The Effects of Salt Stress on Germination, Seedling Growth and Biochemical Responses of Tunisian Squash (Cucurbita maxima Duchesne) Germplasm
Abstract
:1. Introduction
2. Results
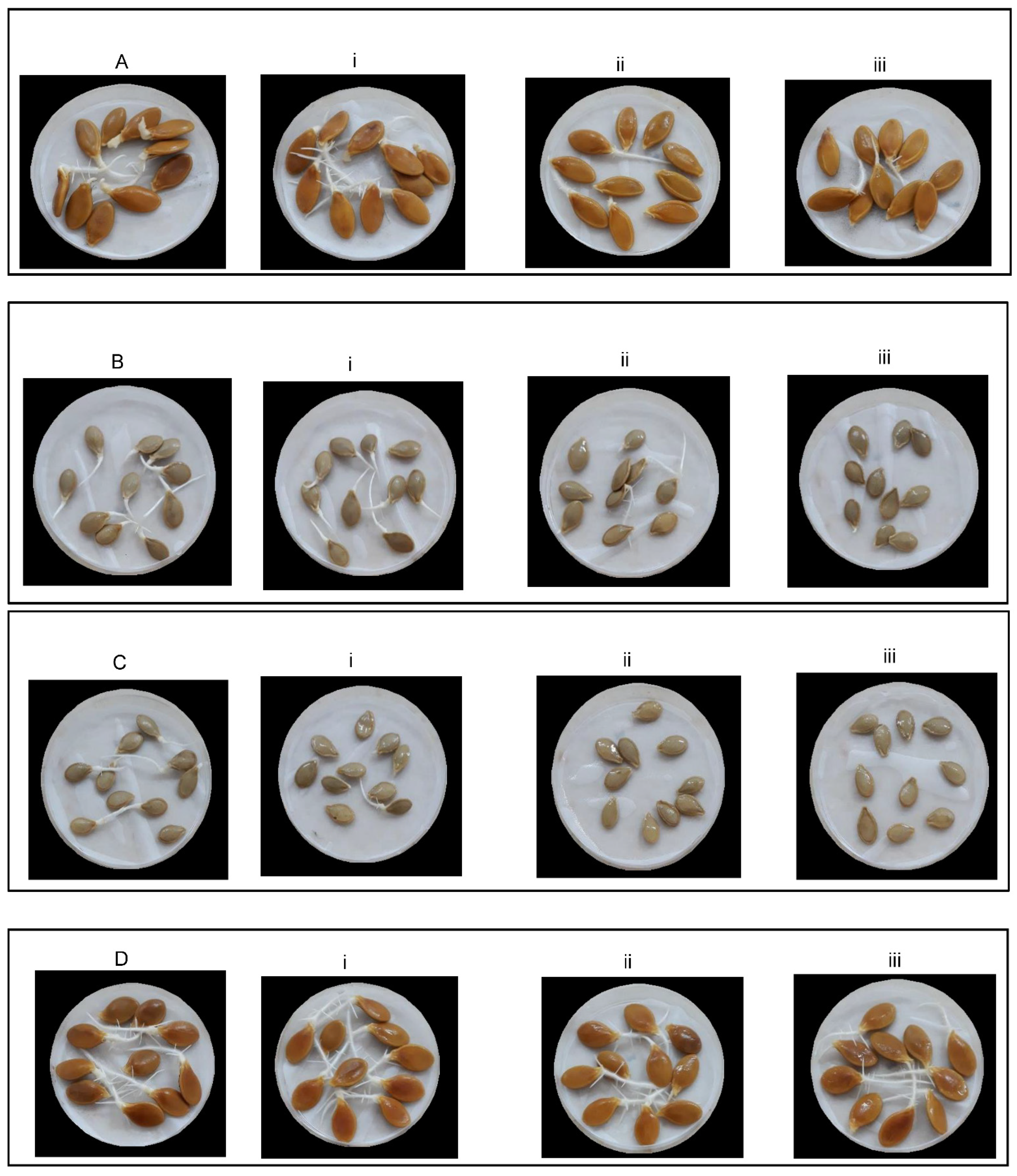
2.1. Effect of Salt Stress Level on Traits Related to Seed Germination and Seedling Growth Potential
2.2. Effect of Landrace on Traits Related to Seed Germination and Seedling Growth Potential
2.3. Effect of the Landrace and the Salt Stress Level on Traits Related to Seed Germination and Seedling Growth Potential
2.4. Effect of the Salt Stress Level on the Content of Malondialdehyde (MDA), Free Proline and Chlorophyll a and b
2.5. Effect of Landrace on the Content of Malondialdehyde, Free Proline and Chlorophyll a and b
2.6. Effect of Landrace and Salt Stress Level on the Content of Malondialdehyde, Free Proline and Chlorophyll a and b
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Salinity Stress Treatments
4.3. Determination of Germination and Seedling Growth Potential under Salt Stress
4.4. Evaluation of Salinity Tolerance Based on Biochemical Parameters
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdein, M.A.E.-H. Genetic Diversity between Pumpkin Accessions Growing in the Northern Border Region in Saudi Arabia Based on Biochemical and Molecular Parameters. Egypt. J. Bot. 2018, 58, 463–476. [Google Scholar] [CrossRef]
- Brown, C.H.; Luedeling, E.; Wichmann, S.; Epps, P. The paleobiolinguistics of domesticated squash (Cucurbita spp.). In Explorations in Ethnobiology: The Legacy of Amadeo Rea; Quinlan, M., Lepofsky, D., Eds.; Society of Ethnobiology, Department of Geography, University of North Texas: Denton, TX, USA, 2013; pp. 132–161. [Google Scholar]
- Ferriol, M.; Picó, B.; Nuez, F. Morphological and Molecular Diversity of a Collection of Cucurbita maxima Landraces. J. Am. Soc. Hortic. Sci. 2004, 129, 60–69. [Google Scholar] [CrossRef]
- Decker-Walters, D.S.; Walters, T.W. The Cambridge World History of Food; Kiple, K., Ornelas, K.C., Eds.; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Le Floch, E.; Boulos, L.; Véla, E. Catalogue Synonymique Commenté de la Flore Tunisie; Ministère de l’Environnement et du Développement durable: Tunis, Tunisie, 2010; p. 500.
