Exogenous Melatonin Activates Antioxidant Systems to Increase the Ability of Rice Seeds to Germinate under High Temperature Conditions
Abstract
:1. Introduction
2. Results
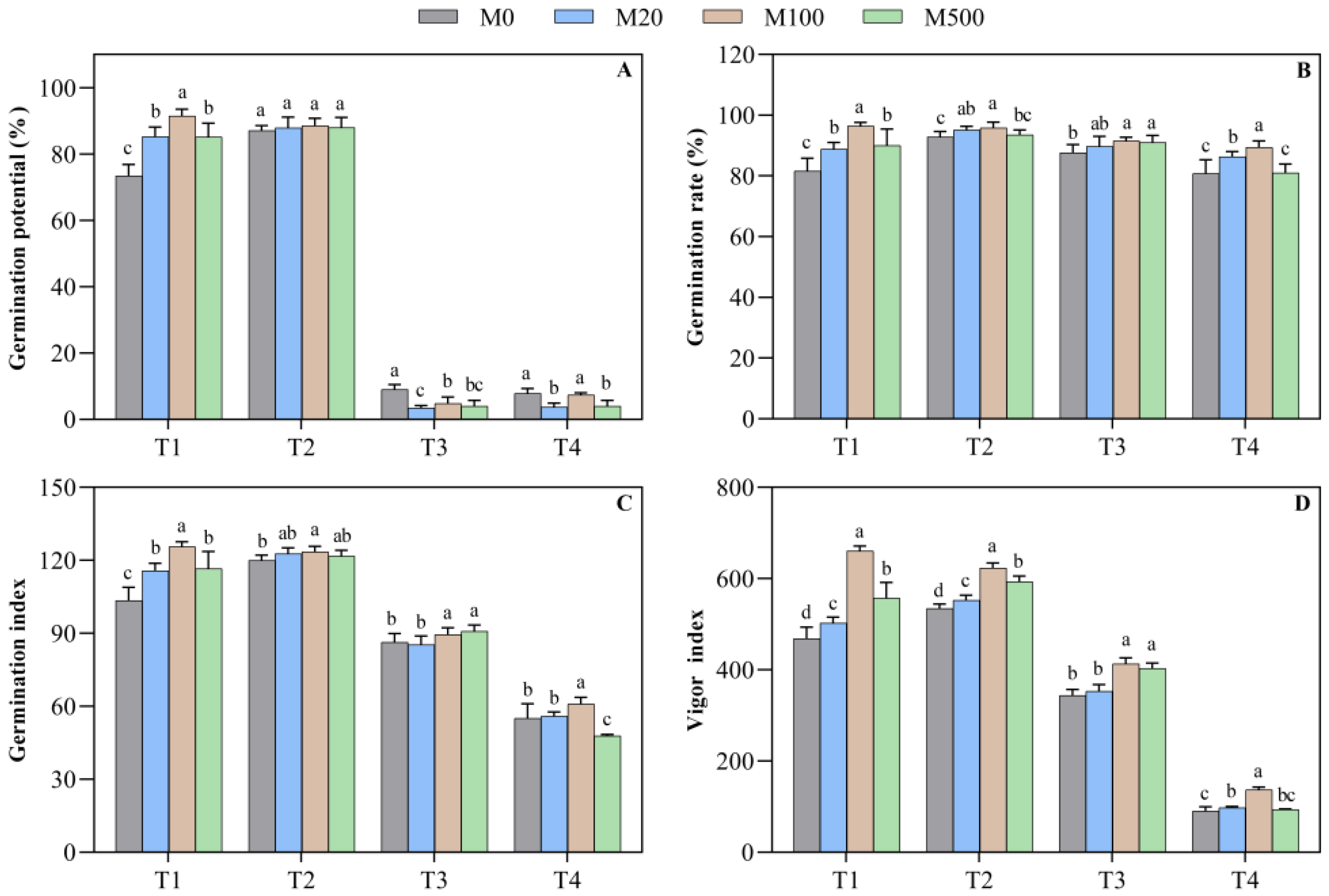
2.1. Germination Characteristics
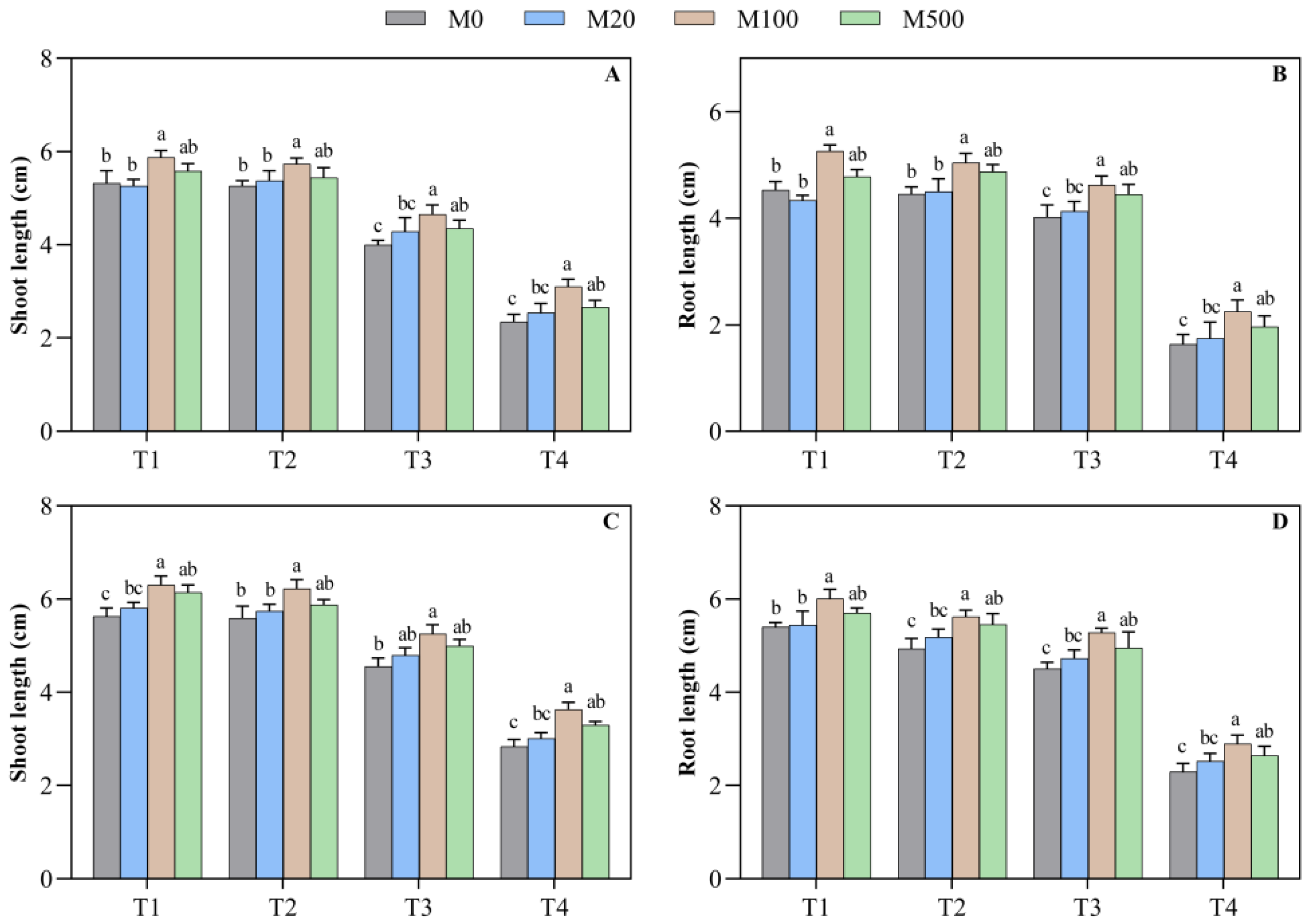
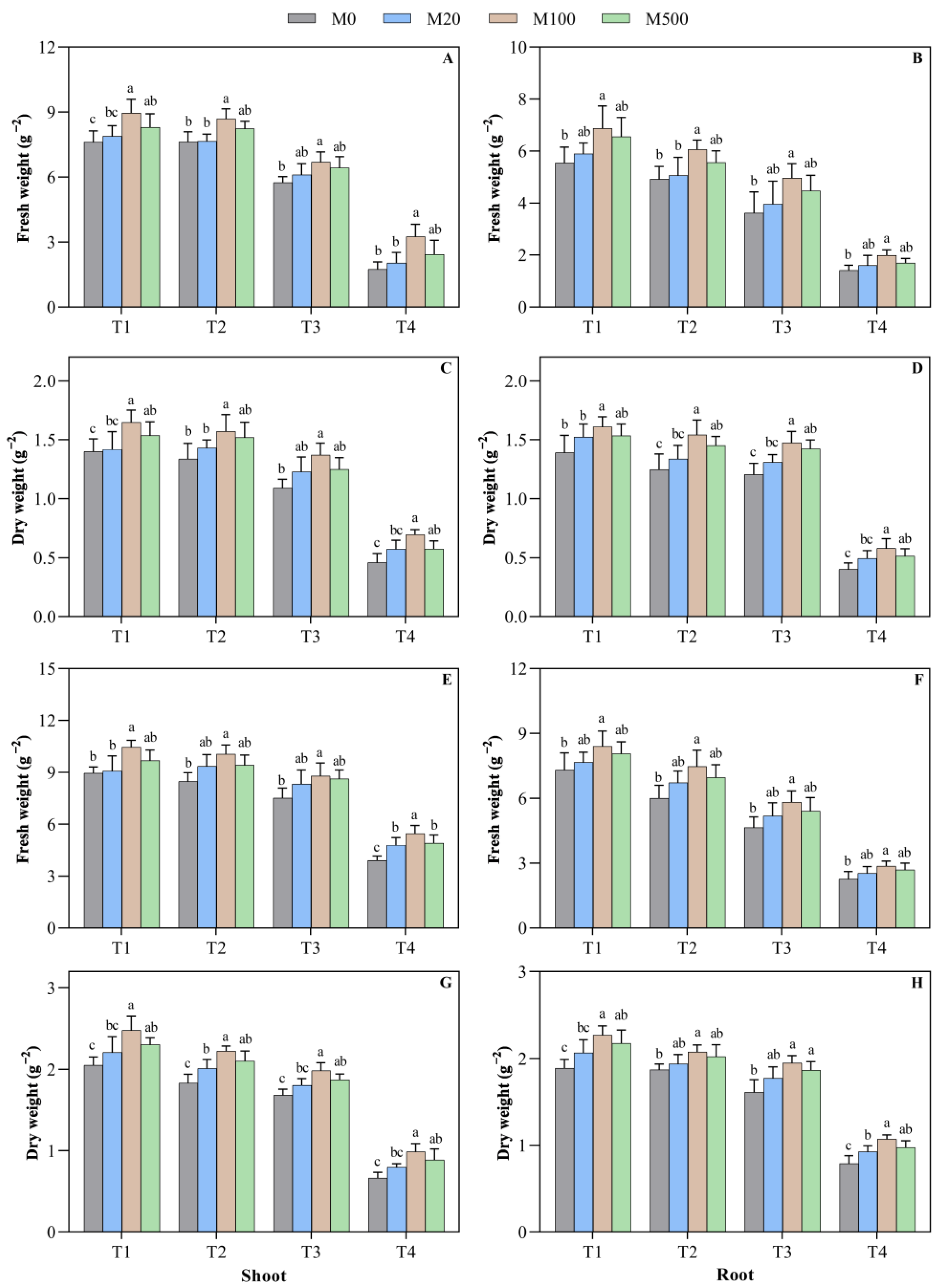
2.2. Biomass of Rice Seed Shoots and Roots
2.3. SOD Activity in Rice Seed Shoots and Roots
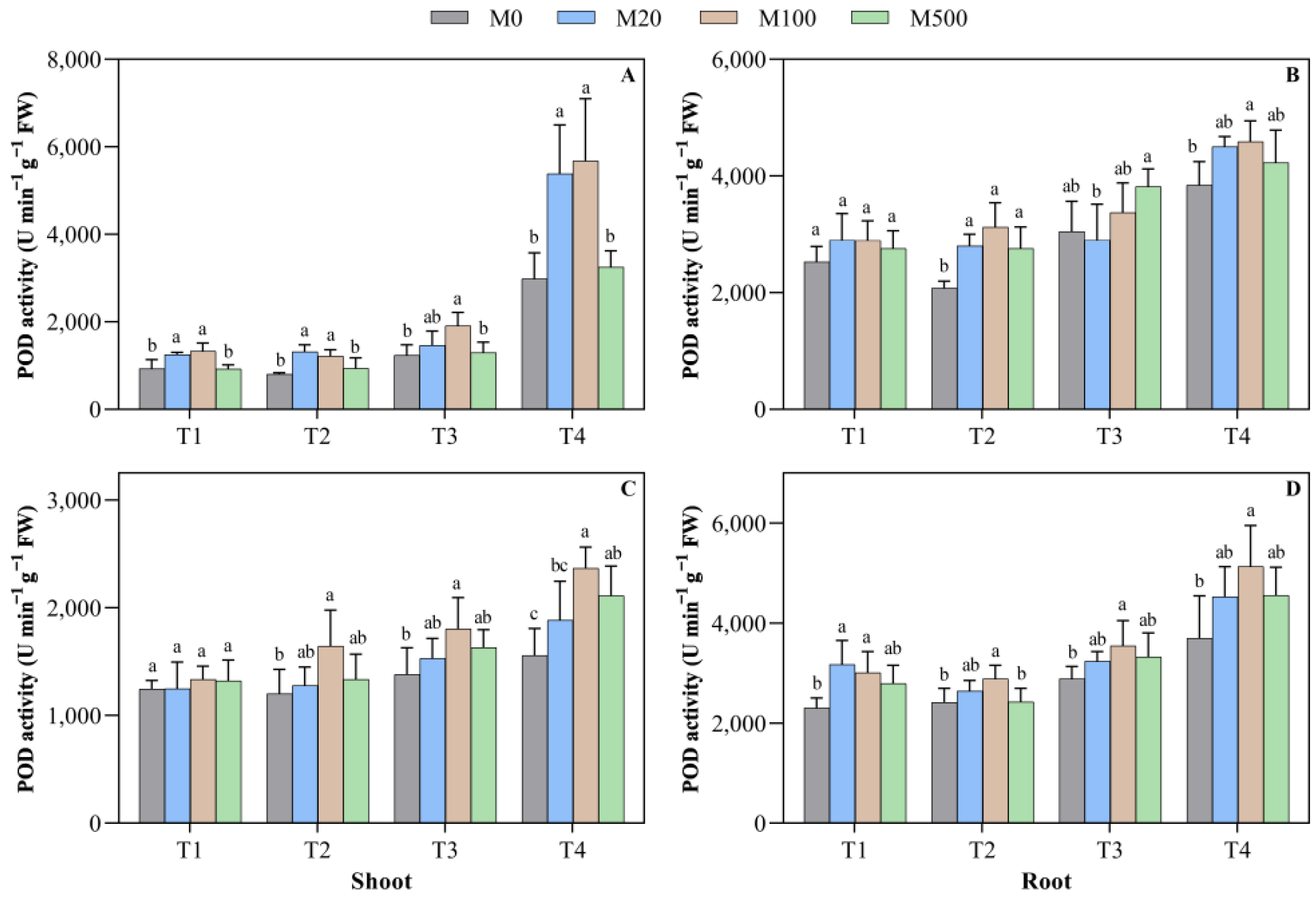
2.4. POD Activity in Rice Seed Shoots and Roots
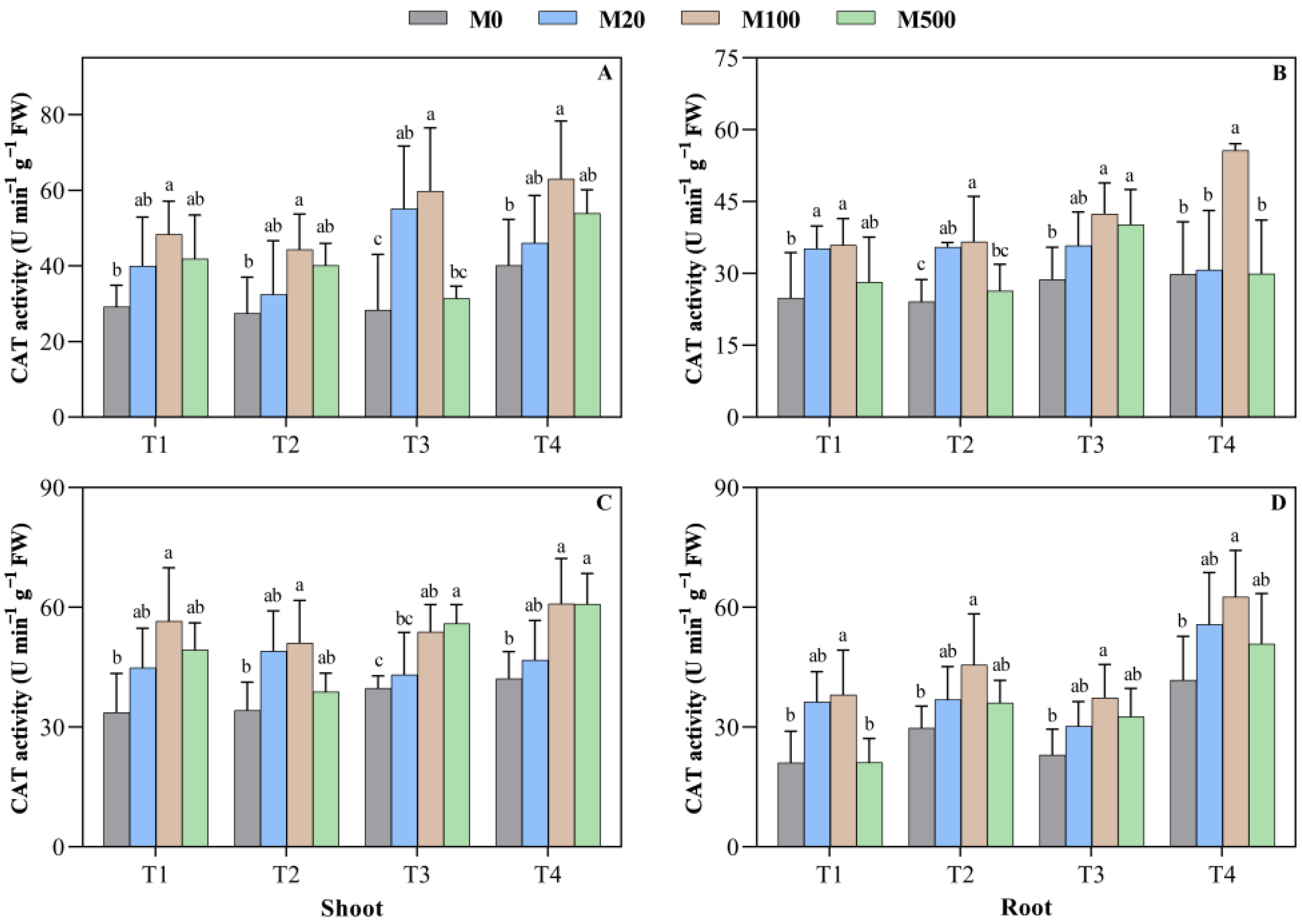
2.5. CAT Activity in Rice Seed Shoots and Roots
2.6. MDA Content in Rice Seed Shoots and Roots
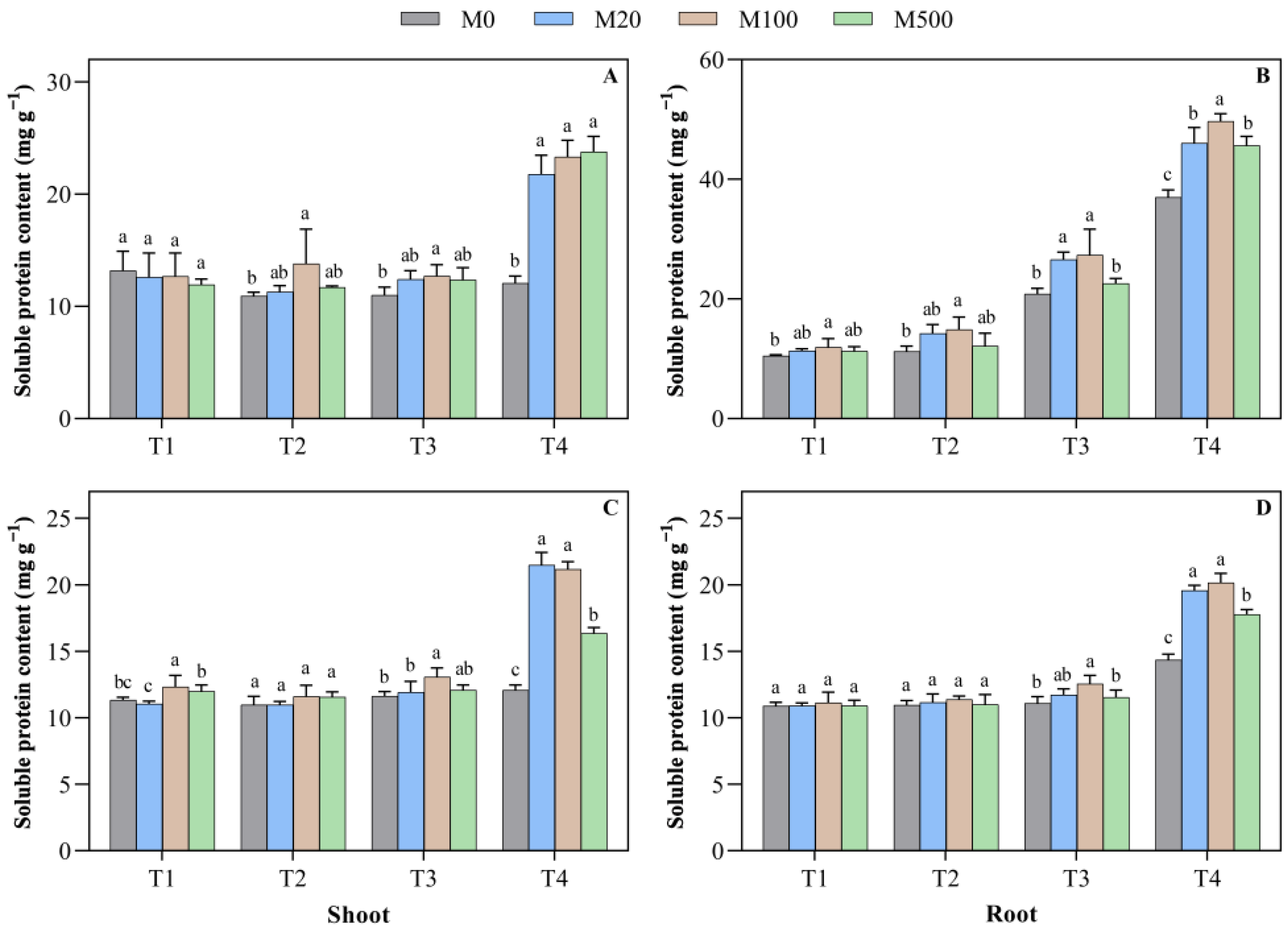
2.7. Soluble Protein Content in Rice Seed Shoots and Roots
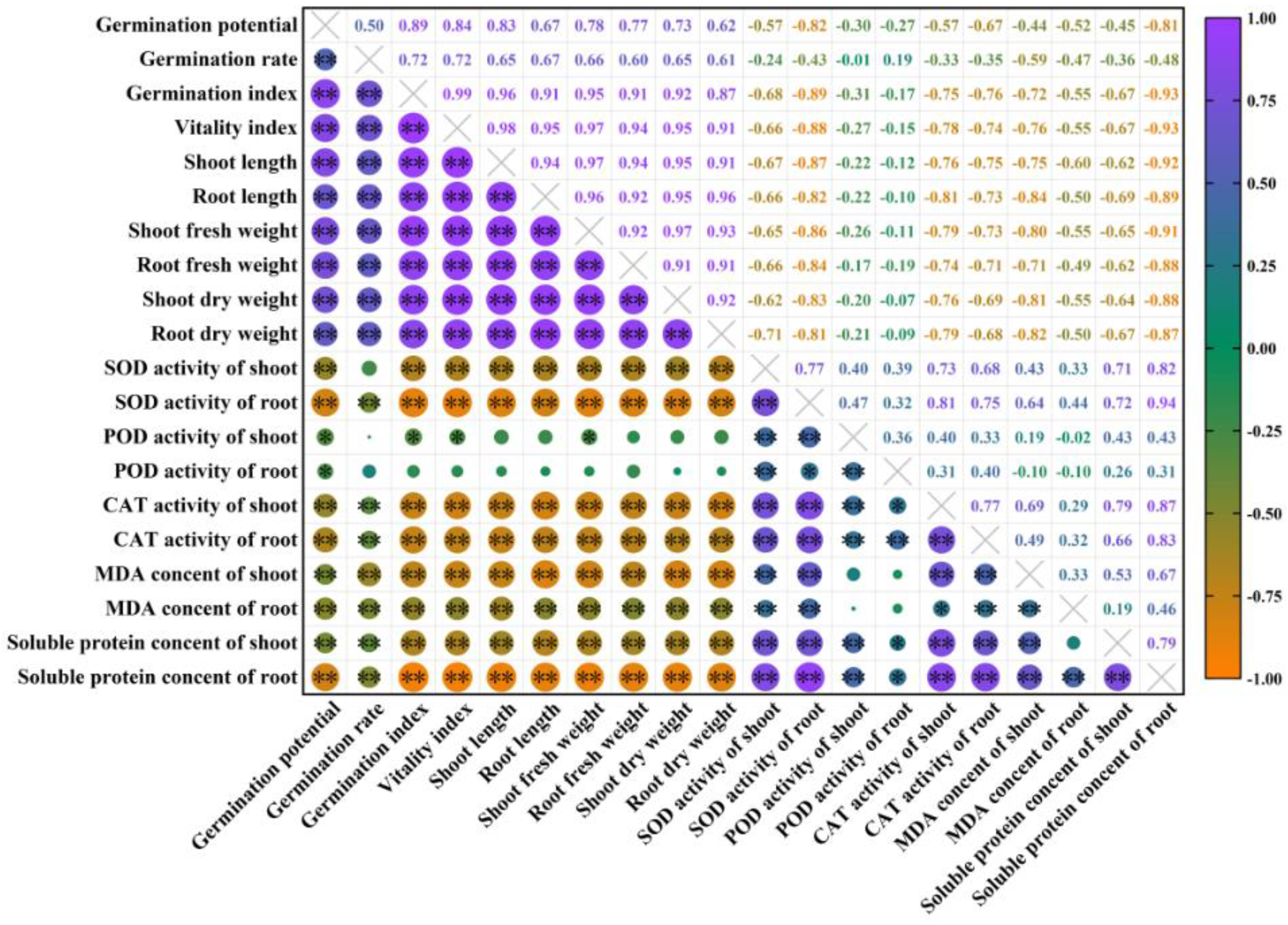
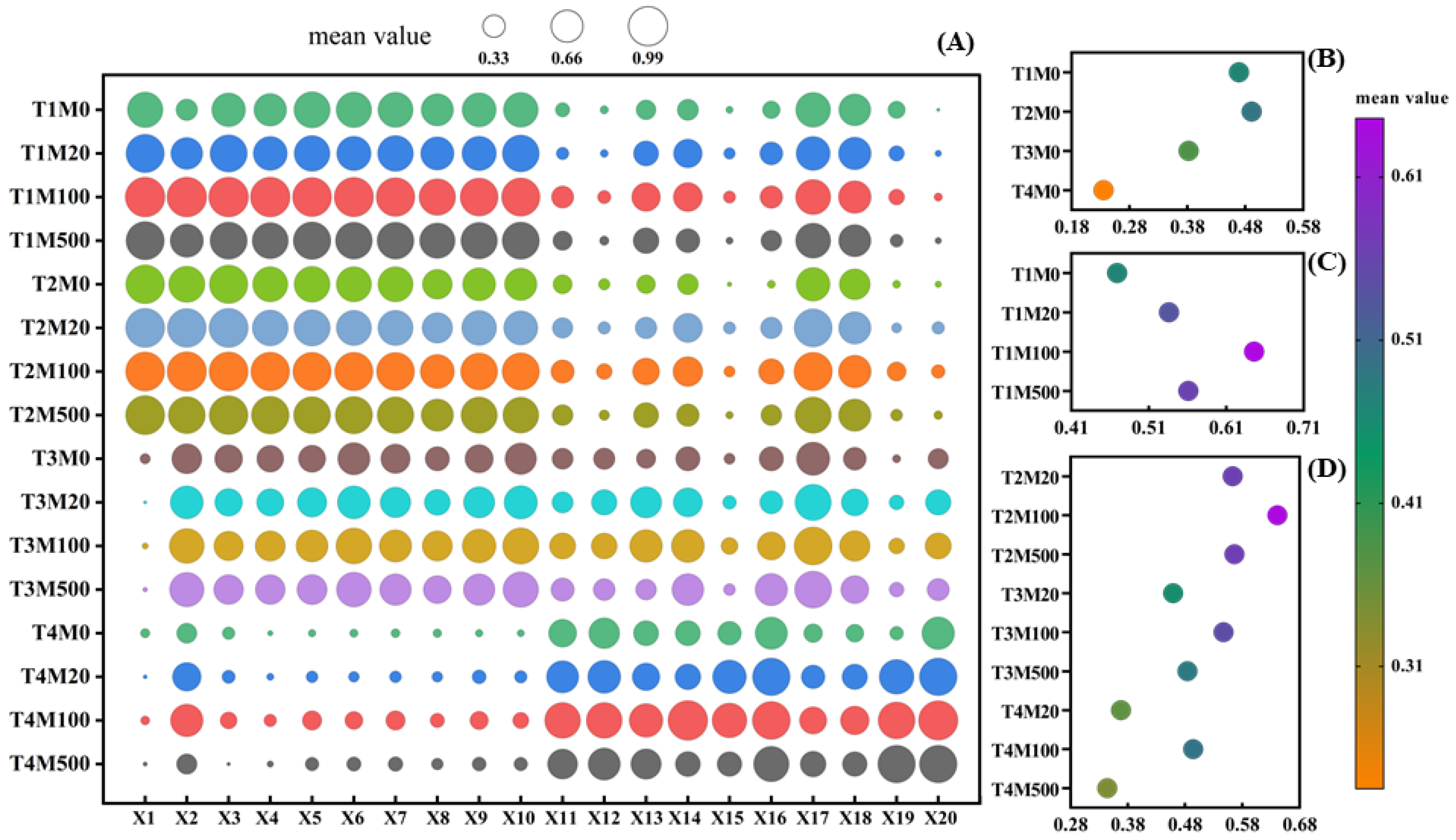
2.8. Gray Relational Grade and Correlational Analysis of Exogenous Melatonin in Rice Seed Germination and Physiological Indicators under High Temperature Stress
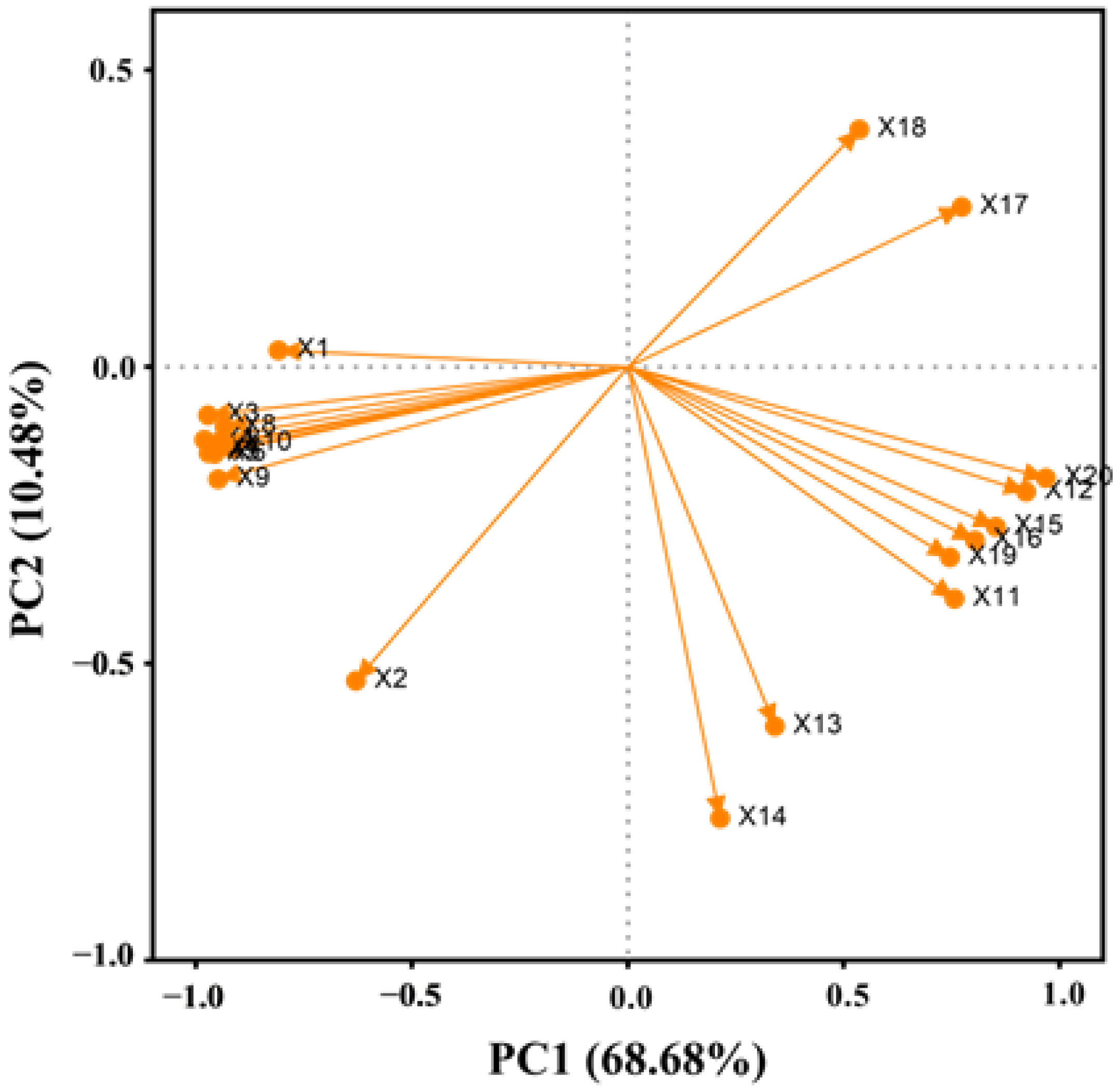
2.9. Principal Component Analysis
2.10. Comparison of the Differences and Comprehensive Evaluation of Tolerance between High Temperature Stress and Exogenous Melatonin Interaction
3. Discussion
4. Materials and Methods
4.1. Test Material
4.2. Experimental Design
4.3. Measurement Items and Methods
