Utility of EST-SNP Markers for Improving Management and Use of Olive Genetic Resources: A Case Study at the Worldwide Olive Germplasm Bank of Córdoba
Abstract
:1. Introduction
2. Results
2.1. Genotyping of WOGBC by Means of EST-SNPs
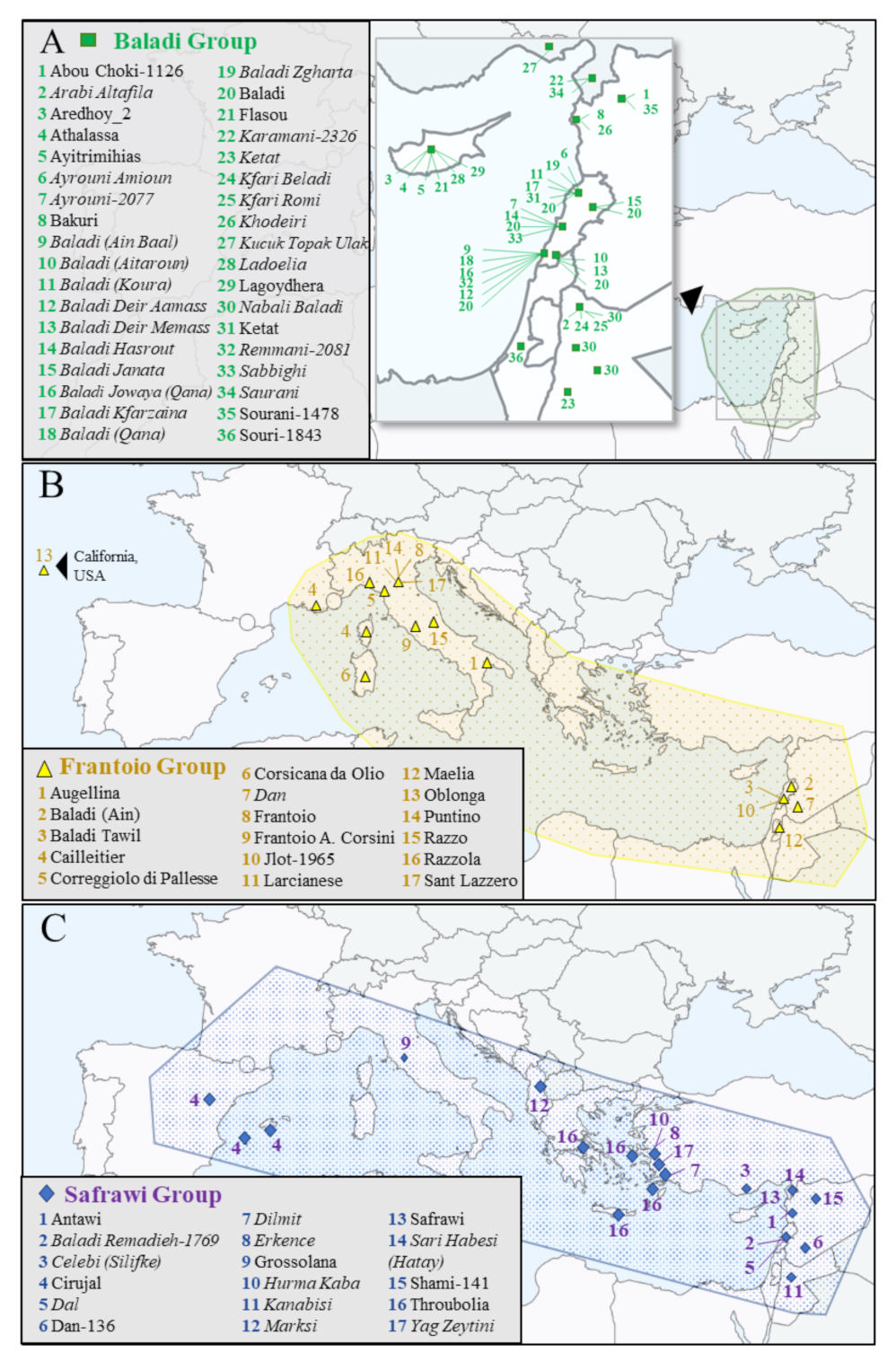
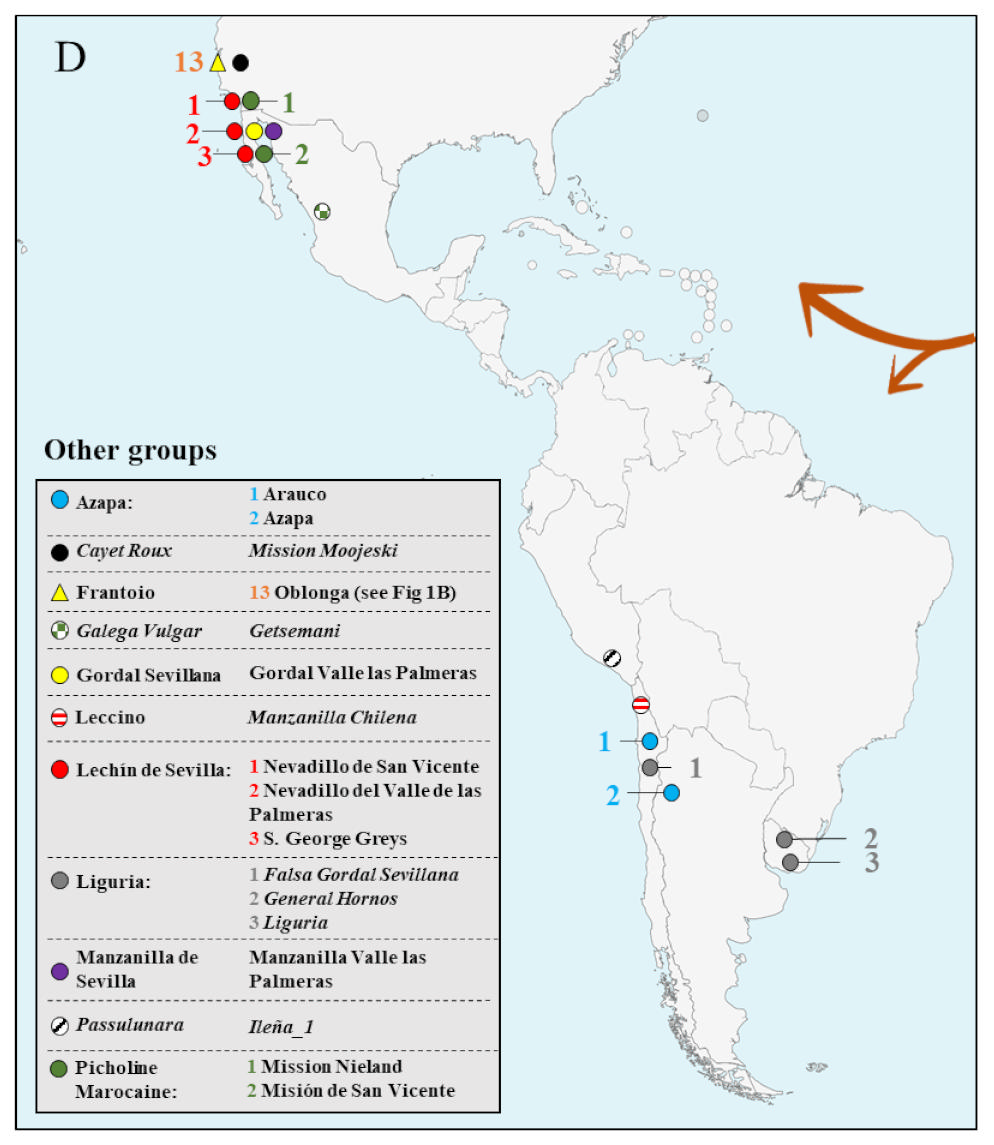
2.2. Evaluation of WOGBC Redundancies
2.3. Evaluation of Homonymy Cases at the WOGBC
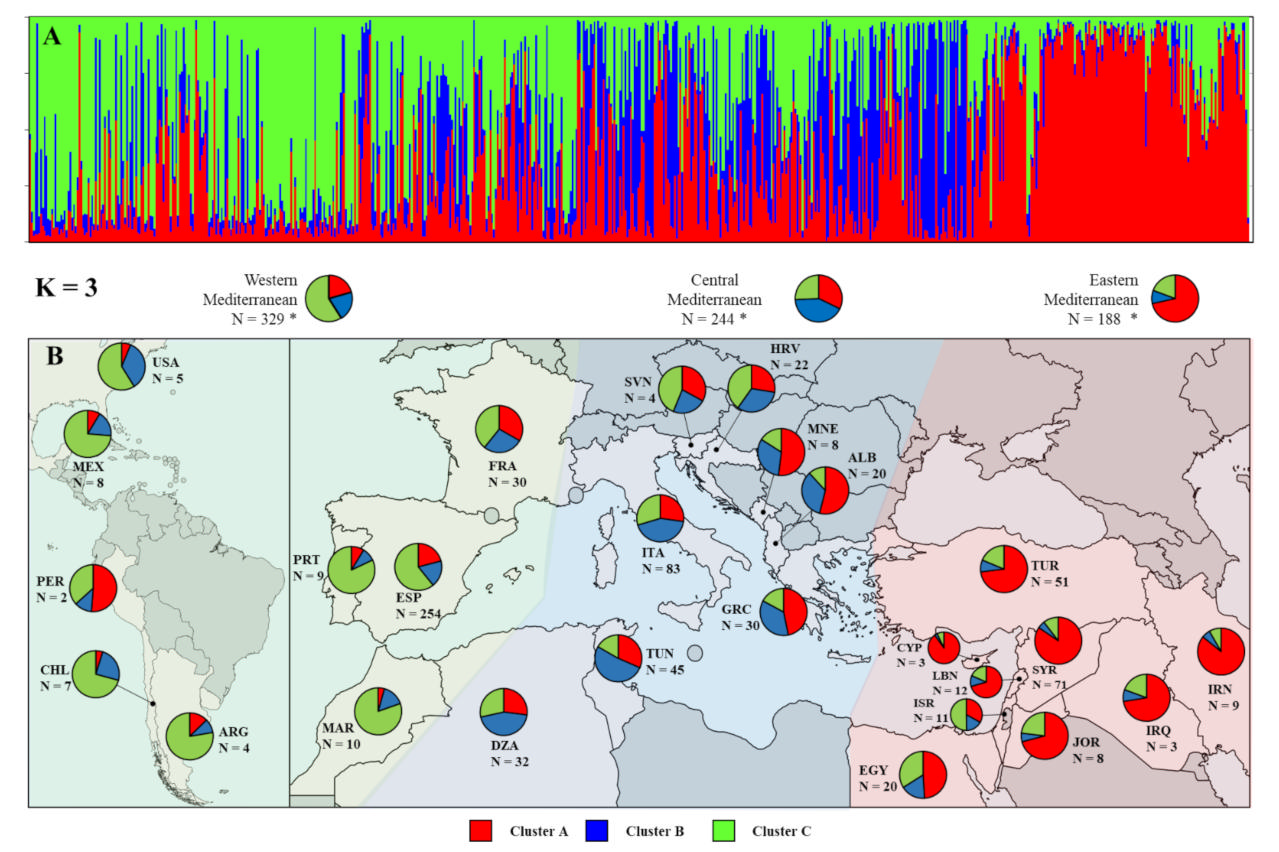
2.4. Assessment of Genetic Diversity and Relationships among Nonredundant Olive Cultivars
3. Discussion
3.1. Utility of the Set of 96 EST-SNP Markers for Olive Cultivar Identification
3.2. Assessment of Redundant Germplasm by Means of EST-SNP Markers
3.3. Discrimination of Homonymy Cases and Naming of Olive Cultivars
3.4. Implementation of a Protocol for Efficient Safeguard and Management of Olive Genetic Resources
3.5. Genetic Diversity and Relationships among Olive Cultivars
4. Materials and Methods
4.1. Plant Material
4.2. EST-SNP Genotyping of WOGBC
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartolini, G. Olive Germplam (Olea europaea L.): Cultivars, Synonyms, Cultivation Area, Collections, Descriptors; Sesto Fiorentino: Istituto per la Valorizzazione del Legno e delle Specie Arboree (IVALSA), Tree and Timber Institute: Trento, Italy, 2008. [Google Scholar]
- Belaj, A.; Gurbuz Veral, M.; Sikaoui, H.; Moukhli, A.; Khadari, B.; Mariotti, R.; Baldoni, L. Olive Genetic Resources. In The Olive Tree Genome, Compendium of Plant Genomes; Rugini, E., Baldoni, L., Muleo, R., Sebastiani, L., Eds.; Compendium of Plant Genomes; Springer International Publishing: Cham, Switzerland, 2016; pp. 27–54. [Google Scholar]
- El Bakkali, A.; Essalouh, L.; Tollon, C.; Rivallan, R.; Mournet, P.; Moukhli, A.; Zaher, H.; Mekkaoui, A.; Hadidou, A.; Sikaoui, L.; et al. Characterization of Worldwide Olive Germplasm Banks of Marrakech (Morocco) and Córdoba (Spain): Towards management and use of olive germplasm in breeding programs. PLoS ONE 2019, 14, e0223716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. OLEA Databases. 2007. Available online: http://www.oleadb.it/ (accessed on 23 March 2022).