- Hamdi, K.; Palma, D.; Angelini, P.; Acciarri, N.; Tarchoun, N.; Sestili, S. Cucurbita maxima Duch. population analysis: Relationship between Tunisian and Italian germplasm. J. Hortic. Sci. Biotechnol. 2020, 95, 496–505. [Google Scholar] [CrossRef]
- Hamdi, K.; Ben-Amor, J.; Mokrani, K.; Mezghanni, N.; Tarchoun, N. Assessment of the genetic diversity of some local squash (Cucurbita maxima Duchesne) populations revealed by agro-morphological and chemical traits. J. New Sci. 2017, 42, 2306–2317. [Google Scholar]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Sium, A.; Shawon, A.; Swapan, K.R.; Sun, H.W.; Kailas, S.D.; Abdullah, S.M. Effect of salinity on the morphological, physiological and biochemical properties of lettuce (Lactuca sativa L.) in Bangladesh. Open Agric. 2019, 4, 361–373. [Google Scholar] [CrossRef]
- Rouphael, Y.; Petropoulos, S.A.; Cardarelli, M.; Colla, G. Salinity as eustressor for enhancing quality of vegetables. Sci. Hortic. 2018, 234, 361–369. [Google Scholar] [CrossRef]
- Rogel, J.A.; Ariza, F.A.; Silla, R.O. Soil salinity and moisture gradients and plant zonation in Mediterranean salt marshes of Southeast Spain. Wetlands 2000, 20, 357–372. [Google Scholar] [CrossRef]
- Rubio, J.S.; García-Sánchez, F.; Rubio, F.; Martínez, V. Yield, blossom-end rot incidence, and fruit quality in pepper plants under moderate salinity are affected by K+ and Ca2+ fertilization. Sci. Hortic. 2009, 119, 79–87. [Google Scholar] [CrossRef]
- Soltabayeva, A.; Ongaltay, A.; Omondi, J.O.; Srivastava, S. Morphological, physiological and molecular markers for salt-stressed plants. Plants 2021, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Petropoulos, S.A. Response and Defence Mechanisms of Vegetable Crops against Drought, Heat and Salinity Stress. Agriculture 2021, 11, 463. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping Active Oxygen Under Control. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Saibi, W.; Feki, K.; Ben Mahmoud, R.; Brini, F. Durum wheat dehydrin (DHN-5) confers salinity tolerance to transgenic Arabidopsis plants through the regulation of proline metabolism and ROS scavenging system. Planta 2015, 242, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Sazzad Hossain, M.; Persicke, M.; Elsayed, A.I.; Kalinowski, J.; Dietz, K.J. Metabolite profiling at the cellular and subcellular level reveals metabolites associated with salinity tolerance in sugar beet. J. Exp. Bot. 2017, 68, 5961–5976. [Google Scholar] [CrossRef] [Green Version]
- Chaves, M.M.; Oliveira, M.M. Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Mahlooji, M.; Seyed Sharifi, R.; Razmjoo, J.; Sabzalian, M.R.; Sedghi, M. Effect of salt stress on photosynthesis and physiological parameters of three contrasting barley genotypes. Photosynthetica 2018, 56, 549–556. [Google Scholar] [CrossRef]
- Orlovsky, N.; Japakova, U.; Zhang, H.; Volis, S. Effect of salinity on seed germination, growth and ion content in dimorphic seeds of Salicornia europaea L. (Chenopodiaceae). Plant. Divers. 2016, 38, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, S.A.; Daferera, D.; Polissiou, M.G.; Passam, H.C. The effect of salinity on the growth, yield and essential oils of turnip-rooted and leaf parsley cultivated within the Mediterranean region. J. Sci. Food Agric. 2009, 89, 1534–1542. [Google Scholar] [CrossRef]
- Golbashy, M.; Ebrahimi, M.; Khavari Khorasani, S.; Mostafavi, K. Effects of drought stress on germination indices of corn hybrids (Zea mays L.). Electrnonic J. Plant. Breed. 2012, 3, 664–670. [Google Scholar]
- Almansouri, M.; Kinet, J.M.; Lutts, S. Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.). Plant. Soil 2001, 231, 243–254. [Google Scholar] [CrossRef]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.; Bano, A.; Babar, M.A. The stimulatory effects of plant growth promoting rhizobacteria and plant growth regulators on wheat physiology grown in sandy soil. Arch. Microbiol. 2019, 201, 769–785. [Google Scholar] [CrossRef] [PubMed]
- Savvas, D.; Lenz, F. Effects of NaCl or nutrient-induced salinity on growth, yield, and composition of eggplants grown in rockwool. Sci. Hortic. 2000, 84, 37–47. [Google Scholar] [CrossRef]