4.3.1. Determination of Relevant Indicators of Seed Germination
4.3.2. Determination of Seed Shoot and Root Growth Indicators
4.3.3. Determination of the Biomass Indicators
4.3.4. Determination of the Physiological and Biochemical Indicators
4.4. Data Processing and Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, M.; Fu, Y.Y.; Mou, Q.S.; An, J.Y.; Zhu, X.B.; Ahmed, T.; Zhang, S.; Basit, F.; Hu, J.; Guan, Y.J. Spermidine induces expression of stress associated proteins (SAPs) genes and protects rice seed from heat stress-induced damage during grain-filling. Antioxidants 2021, 10, 1544. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Gu, Q.Q.; Dong, Q.; Zhang, Z.H.; Lin, C.; Hu, W.M.; Pan, R.H.; Guan, Y.J.; Hu, J. Spermidine enhances heat tolerance of rice seeds by modulating endogenous starch and polyamine metabolism. Molecules 2019, 24, 1395. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.J.; Xu, H.H.; Wang, W.Q.; Li, N.; Wang, W.P.; Møller, L.M.; Song, S.Q. A proteomic analysis of rice seed germination as affected by high temperature and ABA treatment. Physiol. Plant. 2015, 154, 142–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hasanuzzaman, M.; Wen, H.L.; Zhang, J.; Peng, T.; Sun, H.W.; Zhao, Q.Z. High temperature and drought stress cause abscisic acid and reactive oxygen species accumulation and suppress seed germination growth in rice. Protoplasma 2019, 256, 1217–1227. [Google Scholar] [CrossRef]