- Barranco, D.; Cimato, A.; Fiorino, P.; Rallo, L.; Touzani, A.; Castañeda, C.; Serafini, F.; Trujillo, I. World Catalogue of Olive Varieties; International Olive Council: Madrid, Spain, 2000. [Google Scholar]
- Trujillo, I.; Ojeda, M.; Urdiroz, N.; Potter, D.; Barranco, D.; Rallo, L.; Diez, C. Identification of the Worldwide Olive Germplasm Bank of Córdoba (Spain) using SSR and morphological markers. Tree Genet. Genomes 2014, 10, 141–155. [Google Scholar] [CrossRef]
- Caballero, J.M.; Del Río, C.; Barranco, D.; Trujillo, I. The olive world germplasm bank of Córdoba, Spain. Olea 2006, 25, 14–19. [Google Scholar]
- Haouane, H.; El Bakkali, A.; Moukhli, A.; Tollon, C.; Santoni, S.; Oukabli, A.; El Modafar, C.; Khadari, B. Genetic structure and core collection of the World Olive Germplasm Bank of Marrakech: Towards the optimised management and use of Mediterranean olive genetic resources. Genetica 2011, 139, 1083–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díez, M.J.; de la Rosa, L.; Martin, I.; Guasch, L.; Elena Cartea, M.; Mallor, C.; Casals, J.; Simo, J.; Rivera, A.; Anastasio, G.; et al. Plant Genebanks: Present Situation and Proposals for Their Improvement. the Case of the Spanish Network. Front. Plant Sci. 2018, 9, 1794. [Google Scholar] [CrossRef] [Green Version]
- Ninot, A.; Howad, W.; Aranzana, M.J.; Senar, R.; Romero, A.; Mariotti, R.; Baldoni, L.; Belaj, A. Survey of over 4,500 monumental olive trees preserved on-farm in the northeast Iberian Peninsula, their genotyping and characterization. Sci. Hortic. 2018, 231, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Rallo, L.; Barranco, D.; Fernández-Escobar, R. El Cultivo del Olivo; Mundi-Prensa: Madrid, Spain, 2008. [Google Scholar]
- De la Rosa, R.; James, C.M.; Tobutt, K.R. Using microsatellites for paternity testing in olive progenies. Hortscience 2004, 39, 351–354. [Google Scholar] [CrossRef] [Green Version]