- Parvin, K. Response of Tomato Plant Under Salt Stress: Role of Exogenous Calcium. J. Plant. Sci. 2015, 10, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Janghel, D.K.; Kumar, K.; Sunil, R.; Chhabra, A.K. Genetic Diversity Analysis, Characterization and Evaluation of Elite Chickpea (Cicer arietinum L.) Genotypes. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 199–209. [Google Scholar] [CrossRef]
- Foti, C.; Khah, E.M.; Pavli, O.I. Germination profiling of lentil genotypes subjected to salinity stress. Plant. Biol. 2019, 21, 480–486. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Cho, M.C.; Yang, E.Y.; Lee, J.G. Response to salt stress in lettuce: Changes in chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities. Agronomy 2020, 10, 1627. [Google Scholar] [CrossRef]
- Othman, Y.; Al-Karaki, G.; Al-Tawaha, A.R.; Al-Horani, A. Variation in Germination and Ion Uptake in Barley Genotypes under Salinity Conditions. World J. Agric. Sci. 2006, 2, 11–15. [Google Scholar]
- Mehr, Z.S. Salt-induced changes in germination and vegetative stages of Anethum graveolens L. J. Stress Physiol. Biochem. 2013, 9, 189–198. [Google Scholar]
- Kandil, A.; Shareif, A.; Gad, M. Effect of Salinity on Germination and Seeding Parameters of Forage Cowpea Seed. Res. J. Seed Sci. 2016, 10, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Viegas, D.X.; Piñol, J.; Viegas, M.T.; Ogaya, R. Estimating live fine fuels moisture content using meteorologically-based indices. Int. J. Wildl. Fire 2001, 10, 223–240. [Google Scholar] [CrossRef]
- Yildirim, E.; Dursun, A.; Kumlay, M.A.; Güvenç, Í. The effects of different salt, biostimulant and temperature levels on seed germination of some vegetable species. Acta Agrobot. 2013, 55, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Azeem, A.; Javed, Q.; Sun, J.; Nawaz, M.I.; Ullah, I.; Kama, R.; Du, D. Functional traits of okra cultivars (Chinese green and Chinese red) under salt stress. Folia Hortic. 2020, 32, 159–170. [Google Scholar] [CrossRef]
- Pavli, O.; Kempapidis, K.; Maggioros, L.; Foti, C.; Panagiotaki, P.; Khah, E. Response of Lettuce Germplasm to Salt Stress at Different Developmental Stages. Ann. Agric. Crop Sci. 2021, 6, 1088. [Google Scholar] [CrossRef]
- Srivastava, S.; Sharma, P.K. Effect of NaCl on chlorophyll fluorescence and thylakoid membrane proteins in leaves of salt sensitive and tolerant rice (Oryza sativa L) varieties. J. Stress Physiol. Biochem. 2021, 17, 35–44. [Google Scholar]
- Hasan, B.; Higano, Y.; Yabar, H.; Devkota, M.; Lamers, J.P.A. Conservation Agriculture Practices in Salt-Affected, Irrigated Areas of Central Asia: Crop Price and Input Cost Variability Effect on Revenue Risks. Sustain. Agric. Res. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Jamil, M.; Rha, E.-S. The Effect of Salinity (NaCI) on the Germination and Seedling of Sugar Beet (Beta vulgaris L.) and Cabbage (Brassica oleracea L.). Plant. Resour. 2004, 7, 226–232. [Google Scholar]
- Pavli, O.I. Effect of Salinity on Seed Germination and Seedling Development of Soybean Genotypes. Int. J. Environ. Sci. Nat. Resour. 2021, 27, 556210. [Google Scholar] [CrossRef]
- Abdoli, M.; Saeidi, M.; Azhand, M.; Jalali-honarmand, S.; Esfandiari, E.; Shekari, F. The Effects of Different Levels of Salinity and Indole-3-Acetic Acid (IAA) on Early Growth and Germination of Wheat Seedling. J. Stress Physiol. Biochem. 2013, 9, 329–338. [Google Scholar]
- Ouji, A.; El-Bok, S.; Mouelhi, M.; Younes, M.; Kharrat, M. Effect of salinity stress on germination of five Tunisian lentil Lens culinaris L. genotypes. Eur. Sci. J. 2015, 11, 63–75. [Google Scholar]
- Shin, Y.K.; Bhandari, S.R.; Cho, M.C.; Lee, J.G. Evaluation of chlorophyll fluorescence parameters and proline content in tomato seedlings grown under different salt stress conditions. Hortic. Environ. Biotechnol. 2020, 61, 433–443. [Google Scholar] [CrossRef]
- Abbas, W.; Ashraf, M.; Akram, N.A. Alleviation of salt-induced adverse effects in eggplant (Solanum melongena L.) by glycinebetaine and sugarbeet extracts. Sci. Hortic. 2010, 125, 188–195. [Google Scholar] [CrossRef]
- Kaya, M.D.; Ipek, A.; Öztürk, A. Effects of different soil salinity levels on germination and seedling growth of safflower (Carthamus tinctorius L.). Turk. J. Agric. For. 2003, 27, 221–227. [Google Scholar] [CrossRef]