- Mangrauthia, S.K.; Agarwal, S.; Sailaja, B.; Sarla, N.; Voleti, S.R. Transcriptome analysis of Oryza sativa (Rice) seed germination at high temperature shows dynamics of genome expression associated with hormones signalling and abiotic stress pathways. Trop. Plant Biol. 2016, 9, 215–218. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. The physiological function of melatonin in plants. Plant Signal Behav. 2006, 1, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Seo, H.; Park, C.; Park, W.J. Examination of the auxin hypothesis of phytomelatonin action in classical auxin assay systems in maize. J. Plant Physiol. 2016, 190, 67–71. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Hardeland, R.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Melatonin: A hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal Res. 2003, 34, 75–78. [Google Scholar] [CrossRef]
- Han, Q.H.; Huang, B.; Ding, C.B.; Zhang, Z.W.; Chen, Y.E.; Hu, C.; Zhou, L.J.; Huang, Y.; Liao, J.Q.; Yuan, S.; et al. Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.T.; Lu, B.; Ma, T.T.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.C.; Zhang, Y.J.; Bai, Z.Y.; et al. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar] [CrossRef] [Green Version]
- Li, D.X.; Batchelor, W.D.; Zhang, D.; Miao, H.X.; Li, H.Y.; Song, S.J.; Li, R.Q. Analysis of melatonin regulation of germination and antioxidant metabolism in different wheat cultivars under polyethylene glycol stress. PLoS ONE 2020, 15, e237536. [Google Scholar] [CrossRef] [PubMed]
- Butter, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.J.; Ullah, I.; Wang, C.S. Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants 2012, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Guo, S.R.; Sun, J.; Shu, S.; Wang, Y.; El-Yazied, A.A.; Alabdallah, N.M.; Hikal, M.; Mohamed, M.H.M.; Ibrahim, M.F.M.; et al. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.Y.; Wang, K.X.; Yan, M.Y.; Kanwar, M.K.; Li, D.Y.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P.; Zhou, J. Melatonin alleviates high temperature-induced pollen abortion in solanum lycopersicum. Molecules 2018, 23, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, M.H.; Deng, W.W.; Ahammed, G.J.; Wei, J.P.; Yan, P.; Zhang, L.P.; Fu, J.Y.; Han, W.Y. Exogenous melatonin improves tea quality under moderate high temperatures by increasing epigallocatechin-3-gallate and theanine biosynthesis in Camellia sinensis L. J. Plant Physiol. 2020, 253, 153273. [Google Scholar] [CrossRef]