- Besnard, G.; Hernández, P.; Khadari, B.; Dorado, G.; Savolainen, V. Genomic profiling of plastid DNA variation in the Mediterranean olive tree. BMC Plant Biol. 2011, 11, 80. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Ruiz, J.; Leyva-Perez, M.d.l.O.; Gomez-Lama Cabanas, C.; Barroso, J.B.; Luque, F.; Mercado-Blanco, J. The Transcriptome of Verticillium dahliae Responds Differentially Depending on the Disease Susceptibility Level of the Olive (Olea europaea L.) Cultivar. Genes 2019, 10, 251. [Google Scholar] [CrossRef] [Green Version]
- De la Rosa, R.; Talhaoui, N.; Rouis, H.; Velasco, L.; Leon, L. Fruit characteristics and fatty acid composition in advanced olive breeding selections along the ripening period. Food Res. Int. 2013, 54, 1890–1896. [Google Scholar] [CrossRef]
- Atienza, S.G.; de la Rosa, R.; Domínguez-García, M.C.; Martín, A.; Kilian, A.; Belaj, A. Use of DArT markers as a means of better management of the diversity of olive cultivars. Food Res. Int. 2013, 54, 2045–2053. [Google Scholar] [CrossRef]
- Belaj, A.; Satovic, Z.; Rallo, L.; Trujillo, I. Genetic diversity and relationships in olive (Olea europaea L.) germplasm collections as determined by randomly amplified polymorphic DNA. Theor. Appl. Genet. 2002, 105, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Belaj, A.; Dominguez-García, M.d.C.; Gustavo Atienza, S.; Martín Urdiroz, N.; De la Rosa, R.; Satovic, Z.; Martín, A.; Kilian, A.; Trujillo, I.; Valpuesta, V.; et al. Developing a core collection of olive (Olea europaea L.) based on molecular markers (DArTs, SSRs, SNPs) and agronomic traits. Tree Genet. Genomes 2012, 8, 365–378. [Google Scholar] [CrossRef]
- Baldoni, L.; Cultrera, N.G.; Mariotti, R.; Ricciolini, C.; Arcioni, S.; Vendramin, G.G.; Buonamici, A.; Porceddu, A.; Sarri, V.; Ojeda, M.A.; et al. A consensus list of microsatellite markers for olive genotyping. Mol. Breed. 2009, 24, 213–231. [Google Scholar] [CrossRef]
- Beghè, D.; Molano, J.F.G.; Fabbri, A.; Ganino, T. Olive biodiversity in Colombia. A molecular study of local germplasm. Sci. Hortic. 2015, 189, 122–131. [Google Scholar] [CrossRef]
