
We received P. triticina infectious material as a result of annual route inspections of industrial and selection crops of winter wheat in Krasnodar region, Stavropol region and Rostov Region during 2011–2020. Affected leaves were collected from each area, signed and placed in a cold storage in filter paper at 4–6 °C. Then leaf samples were mixed to obtain a population of P. triticina. For further experiments, the pathogen population was propagated on a susceptible cultivar Michigan Amber.
5.3. Efficiency Evaluation of the Lr Genes at the Adult Stage
We carried out the studies at the FSCBPP field site in 2010–2020 using a set of 52 NILs of winter wheat cv. Thatcher containing the following resistance Lr genes: 1, 2a, 2b, 2c, 3, 3bg, 3ka, 9, 10, 11, 12, 13, 14a, 14b, 15, 16, 17, 18, 19, 20, 21, 22a, 22b, 23, 24, 25, 26, 28, 29, 30, 32, 33, 34, 35, 36, 37, 38, 40, 41, 42, 43, 43+24, 44, 45, 47, B, 50, 52 (W), 57 (58), Exch, KR1, KR2, 73.
Seeds of each line were grown at the FSCBPP field site in autumn on plots of 1 sq.m. Every 10 plots, a susceptible variety was grown, which served as a reservoir of infection. Michigan Amber was the control for susceptibility. Plants were inoculated in the booting phase (Z 32). For plant inoculation, we used a mixture of P. triticina urediniospores with talc in a ratio of 1:100 at a load of 5 mg spores/m2 [18]. We scored the plants for infection at the time of the initial manifestation of the disease, and then every seven days until the peak incidence.
The severity of damage (in %) and the reaction of wheat to infection (in points) were assessed by the CIMMYT scale [37]. The following scale was used: 0—no spots or uredinia; R—small uredinia with necrosis; MR—moderate size pustules with necrosis; MS-moderate pustules with chlorosis; S—large pustules. The genes were ranked as follows: highly effective (plants without signs of damage), effective (1-5 R), moderately effective (10–20 MR), ineffective (25 MS and above). Ranking was performed according to the CIMMYT scale [17].
5.4. Efficiency Evaluation of the Lr Genes at the Juvenile Stage
We carried out the studies in greenhouses of FSCBPP in 2011–2020 using a set of 41 NILs of winter wheat cv. Thatcher containing the following resistance Lr genes: 1, 2a, 2c, 3, 3bg, 3ka, 9, 10, 11, 14a, 14b, 15, 16, 17, 18, 19, 20, 21, 23, 24, 25, 26, 28, 29, 30, 32, 33, 36, 38, 40, 41, 42, 43+24, 44, 45, 47, B, 52 (W), Exch, KR1KR2, 50.
The seeds of each line were pre-germinated in Petri dishes, and then sown in pots, 5 plants of each line. Seedlings were grown for 7 days at mean temperature approximately 18- 20 C in isolated greenhouse boxes. Then sets of seedlings of isogenic lines were inoculated with each of the single pustule isolates of the P. triticina population. We described in detail the methods of isolation and reproduction of single pustule isolates of the leaf rust pathogen population in the paper [6]. At 14 days after inoculation, plant damage was scored for infection type (IT): low IT 0–2+ and high IT (3,4) according to Kolmer [36,38]. The effectiveness of the Lr genes in the juvenile stage was assessed by the frequency of virulent P. triticina isolates to the lines carrying the Lr genes.
For our study we used the material and technical base of the unique scientific installation “Phytotron for the isolation, identification, study and maintenance of races, strains, phenotypes of pathogens” (http://ckp-rf.ru/671925 (accessed on 2 February 2021)).
Author Contributions
Conceptualization, G.V. and O.K.; Data curation, G.V.; Investigation, O.K., O.V. and V.A.; Project administration, G.V.; Writing—original draft, O.K.; Writing—review & editing, G.V., V.A. and O.K. All authors have read and agreed to the published version of the manuscript.
Funding
The research was carried out in accordance with the State Assignment of the Ministry of Science and Higher Education of the Russian Federation within the framework of research on the topic No. FGRN-2022-0004.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data obtained is contained in this article.
Acknowledgments
The authors thank E. Kosman, Institute for Cereal Crops Research, School of Plant Science and Food Security, Faculty of life Sciences, Tel Aviv University, for his assistance in data analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAOSTAT Browse Data, By Domain—Production/Crops. FAOSTAT (Food and Agriculture Organisation of the United Nations Statistics Division). 2020. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 18 December 2021).