- Imran, M.; Aaqil Khan, M.; Shahzad, R.; Bilal, S.; Khan, M.; Yun, B.W.; Khan, A.L.; Lee, I.J. Melatonin ameliorates thermotolerance in soybean seedling through balancing redox homeostasis and modulating antioxidant defense, phytohormones and polyamines biosynthesis. Molecules 2021, 26, 5116. [Google Scholar] [CrossRef]
- Li, D.; Guo, Y.; Zhang, D.; He, S.C.; Gong, J.Y.; Ma, H.L.; Gao, X.; Wang, Z.H.; Jiang, L.X.; Dun, X.L.; et al. Melatonin represses oil and anthocyanin accumulation in seeds. Plant Physiol. 2020, 183, 898–914. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Lei, K.Q.; Sun, S.Z.; Zhong, K.T.; Li, S.Y.; Hu, H.; Sun, C.J.; Zheng, Q.M.; Tian, Z.W.; Dai, T.B.; Sun, J.Y. Seed soaking with melatonin promotes seed germination under chromium stress via enhancing reserve mobilization and antioxidant metabolism in wheat. Ecotoxicol Env. Saf. 2021, 220, 112241. [Google Scholar] [CrossRef]
- Ahmad, S.; Muhammad, I.; Wang, G.Y.; Zeeshan, M.; Yang, L.; Ali, I.; Zhou, X.B. Ameliorative effect of melatonin improves drought tolerance by regulating growth, photosynthetic traits and leaf ultrastructure of maize seedlings. BMC Plant Biol. 2021, 21, 368. [Google Scholar] [CrossRef]
- Alam, M.N.; Zhang, L.H.; Yang, L.; Islam, M.R.; Liu, Y.; Luo, H.; Yang, P.F.; Wang, Q.F.; Chan, Z.L. Transcriptomic profiling of tall fescue in response to heat stress and improved thermotolerance by melatonin and 24-epibrassinolide. BMC Genom. 