- Kaya, H.B.; Cetin, O.; Kaya, H.; Sahin, M.; Sefer, F.; Kahraman, A.; Tanyolac, B. SNP Discovery by Illumina-Based Transcriptome Sequencing of the Olive and the Genetic Characterization of Turkish Olive Genotypes Revealed by AFLP, SSR and SNP Markers. PLoS ONE 2013, 8, e73674. [Google Scholar] [CrossRef] [Green Version]
- Biton, I.; Doron-Faigenboim, A.; Jamwal, M.; Mani, Y.; Eshed, R.; Rosen, A.; Sherman, A.; Ophir, R.; Lavee, S.; Avidan, B.; et al. Development of a large set of SNP markers for assessing phylogenetic relationships between the olive cultivars composing the Israeli olive germplasm collection. Mol. Breed. 2015, 35, 107. [Google Scholar] [CrossRef]
- D'Agostino, N.; Taranto, F.; Camposeo, S.; Mangini, G.; Fanelli, V.; Gadaleta, S.; Miazzi, M.M.; Pavan, S.; Di Rienzo, V.; Sabetta, W.; et al. GBS-derived SNP catalogue unveiled wide genetic variability and geographical relationships of Italian olive cultivars. Sci. Rep. 2018, 8, 15877. [Google Scholar] [CrossRef] [Green Version]
- Belaj, A.; de la Rosa, R.; Lorite, I.J.; Mariotti, R.; Cultrera, N.G.M.; Beuzón, C.R.; González-Plaza, J.J.; Muñoz-Mérida, A.; Trelles, O.; Baldoni, L. Usefulness of a New Large Set of High Throughput EST-SNP Markers as a Tool for Olive Germplasm Collection Management. Front. Plant Sci. 2018, 9, 1320. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Niu, E.; Shi, A.; Mou, B. Genetic Diversity Analysis of Olive Germplasm (Olea europaea L.) With Genotyping-by-Sequencing Technology. Front. Genet. 2019, 10, 755. [Google Scholar] [CrossRef] [Green Version]
- Islam, A.S.M.F.; Sanders, D.; Mishra, A.K.; Joshi, V. Genetic Diversity and Population Structure Analysis of the USDA Olive Germplasm Using Genotyping-By-Sequencing (GBS). Genes 2021, 12, 2007. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Merida, A.; Jose Gonzalez-Plaza, J.; Canada, A.; Maria Blanco, A.; del Carmen Garcia-Lopez, M.; Manuel Rodriguez, J.; Pedrola, L.; Dolores Sicardo, M.; Luisa Hernandez, M.; De la Rosa, R.; et al. De Novo Assembly and Functional Annotation of the Olive (Olea europaea) Transcriptome. DNA Res. 2013, 20, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Belaj, A.; Satovic, Z.; Rallo, L.; Trujillo, I. Optimal use of RAPD markers for identifying varieties in olive (Olea europaea L.) germplasm collections. J. Am. Soc. Hortic. Sci. 2004, 129, 266–270. [Google Scholar] [CrossRef] [Green Version]
- Muzzalupo, I.; Vendramin, G.G.; Chiappetta, A. Genetic Biodiversity of Italian Olives (Olea europaea) Germplasm Analyzed by SSR Markers. Sci. World J. 2014, 2014, 296590. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.; Mariotti, R.; Regni, L.; Nasini, L.; Bufacchi, M.; Pandolfi, S.; Baldoni, L.; Proietti, P. The First Molecular Identification of an Olive Collection Applying Standard Simple Sequence Repeats and Novel Expressed Sequence Tag Markers. Front. Plant Sci. 2017, 8, 1283. [Google Scholar] [CrossRef]
- Belaj, A.; de la Rosa, R.; Leon, L.; Gabaldon-Leal, C.; Santos, C.; Porras, R.; de La Cruz-Blanco, M.; Lorite, I.J. Phenological diversity in a World Olive Germplasm Bank: Potential use for breeding programs and climate change studies. Span. J. Agric. Res. 2020, 18, e0701. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Galvez, F.J.; Perez-Mohedano, D.; de la Rosa-Navarro, R.; Belaj, A. High-throughput analysis of the canopy traits in the worldwide olive germplasm bank of Cordoba using very high-resolution imagery acquired from unmanned aerial vehicle (UAV). Sci. Hortic. 2021, 278, 109851. [Google Scholar] [CrossRef]
- Debbabi, O.S.; Miazzi, M.M.; Elloumi, O.; Fendri, M.F.; Ben Amar, F.; Savoia, M.; Sion, S.; Souabni, H.; Mnasri, S.R.; Ben Abdelaali, S.; et al. Recovery, Assessment, and Molecular Characterization of Minor Olive Genotypes in Tunisia. Plants 2020, 9, 382. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Sanz, P.; Lombardo, L.; Lorenzi, S.; Michelotti, F.; Grando, M.S. Genetic Resources of Olea europaea L. in the Garda Trentino Olive Groves Revealed by Ancient Trees Genotyping and Parentage Analysis of Drupe Embryos. Genes 2020, 11, 1171. [Google Scholar] [CrossRef]