- Benidire, L.; Daoui, K.; Fatemi, Z.A.; Achouak, W.; Bouarab, L.; Oufdou, K. Effect of salt stress on germination and seedling of Vicia faba L. J. Mater. Environ. Sci. 2015, 6, 840–851. [Google Scholar]
- Alom, R.; Hasan, M.A.; Islam, M.R.; Wang, Q.F. Germination characters and early seedling growth of wheat (Triticum aestivum L.) genotypes under salt stress conditions. J. Crop Sci. Biotechnol. 2016, 19, 383–392. [Google Scholar] [CrossRef]
- Partheeban, C.; Chandrasekhar, C.N.; Jeyakumar, P.; Ravikesavan, R.; Gnanam, R. Effect of PEG Induced Drought Stress on Seed Germination and Seedling Characters of Maize (Zea mays L.) Genotypes. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1095–1104. [Google Scholar] [CrossRef] [Green Version]
- Hannachi, S.; Van Labeke, M.C. Salt stress affects germination, seedling growth and physiological responses differentially in eggplant cultivars (Solanum melongena L.). Sci. Hortic. 2018, 228, 56–65. [Google Scholar] [CrossRef]
- Du, F.; Shi, H.; Zhang, X.; Xu, X. Responses of reactive oxygen scavenging enzymes, proline and malondialdehyde to water deficits among six secondary successional seral species in Loess Plateau. PLoS ONE 2014, 9, e98872. [Google Scholar] [CrossRef]
- Ma, J.; Du, G.; Li, X.; Zhang, C.; Guo, J. A major locus controlling malondialdehyde content under water stress is associated with Fusarium crown rot resistance in wheat. Mol. Genet. Genom. 2015, 290, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, Z.W.; Han, X.G.; Yang, Y.G.; Busso, C.A.; Zhang, Z.; Yang, Y.F. Effect of mixed salt stress on malondialdehyde, proteins and antioxidant enzymes of Leymus chinensis in three leaf colors. Phyton 2017, 86, 205–213. [Google Scholar]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef]
- Xing, J.-C.; Dong, J.; Wang, M.-W.; Liu, C.; Zhao, B.-Q.; Wen, Z.-G.; Zhu, X.-M.; Ding, H.-R.; Zhao, X.-H.; Hong, L.-Z. Effects of NaCl stress on growth of Portulaca oleracea and underlying mechanisms. Braz. J. Bot. 2019, 42, 217–226. [Google Scholar] [CrossRef]
- Borsai, O.; Al Hassan, M.; Negrușier, C.; Raigón, M.D.; Boscaiu, M.; Sestraș, R.E.; Vicente, O. Responses to salt stress in portulaca: Insight into its tolerance mechanisms. Plants 2020, 9, 1660. [Google Scholar] [CrossRef]
- Jaarsma, R.; de Vries, R.S.M.; de Boer, A.H. Effect of Salt Stress on Growth, Na+ Accumulation and Proline Metabolism in Potato (Solanum tuberosum) Cultivars. PLoS ONE 2013, 8, e60183. [Google Scholar] [CrossRef] [PubMed]
- Sarabi, B.; Bolandnazar, S.; Ghaderi, N.; Ghashghaie, J. Genotypic differences in physiological and biochemical responses to salinity stress in melon (Cucumis melo L.) plants: Prospects for selection of salt tolerant landraces. Plant. Physiol. Biochem. 2017, 119, 294–311. [Google Scholar] [CrossRef]
- De la Torre-González, A.; Montesinos-pereira, D.; Blasco, B.; Ruiz, J.M. Influence of the proline metabolism and glycine betaine on tolerance to salt stress in tomato (Solanum lycopersicum L.) commercial genotypes. J. Plant. Physiol. 2018, 231, 329–336. [Google Scholar] [CrossRef]
- Zhani, K.; Mariem, B.F.; Fardaous, M.; Cherif, H.; Zhani, K.; Mariem, B.F.; Fardaous, M.; Cherif, H. Impact of salt stress (NaCl) on growth, chlorophyll content and fluorescence of Tunisian cultivars of chili pepper (Capsicum frutescens L.). J. Stress Physiol. Biochem. 2012, 8, 236–252. [Google Scholar]
- Seroczyńska, A.; Antczak, A.; Kamińska, K.; Korytowska, M.; Korzeniewska, A.; Niemirowicz-szczytt, K.; Radomski, A.; Zawadzki, J. Evaluation of the selected forms of winter squash (Cucurbita maxima Duch.) for the content of free sugars and polysaccharides. Polish J. Agron. 2014, 16, 69–73. [Google Scholar]
- Xu, C.; Mou, B. Evaluation of lettuce genotypes for salinity tolerance. HortScience 2015, 50, 1441–1446. [Google Scholar] [CrossRef] [Green Version]
- Ekinci, M.; Yildirim, E.; Dursun, A.; Turan, M. Mitigation of salt stress in lettuce (Lactuca sativa L. var. Crispa) by seed and foliar 24-epibrassinolide treatments. HortScience 2012, 47, 631–636. [Google Scholar] [CrossRef]
- ECPGR. Minimum descriptors for Cucurbita spp., cucumber, melon and watermelon. Cucurbits Work. Gr. 2008, 15, 1–13. [Google Scholar]
- Monneveaux, P.; Nemmar, M. Contribution à l’étude de la résistance à la sécheresse chez le blé tendre (Triticum aestivum L.) et chez le blé dur (Triticum durum Desf.): Étude de l’accumulation de la proline au cours du cycle de développement. Agronomie 1986, 6, 583–590. [Google Scholar] [CrossRef]