2018, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Ma, G.J.; Zhang, M.D.; Xu, J.L.; Zhou, W.X.; Cao, L.W. Transcriptomic analysis of short-term heat stress response in Pinellia ternata provided novel insights into the improved thermotolerance by spermidine and melatonin. Ecotoxicol Environ. Saf. 2020, 202, 110877. [Google Scholar] [CrossRef]
- Wang, Z.H.; Wu, X.S.; Chang, X.P.; Li, R.Z.; Jing, R.L. Chlorophyll content and chlorophyll fluorescence kinetics parameters of flag leaf and their gray relational grade with yield in wheat. Acta Agron. Sin. 2010, 36, 217–227. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309. [Google Scholar] [CrossRef]
- Kochba, J.; Lavee, S.; Spiegel-Roy, P. Differences in peroxidase activity and isoenzymes in embryogenic ane non-embryogenic ‘Shamouti’ orange ovular callus lines. Plant Cell Physiol. 1977, 18, 463–467. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Mark Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Su, D.W.; Mei, L.; Lin, H.; Luo, H.L.; Zhang, L.L.; Song, J.; Song, J.; Liu, J.; Lin, Z.X. Grey correlation analysis of physiological traits related to drought tolerance in pennisetum sp. Agric. Basic Sci. Technol. 2017, 18, 1158–1163. [Google Scholar]