- Belaj, A.; Muñoz-Diez, C.; Baldoni, L.; Satovic, Z.; Barranco, D. Genetic diversity and relationships of wild and cultivated olives at regional level in Spain. Sci. Hortic. 2010, 124, 323–330. [Google Scholar] [CrossRef]
- di Rienzo, V.; Sion, S.; Taranto, F.; D’Agostino, N.; Montemurrola, C.; Fanelli, V.; Sabetta, W.; Boucheffa, S.; Tamendjari, A.; Pasqualone, A.; et al. Genetic flow among olive populations within the Mediterranean basin. PeerJ 2018, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díez, C.M.; Trujillo, I.; Barrio, E.; Belaj, A.; Barranco, D.; Rallo, L. Centennial olive trees as a reservoir of genetic diversity. Ann. Bot. 2011, 108, 797–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barazani, O.; Westberg, E.; Hanin, N.; Dag, A.; Kerem, Z.; Tugendhaft, Y.; Hmidat, M.; Hijawi, T.; Kadereit, J.W. A comparative analysis of genetic variation in rootstocks and scions of old olive trees—A window into the history of olive cultivation practices and past genetic variation. BMC Plant Biol. 2014, 14, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besnard, G.; Baradat, P.; Bervillé, A. Genetic relationships in the olive (Olea europaea L.) reflect multilocal selection of cultivars. Theor. Appl. Genet. 2001, 102, 251–258. [Google Scholar] [CrossRef]
- Besnard, G.; Khadari, B.; Navascués, M.; Fernández-Mazuecos, M.; El Bakkali, A.; Arrigo, N.; Baali-Cherif, D.; de Caraffa, V.B.-B.; Santoni, S.; Vargas, P.; et al. The complex history of the olive tree: From Late Quaternary diversification of Mediterranean lineages to primary domestication in the northern Levant. Proc. R. Soc. B-Biol. Sci. 2013, 280. [Google Scholar] [CrossRef] [Green Version]