- Curtis, O.F.; Shetty, K. Growth medium effects on vitrification, total phenolics, chlorophyll, and water content of in vitro propagated oregano clones. Acta Hortic. 1996, 426, 489–504. [Google Scholar] [CrossRef]
| S.O.V. | DF | GP | SL | RL | SFW | RFW | SRR | GR | SLR | RLR | GSTI | SLSTI | RLSTI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accession | 14 | 4066.8 | 341.0 | 152.9 | 2.6 | 0.05 | 54.8 | 3801.8 | 487.9 | 115.3 | 0.39 | 26,109.5 | 18,310.1 |
| Salinity | 3 | 17,405.8 | 1103.6 | 697.3 | 8.4 | 0.25 | 139.3 | 7553.0 | 22.9 | 227.9 | 0.98 | 1484.6 | 44,510.7 |
| Accession x Salinity | 42 | 620.4 | 46.7 | 14.1 | 0.2 | 0.004 | 18.9 | 455.4 | 9.1 | 6.6 | 0.05 | 669.1 | 1321.4 |
| CV (%) | 13.3 | 9.0 | 11.3 | 17.0 | 24.9 | 20.6 | 10.2 | 20.7 | 21.7 | 29.69 | 15.4 | 16.6 |
| NaCl Concentration (mM) | GP (%) | SL (mm) | RL (mm) | SFW (g) | RFW (g) | SRR | GR (%) | SLR (%) | RRL (%) | GSTI (%) | SLSTI (%) | RLSTI (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 86.3 ± 1.08 a | 12.3 ± 0.24 a | 7.4 ± 0.18 a | 0.9 ± 0.02 a | 0.14 a ± 0.004 a | 1.3 ± 0.02 c | - | - | - | - | - | - |
| 100 | 23.3 ± 1.50 b | 7.1 ± 0.33 b | 4.7 ± 0.24 b | 0.6 ± 0.03 b | 0.08 ± 0.004 b | 1.7 ± 0.06 b | 63.0 ± 1.49 c | 5.2 ± 0.37 b | 2.7 ± 0.22 c | 0.27 ± 0.016 a | 60.1 ± 2.83 a | 63.8 ± 2.94 a |
| 200 | 15.0 ± 1.08 c | 6.4 ± 0.31 c | 3.3 ± 0.20 c | 0.5 ± 0.05 c | 0.06 ± 0.003 c | 1.8 ± 0.10 b | 71.3 ± 1.18 b | 5.9 ± 0.35 a | 4.1 ± 0.19 b | 0.17 ± 0.011 b | 54.2 ± 2.58 b | 44.0 ± 2.34 b |
| 300 | 8.4 ± 0.67 d | 6.3 ± 0.30 c | 2.0 ± 0.14 d | 0.3 ± 0.01 d | 0.04 ± 0.003 d | 3.6 ± 0.27 a | 77.9 a ± 1.02 a | 5.9 ± 0.39 a | 5.3 ± 0.16 a | 0.09 ± 0.006 v | 54.6 ± 2.84 b | 27.5 ± 1.77 c |
| F Value | 8816 ** | 2141.20 ** | 2879.11 ** | 815.17 ** | 649.38 ** | 745.38 ** | 362.56 ** | 143.95 ** | 791.03 ** | 296.89 ** | 19.75 ** | 16.58 ** |
| Accession | GP (%) | SL (mm) | RL (mm) | SFW (g) | RFW (g) | SRR | GR (%) | SLR (%) | RRL (%) | GSTI (%) | SLSTI (%) | RLSTI (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| “745” | 42.0 ± 4.72 bc | 8.0 ± 0.18 gh | 4.7 ± 0.29 de | 0.6 ± 0.02 de | 0.07 ± 0.008 e | 1.8 ± 0.58 h | 74.6 ± 3.55 c | 6.0 ± 1.11 d | 2.0 ± 0.56 h | 0.24 ± 0.04 e | 56.67 ± 7.45 e | 68.9 ± 7.47 b |
| “746” | 48.4 ± 5.36 a | 8.3 ± 0.32 g | 4.5 ± 0.24 e | 0.6 ± 0.007 de | 0.02 ± 0.005 h | 2.4 ± 0.26 e | 67.0 ± 3.15 de | 2.1 ± 0.69 h | 3.6 ± 0.74 e | 0.32 ± 0.03 ab | 80.85 ± 5.66 a | 49.8 ± 7.55 e |
| “747” | 49.3 ± 4.81 a | 7.8 ± 0.16 h | 3.0 ± 0.26 g | 0.6 ± 0.03 de | 0.03 ± 0.005 fg | 3.6 ± 0.36 b | 64.0 ± 2.64 ef | 1.6 ± 0.25 h | 2.5 ± 0.61 fh | 0.34 ± 0.07 a | 82.25 ± 7.23 a | 44.3 ± 8.50 ef |
| “748” | 41.5 ± 7.02 cd | 9.3 ± 1.11 d | 4.7 ± 0.66 de | 0.8 ± 0.05 b | 0.10 ± 0.008 cd | 2.1 ± 0.72 f | 64.6 ± 2.49 ef | 4.8 ± 0.89 e | 2.0 ± 0.55 gh | 0.29 ± 0.02 cd | 64.20 ± 5.15 d | 67.6 ± 8.47 b |
| “749” | 40.0 ± 4.93 cd | 9.3 ± 0.29 de | 4.0 ± 0.41 f | 0.6 ef ± 0.01 ef | 0.10 ± 0.006 cd | 2.9 ± 0.32 d | 60.0 ± 3.56 g | 4.1 ± 0.81 f | 4.1 ± 0.64 d | 0.30 ± 0.02 bc | 72.54 ± 6.80 b | 42.9 ± 7.89 gh |
| “750” | 39.5 ± 5.05 d | 5.3 ± 0.81 i | 3.0 ± 0.51 g | 0.8 ± 0.05 c | 0.10 ± 0.006 cd | 3.3 ± 0.24 c | 71.3 ± 4.40 c | 2.0 ± 0.66 h | 2.8 ± 0.52 f | 0.23 ± 0.03 e | 73.00 ± 6.77 b | 44.7 ± 8.12 fg |
| “751” | 31.2 ± 4.83 e | 9.8 ± 0.35 c | 8.0 ± 0.36 b | 0.9 ± 0.02 b | 0.09 ± 0.004 d | 1.2 ± 0.23 i | 65.0 ± 5.06 ef | 10.6 ± 1.35 c | 5.3 ± 0.79 c | 0.19 ± 0.05 f | 40.54 ± 5.44 f | 57.4 ± 7.36 c |
| “752” | 42.7 ± 4.10 b | 8.8 ± 0.37 f | 5.8 ± 0.36 c | 1.1 ± 0.03 a | 0.15 ± 0.006 a | 1.5 ± 0.34 h | 71.0 ± 2.64 cd | 3.8 ± 0.85 f | 2.3 ± 0.50 gh | 0.26 ± 0.02 de | 67.43 ± 6.70 cd | 70.5 ± 5.47 b |
| “753” | 28.7 ± 5.47 f | 9.0 ± 0.24 ef | 3.0 ± 0.31 g | 0.6 ± 0.05 d | 0.11 ± 0.005 b | 4.5 ± 0.52 a | 61.7 ± 4.04 fg | 3.0 ± 0.80 h | 3.4 ± 0.71 e | 0.18 ± 0.04 f | 81.11 ± 8.30 a | 32.6 ± 4.55 h |
| “1004” | 23.2 ± 5.09 gh | 10.9 ± 0.24 b | 4.7 ± 0.57 d | 0.5 ± 0.02 f | 0.10 ± 0.006 cd | 3.1 ± 0.21 c | 66.3 ± 3.20 e | 3.6 ± 0.84 f | 4.1 ± 0.82 d | 0.09 ± 0.03 h | 73.98 ± 6.21 b | 50.8 ± 8.24 de |
| “1005” | 22.0 ± 3.23 gh | 10.7 ± 0.22 b | 5.9 ± 0.62 c | 0.6 ± 0.04 de | 0.10 ± 0.006 cd | 1.9 ± 0.18 fg | 54.7 ± 6.80 h | 4.2 ± 0.91 f | 4.2 ± 0.80 d | 0.13 ± 0.20 g | 69.99 ± 5.41 bc | 54.6 ± 4.91 cd |