- Hudokormova, Z.N. Retrospective Analysis of the Development of Leaf Rust (Puccinia triticina Rob. Ex Desm. f. sp. tritici Erikss.) and Wheat Resistance and Triticale to the Pathogen. Dissertation of Candidate of Agricultural Sciences; All-Russian Research Institute of Rice: Krasnodar, Russia, 2008; 25p. [Google Scholar]
- Gultyaeva, E.I.; Aristova, M.K.; Shaidayuk, E.L.; Mironenko, N.V.; Kazartsev, I.A.; Akhmetova, A.; Kosman, E. Genetic differentiation of Puccinia triticina Erikss. in Russia. Russ. J. Genet. 2017, 53, 998–1005. [Google Scholar] [CrossRef]
- Kolomiets, T.M.; Zhemchuzhina, A.I.; Kiseleva, M.I.; Zhemchuzhina, N.S. Population and Genetic monitoring the Puccinia triticina to provide food safety of Russia. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 663, p. 012006. [Google Scholar] [CrossRef]
- Kon’kova, E.A. Population structure of Puccinia triticina Erikss. on crops of winter and spring soft wheat in the Saratov region. Plant Prot. News 2018, 4, 44–49. [Google Scholar]
- Volkova, G.V.; Vaganova, O.F.; Kudinova, O.A. Virulence of Puccinia triticina in the North Caucasus region of Russia. Span. J. Agric. Res. 2020, 18, e10SC01. [Google Scholar] [CrossRef]
- Skolotneva, E.S.; Kosman, E.; Patpour, M.; Kelbin, V.N.; Morgounov, A.I.; Shamanin, V.P.; Salina, E.A. Virulence phenotypes of Siberian wheat stem rust population in 2017–2018. Front. Agron. 2020, 2, 6. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Dubcovsky, J.; Rogers, W.J.; Morris, C.; Xia, X.C. Catalogue of Gene Symbols for Wheat: 2017 Supplement. Available online: https://shigen.nig.ac.jp/wheat/komugi/genes/macgene/supplement2017.pdf (accessed on 16 November 2021).
- And, M.D.; Linde, C. Pathogen population genetics, evolutionary potential, and durable resistance. Annu. Rev. Phytopathol. 2003, 40, 349–379. [Google Scholar]
- Kumar, S.; Phogat, B.S.; Vikas, V.K.; Sharma, A.K.; Saharan, M.S.; Singh, A.K.; Kumari, J.; Singh, R.; Jacob, S.R.; Singh, G.P.; et al. Mining of Indian wheat germplasm collection for adult plant resistance to leaf rust. PLoS ONE 2019, 14, e0213468. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Espino, J.; Singh, R.P.; Germán, S.; McCallum, B.D.; Park, R.F.; Chen, W.Q.; Bhardwaj, S.C.; Goyeau, H. Global status of wheat leaf rust caused by Puccinia triticina. Euphytica 2011, 179, 143–160. [Google Scholar] [CrossRef]
- Warren, R.M.; Cuddy, W.; Park, R.F.; Craigie, R.; Chng, S.F. Recent pathotype development of New Zealand cereal rust populations. N. Z. Plant Prot. 2018, 71, 314–324. [Google Scholar] [CrossRef]
- Lagudah, E.S. Molecular genetics of race non-specific rust resistance in wheat. Euphytica 2011, 179, 81–91. [Google Scholar] [CrossRef]
- Singh, R.P.; Huerta-Espino, J.; Bhavani, S.; Herrera-Foessel, S.A.; Singh, D.; Singh, P.K.; Velu, G.; Mason, R.E.; Jin, Y.; Njau, P.; et al. Race non-specific resistance to rust diseases in CIMMYT spring wheats. Euphytica 2011, 179, 175–186. [Google Scholar] [CrossRef]
- Singh, R.P.; Huerta-Espino, J.; Rajaram, S. Achieving near immunity to leaf and stripe rusts in wheat by combining slow rusting resistance genes. Acta Phytopathol. Entomol. Hung. 2000, 35, 133–139. [Google Scholar]
- Ellis, J.G.; Lagudah, E.S.; Spielmeyer, W.; Dodds, P.N. The past, present and future of breeding rust resistant wheat. Front. Plant Sci. 2014, 5, 641. [Google Scholar] [CrossRef] [Green Version]
- Duveiller, E.; Singh, R.P.; Singh, P.K.; Dababat, A.A.; Mezzalama, M. Wheat Diseases and Pests: A Guide for Field Identification; CIMMYT: Mexico City, Mexico, 2012; 138p. [Google Scholar]
- Anpilogova, L.K.; Volkova, G.V. Methods for Creating Artificial Infectious Backgrounds and Assessing Wheat Varieties for Resistance to Harmful Diseases (Head Fusarium, Rust, Powdery Mildew) Recommendations; LLC “Innovative Plant Protection Center”: Saint-Petersburg, Russia, 2000; 28p. [Google Scholar]
- Sochalova, L.P.; Khristov, Y.A. Influence of variety genotype on population structure of wheat leaf rust pathogen Puccinia recondita. Sib. Bull. Agric. Sci. 2009, 10, 61–67. [Google Scholar]
- Tyunin, V.A.; Schreider, E.R.; Gultyaeva, E.I.; Shaydayuk, E.L. Characteristics of the virulence of Puccinia triticina populations and prospects for the use of the Lr24, Lr25, and LrSp genes in spring soft wheat breeding in the Southern Urals. Vavilov J. Genet. Sel. 2017, 21, 523–529. [Google Scholar] [CrossRef]