- Sun, Q.; Hu, J.J. Research Technology of Plant Physiology; Northwest University of Agriculture and Forestry Science and Technology Press: Yang Ling, China, 2005; p. 67. [Google Scholar]




| Index | High Temperature Treatment | MT Treatment | Interactive Treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Df | F | P | Df | F | P | Df | F | P | |
| X1 | 3 | 15,998.1 | <0.001 | 3 | 19.7 | <0.001 | 9 | 35.6 | <0.001 |
| X2 | 3 | 72.3 | <0.001 | 3 | 42.8 | <0.001 | 9 | 7.4 | <0.001 |
| X3 | 3 | 2524.2 | <0.001 | 3 | 35.0 | <0.001 | 9 | 15.7 | <0.001 |
| X4 | 3 | 8352.8 | <0.001 | 3 | 345.3 | <0.001 | 9 | 39.3 | <0.001 |
| X5 | 3 | 846.3 | <0.001 | 3 | 32.5 | <0.001 | 9 | 0.8 | 0.643 |
| X6 | 3 | 850.7 | <0.001 | 3 | 42.1 | <0.001 | 9 | 1.0 | 0.45 |
| X7 | 3 | 492.9 | <0.001 | 3 | 19.1 | <0.001 | 9 | 0.3 | 0.962 |
| X8 | 3 | 192.5 | <0.001 | 3 | 11.5 | <0.001 | 9 | 0.4 | 0.92 |
| X9 | 3 | 269.9 | <0.001 | 3 | 16.0 | <0.001 | 9 | 0.3 | 0.964 |
| X10 | 3 | 376 | <0.001 | 3 | 18.1 | <0.001 | 9 | 0.5 | 0.89 |
| X11 | 3 | 41.8 | <0.001 | 3 | 8.3 | <0.001 | 9 | 0.8 | 0.591 |
| X12 | 3 | 169.4 | <0.001 | 3 | 6.0 | 0.001 | 9 | 0.7 | 0.743 |
| X13 | 3 | 4.6 | 0.006 | 3 | 10.0 | <0.001 | 9 | 1.3 | 0.25 |
| X14 | 3 | 3.0 | 0.039 | 3 | 11.8 | <0.001 | 9 | 2.0 | 0.061 |
| X15 | 3 | 146.3 | <0.001 | 3 | 16.5 | <0.001 | 9 | 5.3 | <0.001 |
| X16 | 3 | 56.9 | <0.001 | 3 | 7.7 | <0.001 | 9 | 1.8 | 0.091 |