| “1006” | 28.1 ± 4.95 f | 13.4 ± 0.85 a | 8.3 ± 0.38 a | 0.4 ± 0.03 g | 0.10 ± 0.006 cd | 1.7 ± 0.16 gh | 92.3 ± 2.77 a | 2.8 ± 0.60 g | 2.1 ± 0.58 gh | 0.15 ± 0.01 i | 81.98 ± 7.16 a | 81.6 ± 7.93 a |
| “1007” | 21.5 ± 6.76 h | 2.7 ± 0.34 k | 1.8 ± 0.30 i | 0.2 ± 0.03 h | 0.04 ± 0.007 f | 0.4 ± 0.10 j | 86.0 ± 2.58 b | 10.7 ± 1.42 c | 7.4 ± 0.88 b | 0.03 ± 0.01 i | 0.00 ± 0.00 g | 0.0 ± 0.00 i |
| “1008” | 16.7 ± 6.30 i | 2.9 ± 0.78 k | 1.3 ± 0.54 j | 0.1 ± 0.06 i | 0.03 ± 0.001 h | 0.5 ± 0.13 j | 67.0 ± 4.99 de | 11.6 ± 1.54 b | 5.3 ± 0.84 c | 0.04 ± 0.01 i | 0.00 ± 0.00 g | 0.0 ± 0.00 i |
| “1009” | 24.0 ± 4.94 g | 3.8 ± 0.38 j | 2.3 ± 0.21 h | 0.2 ± 0.04 h | 0.03 ± 0.006 h | 0.4 ± 0.10 j | 96.0 ± 4.40 a | 15.1 ± 1.66 a | 9.1 ± 0.96 a | 0.03 ± 0.01 i | 0.00 ± 0.00 g | 0.0 ± 0.00 i |
| CV % | 13.54 | 8.96 | 11.32 | 16.98 | 23.92 | 20.63 | 10.23 | 20.69 | 21.74 | 26.69 | 15.39 | 16.62 |
| NaCl Concentration (mM) | Accession | GP (%) | SL (mm) | RL (mm) | SFW (g) | RFW (g) | SRR |
|---|---|---|---|---|---|---|---|
| Control | “745” | 97.9 ± 1.5 | 12.5 ± 1.6 | 6.2 ± 0.8 | 0.9 ± 0.2 | 0.15 ± 0.02 | 2.0 ± 0.4 |
| “746” | 98.7 ± 1.5 | 9.9 ± 1.8 | 7.2 ± 0.7 | 0.8 ± 0.1 | 0.04 ± 0.01 | 1.4 ± 0.2 | |
| “747” | 97.3 ± 2.3 | 9.1 ± 0.7 | 4.9 ± 0.5 | 0.7 ± 0.4 | 0.07 ± 0.03 | 1.9 ± 0.2 | |
| “748” | 90.0 ± 8.7 | 12.9 ± 1.8 | 6.2 ± 0.6 | 1.1 ± 0.3 | 0.15 ± 0.02 | 2.1 ± 0.4 | |
| “749” | 85.0 ± 4.3 | 12.4 ± 1.6 | 7.0 ± 1.2 | 0.76 ± 0.06 | 0.15 ± 0.01 | 1.8 ± 0.4 | |
| “750” | 93.0 ± 2.6 | 6.8 ± 0.7 | 5.1 ± 0.3 | 1.1 ± 0.1 | 0.16 ± 0.02 | 1.3 ± 0.2 | |
| “751” | 80.0 ± 4.3 | 17.8 ± 1.8 | 12.0 ± 2.0 | 1.3 ± 0.2 | 0.14 ± 0.02 | 1.5 ± 0.1 | |
| “752” | 96.0 ± 2.6 | 11.7 ± 1.1 | 7.5 ± 0.6 | 1.7 ± 0.2 | 0.19 ± 0.05 | 1.4 ± 0.2 | |
| “753” | 75.0 ± 4.3 | 10.4 ± 0.4 | 5.6 ± 0.3 | 0.83 ± 0.03 | 0.18 ± 0.02 | 1.9 ± 0.1 | |
| “1004” | 73.0 ± 5.7 | 13.5 ± 1.1 | 7.7 ± 1.4 | 0.63 ± 0.05 | 0.15 ± 0.01 | 1.8 ± 0.3 | |
| “1005” | 63.0 ± 6.7 | 13.9 ± 1.0 | 9.1 ± 1.1 | 0.8 ± 0.1 | 0.15 ± 0.03 | 1.5 ± 0.2 | |
| “1006” | 97.3 ± 1.9 | 15.5 ± 0.5 | 9.9 ± 0.4 | 0.69 ± 0.07 | 0.16 ± 0.02 | 1.6 ± 0.1 | |
| “1007” | 86.0 ± 5.2 | 10.7 ± 0.4 | 7.4 ± 0.4 | 0.86 ± 0.03 | 0.17 ± 0.02 | 1.4 ± 0.1 | |
| “1008” | 67.0 ± 5.7 | 11.6 ± 0.8 | 5.3 ± 0.3 | 0.52 ± 0.03 | 0.16 ± 0.02 | 2.2 ± 0.2 | |
| “1009” | 96.0 ± 2.6 | 15.1 ± 0.8 | 9.1 ± 0.3 | 0.73 ± 0.09 | 0.10 ± 0.01 | 1.7 ± 0.1 | |
| 100 | “745” | 50.0 ± 5.7 | 7.7 ± 0.6 | 5.0 ± 0.5 | 0.7 ± 0.04 | 0.08 ± 0.01 | 1.5 ± 0.2 |
| “746” | 40.0 ± 4.3 | 9.0 ± 0.6 | 5.7 ± 0.5 | 0.6 ± 0.2 | 0.03 ± 0.05 | 1.6 ± 0.2 | |
| “747” | 45.0 ± 4.3 | 8.0 ± 0.4 | 3.8 ± 0.4 | 0.68 ± 0.04 | 0.04 ± 0.01 | 2.1 ± 0.2 | |
| “748” | 35.0 ± 3.3 | 8.6 ± 0.5 | 5.3 ± 0.2 | 0.9 ± 0.2 | 0.10 ± 0.03 | 1.6 ± 0.1 | |
| “749” | 40.0 ± 4.8 | 9.0 ± 0.9 | 4.4 ± 0.3 | 0.64 ± 0.03 | 0.10 ± 0.01 | 2.0 ± 0.3 | |
| “750” | 30.0 ± 5.3 | 4.8 ± 0.9 | 4.5 ± 0.3 | 0.92 ± 0.06 | 0.11 ± 0.01 | 1.1 ± 0.2 | |
| “751” | 30.0 ± 5.3 | 8.5 ± 0.5 | 9.3 ± 0.3 | 1.00 ± 0.04 | 0.11 ± 0.01 | 0.9 ± 0.1 | |
| “752” | 35.0 ± 4.3 | 8.9 ± 0.5 | 6.3 ± 0.3 | 1.29 ± 0.08 | 0.17 ± 0.01 | 1.4 ± 0.1 | |
| “753” | 25.0 ± 3.3 | 7.6 ± 0.4 | 3.5 ± 0.3 | 0.71 ± 0.04 | 0.12 ± 0.01 | 2.2 ± 0.2 | |
| “1004” | 25.0 ± 3.3 | 10.4 ± 0.7 | 6.2 ± 0.3 | 0.58 ± 0.02 | 0.11 ± 0.01 | 1.7 ± 0.1 | |
| “1005” | 15.0 ± 2.6 | 11.1 ± 0.8 | 6.5 ± 0.2 | 0.69 ± 0.02 | 0.10 ± 0.01 | 1.7 ± 0.2 | |
| “1006” | 10.0 ± 4.4 | 12.2 ± 0.5 | 9.4 ± 0.3 | 0.44 ± 0.03 | 0.10 ± 0.01 | 1.3 ± 0.1 | |
| “1007” | 0 | 0 | 0 | 0 | 0 | 0 | |
| “1008” | 0 | 0 | 0 | 0 | 0 | 0 | |
| “1009” | 0 | 0 | 0 | 0 | 0 | 0 | |
| 200 | “745” | 15.0 ± 4.3 | 6.6 ± 0.4 | 4.1 ± 0.2 | 0.53 ± 0.03 | 0.04 ± 0.00 | 1.6 ± 0.1 |
| “746” | 35.0 ± 4.3 | 7.7 ± 0.6 | 3.4 ± 0.3 | 0.50 ± 0.04 | 0.01 ± 0.00 | 2.3 ± 0.3 | |
| “747” | 35.0 ± 4.3 | 7.5 ± 0.4 | 2.2 ± 0.3 | 0.55 ± 0.03 | 0.01 ± 0.00 | 3.5 ± 0.5 | |
| “748” | 25.0 ± 3.2 | 8.2 ± 0.3 | 4.1 ± 0.2 | 0.74 ± 0.03 | 0.08 ± 0.03 | 2.0 ± 0.1 | |
| “749” | 25.0 ± 3.2 | 8.2 ± 0.7 | 3.0 ± 0.3 | 0.49 ± 0.14 | 0.08 ± 0.01 | 2.8 ± 0.3 | |
| “750” | 25.0 ± 3.2 | 3.7 ± 0.5 | 1.6 ± 0.2 | 0.64 ± 0.02 | 0.08 ± 0.01 | 2.3 ± 0.3 | |
| “751” | 10.0 ± 4.2 | 7.2 ± 0.4 | 6.4 ± 0.3 | 0.85 ± 0.04 | 0.08 ± 0.01 | 1.1 ± 0.1 | |
| “752” | 25.0 ± 3.3 | 7.6 ± 0.4 | 5.9 ± 0.3 | 0.88 ± 0.03 | 0.13 ± 0.01 | 1.3 ± 0.1 | |