- Zhemchuzhina, A.I.; Kiseleva, M.I.; Zhemchuzhina, N.S.; Belyakova, S.V. Virulence of populations of Puccinia triticina Erikss. in the Nonchernozem zone of Russia. Agrar. Sci. 2019, 1, 137–141. [Google Scholar] [CrossRef]
- Gultyaeva, E.I.; Sibikeev, S.N.; Druzhin, A.E.; Shaydayuk, E.L. Expansion of the genetic diversity of spring soft wheat varieties for leaf rust resistance (Puccinia triticina Eriks.) in the Lower Volga region. Agric. Biol. 2020, 55, 27–44. [Google Scholar] [CrossRef]
- Gill, H.S.; Li, C.; Sidhu, J.S.; Liu, W.; Wilson, D.; Bai, G.; Gill, B.S.; Sehgal, S.K. Fine mapping of the wheat leaf rust resistance gene Lr42. Int. J. Mol. Sci. 2019, 20, 2445. [Google Scholar] [CrossRef] [Green Version]
- Babayants, O.; Babayants, L.; Gorash, A.; Vasilev, A.; Traskovetskaya, V.; Galaev, A. Physiologic specialization of Puccinia triticina Erikss. and effectiveness of Lr-genes in the south of Ukraine during 2013–2014. Chil. J. Agric. Res. 2015, 75, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Anpilogova, L.K.; Volkova, G.V.; Vaganova, O.F.; Avdeeva, Y.V. Scientifically substantiated stages of immunological studies necessary to create rust-resistant wheat varieties. Agro XXI 2009, 10, 6. [Google Scholar]
- Ulukan, H. Wild wheats (Triticum spp.) and relatives in wheat rust diseases (Puccinia spp.) from a wheat breeder’s perspective: A general evaluation. Int. J. Agric. Biol. 2020, 23, 121–130. [Google Scholar] [CrossRef]
- Skolotneva, E.S.; Leonova, I.N.; Bukatich, E.Y.; Boiko, N.I.; Piskarev, V.V.; Salina, E.A. Effectiveness of leaf rust resistance genes against Puccinia triticina populations in Western Siberia during 2008–2017. J. Plant Dis. Prot. 2018, 125, 549–555. [Google Scholar] [CrossRef]
- Figlan, S.; Ntushelo, K.; Mwadzingeni, L.; Terefe, T.; Tsilo, T.J.; Shimelis, H. Breeding Wheat for Durable Leaf Rust Resistance in Southern Africa: Variability, Distribution, Current Control Strategies, Challenges and Future Prospects. Front. Plant Sci. 2020, 11, 549. [Google Scholar] [CrossRef]
- Khadegah Najeeb, M.A.; Thabet, M.; Negm, S.S.; El-Deeb, S.H. Monitoring of Puccinia triticina Erikss. physiologic races and effectiveness of Lr-genes in Egyptian wheat during 2014-2016 growing seasons. Int. J. Agric. Technol. 2019, 15, 35–54. [Google Scholar]
- Imbaby, I.A.; Mahmoud, M.A.; Hassan, M.E.M.; Abd-El-Aziz, A.R.M. Identification of Leaf Rust Resistance Genes in Selected Egyptian Wheat Cultivars by Molecular Markers. Sci. World J. 2014, 2014, 574285. [Google Scholar] [CrossRef]
- Nemati, Z.; Mostowfizadeh-Ghalamfarsa, R.; Dadkhodaie, A.; Mehrabi, R.; Steffenson, B.J. Virulence of Leaf Rust Physiological Races in Iran From 2010 to 2017. Plant Dis. 2020, 104, 363–372. [Google Scholar] [CrossRef]
- Park, R.F.; Bariana, H.S.; Wellings, C.R.; Wallwork, H. Detection and occurrence of a new pathotype of Puccinia triticina with virulence for Lr24 in Australia. Aust. J. Agric. Res. 2002, 53, 1069–1076. [Google Scholar] [CrossRef]
- Gessese, M.K. Description of Wheat Rusts and Their Virulence Variations Determined through Annual Pathotype Surveys and Controlled Multi-Pathotype Tests. Adv. Agric. 2019, 2019, 2673706. [Google Scholar] [CrossRef]
- Kosman, E.; Ben-Yehuda, P.; Manisterski, J.; Sela, H. Diversity of virulence phenotypes among annual populations of Puccinia triticina originating from common wheat in Israel during the period 2000–2015. Plant Pathol. 2019, 68, 1741–1748. [Google Scholar] [CrossRef]
- D’yakov, Y.T. Population Biology of Phytopathogenic Fungi; Muravey: Moscow, Russia, 1998; 382p. [Google Scholar]
- Kolmer, J.A.; Hughes, M.E. Physiologic specialization of Puccinia triticina on wheat in the United States in 2015. Plant Dis. 2017, 101, 1968–1973. [Google Scholar] [CrossRef] [Green Version]
- Roelfs, A.P.; Singh, R.P.; Saari, E.E. Rust Diseases of Wheat: Concept and Methods of Disease Management; CIMMYT: Veracruz, Mexico, 1992; 81p. [Google Scholar]
- Kolmer, J.A. Virulence of Puccinia triticina, the wheat leaf rust fungus, in the United States in 2017. Plant Dis. 2019, 103, 2113–2120. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).