| X17 | 3 | 58.9 | <0.001 | 3 | 6.0 | 0.001 | 9 | 0.9 | 0.563 |
| X18 | 3 | 8.4 | <0.001 | 3 | 2.3 | 0.093 | 9 | 0.4 | 0.947 |
| X19 | 3 | 126.8 | <0.001 | 3 | 22.1 | <0.001 | 9 | 14.1 | <0.001 |
| X20 | 3 | 1211.2 | <0.001 | 3 | 34.9 | <0.001 | 9 | 6.4 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Deng, L.; Zhou, L.; Chen, G.; Wang, Y. Exogenous Melatonin Activates Antioxidant Systems to Increase the Ability of Rice Seeds to Germinate under High Temperature Conditions. Plants 2022, 11, 886. https://doi.org/10.3390/plants11070886
Yu Y, Deng L, Zhou L, Chen G, Wang Y. Exogenous Melatonin Activates Antioxidant Systems to Increase the Ability of Rice Seeds to Germinate under High Temperature Conditions. Plants. 2022; 11(7):886. https://doi.org/10.3390/plants11070886
Chicago/Turabian StyleYu, Yufeng, Liyuan Deng, Lu Zhou, Guanghui Chen, and Yue Wang. 2022. "Exogenous Melatonin Activates Antioxidant Systems to Increase the Ability of Rice Seeds to Germinate under High Temperature Conditions" Plants 11, no. 7: 886. https://doi.org/10.3390/plants11070886
APA StyleYu, Y., Deng, L., Zhou, L., Chen, G., & Wang, Y. (2022). Exogenous Melatonin Activates Antioxidant Systems to Increase the Ability of Rice Seeds to Germinate under High Temperature Conditions. Plants, 11(7), 886. https://doi.org/10.3390/plants11070886