| “753” | 10.0 ± 3.3 | 8.3 ± 0.3 | 2.2 ± 0.2 | 0.57 ± 0.03 | 0.09 ± 0.03 | 3.9 ± 0.3 | |
| “1004” | 5.0 ± 1.7 | 9.8 ± 0.7 | 3.4 ± 0.3 | 0.53 ± 0.02 | 0.08 ± 0.01 | 2.9 ± 0.4 | |
| “1005” | 10.0 ± 4.2 | 9.8 ± 0.3 | 4.7 ± 0.1 | 0.51 ± 0.03 | 0.08 ± 0.01 | 2.1 ± 0.1 | |
| “1006” | 5.0 ± 1.7 | 10.8 ± 0.5 | 8.5 ± 0.4 | 0.33 ± 0.02 | 0.08 ± 0.01 | 1.3 ± 0.1 | |
| “1007” | 0 | 0 | 0 | 0 | 0 | 0 | |
| “1008” | 0 | 0 | 0 | 0 | 0 | 0 | |
| “1009” | 0 | 0 | 0 | 0 | 0 | 0 | |
| 300 | “745” | 5.0 ± 1.7 | 5.3 ± 0.6 | 3.5 ± 0.3 | 0.33 ± 0.03 | 0.02 ± 0.00 | 1.5 ± 0.2 |
| “746” | 20.0 ± 3.3 | 6.7 ± 0.7 | 1.6 ± 0.3 | 0.50 ± 0.01 | 0.01 ± 0.00 | 4.0 ± 0.9 | |
| “747” | 25.0 ± 3.3 | 6.7 ± 0.4 | 1.0 ± 0.2 | 0.47 ± 0.03 | 0.01 ± 0.00 | 7.0 ± 1.4 | |
| “748” | 17.0 ± 4.1 | 7.7 ± 0.3 | 3.0 ± 0.3 | 0.5 ± 0.1 | 0.07 ± 0.01 | 2.6 ± 0.2 | |
| “749” | 10.0 ± 3.2 | 7.5 ± 0.5 | 1.6 ± 0.2 | 0.35 ± 0.03 | 0.05 ± 0.01 | 5.0 ± 1.0 | |
| “750” | 10.0 ± 3.2 | 6.0 ± 1.0 | 0.7 ± 0.1 | 0.32 ± 0.04 | 0.06 ± 0.02 | 8.5 ± 1.3 | |
| “751” | 5.0 ± 1.7 | 5.6 ± 0.6 | 4.4 ± 0.3 | 0.35 ± 0.03 | 0.04 ± 0.01 | 1.3 ± 0.1 | |
| “752” | 15.0 ± 4.3 | 6.9 ± 0.4 | 3.6 ± 0.2 | 0.64 ± 0.03 | 0.12 ± 0.01 | 1.9 ± 0.1 | |
| “753” | 5.0 ± 1.7 | 9.4 ± 0.3 | 0.9 ± 0.1 | 0.38 ± 0.02 | 0.05 ± 0.02 | 10.3 ± 1.5 | |
| “1004” | 5.0 ± 1.7 | 9.8 ± 0.7 | 1.6 ± 0.2 | 0.35 ± 0.02 | 0.05 ± 0.01 | 6.2 ± 0.8 | |
| “1005” | 5.0 ± 1.7 | 8.1 ± 0.5 | 3.5 ± 0.2 | 0.3 ± 0.1 | 0.05 ± 0.03 | 2.3 ± 0.1 | |
| “1006” | 5.0 ± 1.7 | 15.2 ± 0.5 | 5.4 ± 0.3 | 0.21 ± 0.02 | 0.06 ± 0.02 | 2.8 ± 0.1 | |
| “1007” | 0 | 0 | 0 | 0 | 0 | 0 | |
| “1008” | 0 | 0 | 0 | 0 | 0 | 0 | |
| “1009” | 0 | 0 | 0 | 0 | 0 | 0 |
| NaCl concentration (mM) | MDA (µmol g−1) | Proline (µg mg−1) | Chl a (mg mg−1) | Chl b (mg mg−1) |
|---|---|---|---|---|
| Control | 1.17 ± 0.53 b | 0.82 ± 0.06 a | 30.09 ± 2.87 b | 60.64 ± 5.96 b |
| 100 | 1.75 ± 0.30 a | 0.51 ± 0.02 b | 35.47 ± 3.90 a | 69.88 ± 7.68 a |
| 200 | 1.43 ± 0.21 b | 0.82 ± 0.04 a | 25.70 ± 2.43 d | 52.44 ± 5.50 d |
| 300 | 1.34 ± 0.18 b | 0.92 ± 0.05 a | 28.91 ± 2.46 c | 56.34 ± 5.98 c |
| F Value | 33.6 ** | 471.2 ** | 552.3 ** | 737.5 ** |
| SD |
| Accession | MDA (µmol g−1) | Proline (µg mg−1) | Chl a (mg mg−1) | Chl b (mg mg−1) |
|---|---|---|---|---|
| “746” | 1.6 ± 0.12 a | 1.0 ± 0.06 a | 12.5 ± 1.50 d | 25.7 ± 2.08 d |
| “747” | 0.8 ± 0.19 b | 0.8 ± 0.02 b | 14.8 ± 1.16 c | 27.9 ± 2.15 c |
| “748” | 0.8 ± 0.12 b | 0.5 ± 0.04 d | 41.8 ± 1.50 b | 77.0 ± 2.08 b |
| “751” | 0.9 ± 0.26 b | 0.6 ± 0.05 c | 51.1 ± 1.29 a | 108.7 ± 3.82 a |
| F Value | 15.5 ** | 1466.3 ** | 12473.9 ** | 21337.9 ** |
| SD |
| Accession | NaCl Concentration (mM) | MDA (µmol g−1) | Proline (µg mg−1) | Chl a (mg mg−1) | Chl b (mg mg−1) |
|---|---|---|---|---|---|
| “746” | Control | 0.88 ± 0.03 | 1.42 ± 0.03 | 14.7 ± 0.2 | 31.1 ± 0.7 |
| 100 | 4.24 ± 0.03 | 0.74 ± 0.01 | 11.6 ± 0.2 | 24.4 ± 0.5 | |
| 200 | 0.58 ± 0.08 | 1.13 ± 0.05 | 9.9 ± 0.2 | 20.7 ± 0.3 | |
| 300 | 0.68 ± 0.09 | 0.71 ± 0.08 | 13.7 ± 0.1 | 26.6 ± 0.8 | |
| “747” | Control | 0.9 ± 0.1 | 0.59 ± 0.02 | 13.7 ± 0.1 | 26.0 ± 0.3 |
| 100 | 1.9 ± 0.6 | 0.44 ± 0.01 | 13.2 ± 0.2 | 25.8 ± 0.2 | |
| 200 | 0.36 ± 0.06 | 0.56 ± 0.02 | 15.6 ± 0.4 | 29.5 ± 0.5 | |
| 300 | 0.5 ± 0.2 | 0.8 ± 0.009 | 16.8 ± 0.4 | 30.2 ± 1.1 | |
| “748” | Control | 1.8 ± 0.4 | 0.47 ± 0.05 | 37.5 ± 0.6 | 72.2 ± 0.9 |
| 100 | 0.65 ± 0.03 | 0.48 ± 0.04 | 57.0 ± 0.8 | 103.6 ± 1.5 | |
| 200 | 0.60 ± 0.04 | 0.55 ± 0.05 | 30.2 ± 0.6 | 55.2 ± 0.6 | |
| 300 | 0.20 ± 0.07 | 0.40 ± 0.01 | 42.5 ± 0.8 | 76.9 ± 0.9 | |
| “751” | Control | 1.1 ± 0.2 | 0.63 ± 0.09 | 54.5 ± 0.5 | 113.3 ± 1.7 |
| 100 | 0.2 ± 0.1 | 0.46 ± 0.01 | 60.0 ± 3.8 | 125.7 ± 3.9 | |
| 200 | 0.20 ± 0.03 | 1.06 ± 0.05 | 47.1 ± 0.4 | 104.3 ± 3.5 | |
| 300 | 1.56 ± 0.20 | 0.98 ± 0.03 | 42.7 ± 0.3 | 91.7 ± 2.4 |
| Landrace Inventory Number | Local Name | Origin | Latitude | Longitude | Short Description |
|---|---|---|---|---|---|
| NGBTUN745 (“745”) | Batati Green | Ariana (Kalaat Andalous) | 37°033″ N | 10°11′7″ E | Globular fruit, light green skin, green flesh |
| NGBTUN746 (“746”) | Batati orange | Siliana (SidiHamada) | 35°57′28″ N | 9°32′57″ E | Globular fruit, orange skin, light orange flesh |
| NGBTUN747 (“747”) | Galaoui | Ariana (Kalaa Andalous) | 37°033″ N | 10°11′7″ E | Raised fruit with basal tip, green skin, green flesh |
| NGBTUN748 (“748”) | Karkoubi orange | Sousse (SidiBouali) | 35°54′22.21″ N | 10°32′47.81″ E | Flattened fruit, dark yellow skin, yellow flesh |
| NGBTUN749 (“749”) | Batati yellow spotted with white | Siliana (SidiHamada) | 35°57′28″ N | 9°32′57″ E | Globular fruit, orange skin spotted with white, orange flesh |
| NGBTUN750 (“750”) | Batati white | Monastir (Sahline) | 35°45′05″ N | 10°42′39″ E | Globular fruit, white skin, white flesh |
| NGBTUN751 (“751”) | Bejaoui Green | Siliana (SidiHamada) | 35°57′28″ N | 9°32′57″ E | Flattened fruit, dark green skin, light green flesh |
| NGBTUN752 (“752”) | Batati yellow | Siliana (North) | 35°57′28″ N | 9°32′57″ E | Globular fruit, yellow skin, light orange flesh |
| NGBTUN753 (“753”) | Béjaoui Green | Siliana (South) | 35°57′28″ N | 9°32′57″ E | Flattened fruit, dark green skin, light green flesh |
| NGBTUN1004 (“1004”) | Galaoui large seeds | Ariana (Kalaat Andalous) | 37°033″ N | 10°11′7″ E | Turbinate interior fruit with basal tip, green skin, white green flesh |
| NGBTUN1005 (“1005”) | Galaoui smoll seeds | Ariana (Kalaat Andalous) | 37°033″ N | 10°11′7″ E | Turbinate interior fruit with a big basal tip, green skin, white green flesh |
| NGBTUN1006 (“1006”) | Karkoubi orange | Monastir (Sahline) | 35°45′05″ N | 10°42′39″ E | Flattened fruit, dark yellow skin, yellow flesh |
| NGBTUN1007 (“1007”) | Batati Green | Siliana | 35°57′28″ N | 9°32′57″ E | Rounded fruit, green skin, green flesh |
| NGBTUN1008 (“1008”) | Batati Green | Monastir (Teboulba) | 35°45′05″ N | 10°42′39″ E | Globular fruit, flat stem end, green skin, light green flesh |
| NGBTUN1009 (“1009”) | Bejaoui spotted with yellow | Siliana (SidiHamada) | 35°57′28″ N | 9°32′57″ E | Globular fruit with flat stem end, spotted with yellow light green skin, light green flesh |
| Trait | Unit | Description/Formula | Reference |
|---|---|---|---|
| Germination percentage (GP) | % | Scott et al. (1984) | |
| Shoot length (SL) | mm | At the day of germination | Sivakumar et al. (2020) |
| Root length (RL) | mm | At the day of germination | Sivakumar et al. (2020) |
| Shoot fresh weight (SFW) | g | Recorded by using a sensitive balance (Sartorius AC 1215, Germany) | Jamil et al. (2006) |
| Root fresh weight (RFW) | g | Recorded by using a sensitive balance (Sartorius AC 1215, Germany) | Jamil et al. (2006) |
| Shoot length/Root length Ratio (SRR) | - | Ratio of SL to RL | Thabet et al. (2018) |
| Germination reduction (GR) | % | GR = GP of controls − GP of stress plants | Thabet et al. (2018) |
| Shoot length reduction (SLR) | mm | SLR = SL of controls − SL of stress plants | Partheeban et al. (2017) |
| Root length reduction (RLR) | mm | RLR = RL of controls − RL of stress plants | Thabet et al. (2018) |
| Germination stress tolerance index (GSTI) | % | Partheeban et al. (2017) | |
| Shoot length stress tolerance index (SLSTI) | % | Partheeban et al. (2017) | |
| Root length stress tolerance index (RLSTI) | % | Partheeban et al. (2017) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarchoun, N.; Saadaoui, W.; Mezghani, N.; Pavli, O.I.; Falleh, H.; Petropoulos, S.A. The Effects of Salt Stress on Germination, Seedling Growth and Biochemical Responses of Tunisian Squash (Cucurbita maxima Duchesne) Germplasm. Plants 2022, 11, 800. https://doi.org/10.3390/plants11060800
Tarchoun N, Saadaoui W, Mezghani N, Pavli OI, Falleh H, Petropoulos SA. The Effects of Salt Stress on Germination, Seedling Growth and Biochemical Responses of Tunisian Squash (Cucurbita maxima Duchesne) Germplasm. Plants. 2022; 11(6):800. https://doi.org/10.3390/plants11060800
Chicago/Turabian StyleTarchoun, Neji, Wassim Saadaoui, Najla Mezghani, Ourania I. Pavli, Hanen Falleh, and Spyridon A. Petropoulos. 2022. "The Effects of Salt Stress on Germination, Seedling Growth and Biochemical Responses of Tunisian Squash (Cucurbita maxima Duchesne) Germplasm" Plants 11, no. 6: 800. https://doi.org/10.3390/plants11060800
APA StyleTarchoun, N., Saadaoui, W., Mezghani, N., Pavli, O. I., Falleh, H., & Petropoulos, S. A. (2022). The Effects of Salt Stress on Germination, Seedling Growth and Biochemical Responses of Tunisian Squash (Cucurbita maxima Duchesne) Germplasm. Plants, 11(6), 800. https://doi.org/10.3390/plants11060800