- Trigui, A.; Msallem, M.; Yengui, A.; Belguith, H.; Khecherem, J.; Meliène, A.; Malek, S.; Bousselmi, A.; Samet, A.; Trabelsi, E.B. Oliviers de Tunisie: Catalogue des Variétés Authochtones & Types Locaux: Identification; Volume Variétale and Charactérisation Morpho-pomologique des Ressources Génétiques Oléicoles de Tunisie; Institut de l'Oliviere, Ministère de l'Agriculture IRESA: Sfax, Republique Tunisienne, 2002; Volume 1. [Google Scholar]
- Barranco, D.; Trujillo, I.; Rallo, L. Elaiografía Hispánica. In Variedades de Olivo en España; Rallo, L., Barranco, D., Caballero, J.M., Del Río, C., Martín, A., Tous, J., Trujillo, I., Eds.; Junta de Andalucía. Ministerio de Agricultura, Pesca y Alimentación. Ediciones Mundi-Prensa: Madrid, Spain, 2005; pp. 80–231. [Google Scholar]
- Mendil, M.; Sebai, A. Catalogue des Variétés Algériennes de l'Olivier. Ministère de l'Agriculture et du Développement Rural; Institut Technique de l'Arboriculture Fruitiére et de la Vigne: Tessala El Merdja, Algeria, 2006; p. 98. [Google Scholar]
- Kostelenos, G.D. Elements of Olive Culture. History, Description, and Geographical Distribution of Olive Varieties in Greece; Embryo Publications: Athens, Greece, 2011. (In Greek) [Google Scholar]
- Muzzalupo, I. Olive Germplasm—The Olive Cultivation, Table Olive and Olive Oil Industry in Italy; BoD–Books on Demand: Norderstedt, Germany, 2012. [Google Scholar]
- Kaya, H.; Sefer, F.; Mete, N.; Çetin, Ö.; Hakan, M.; Sahin, M.; Güloglu, U.; Uluçay, N.; Gürbüz Veral, M.; Savran, M.K. Turkish Olives Variety Catalogue (In Turkish); Olive Research Institute: Bornova, Izmir, Turkey, 2015. [Google Scholar]
- Hosseini-Mazinani, M.; Mariotti, R.; Torkzaban, B.; Sheikh-Hassani, M.; Ataei, S.; Cultrera, N.G.M.; Pandolfi, S.; Baldoni, L. High Genetic Diversity Detected in Olives beyond the Boundaries of the Mediterranean Sea. PLoS ONE 2014, 9, e93146. [Google Scholar] [CrossRef] [Green Version]
- Lazović, B.; Adakalić, M.; Pucci, C.; Perović, T.; Bandelj, D.; Belaj, A.; Mariotti, R.; Baldoni, L. Characterizing ancient and local olive germplasm from Montenegro. Sci. Hortic. 2016, 209, 117–123. [Google Scholar] [CrossRef]
- Kaniewski, D.; van Campo, E.; Boiy, T.; Terral, J.-F.; Khadari, B.; Besnard, G. Primary domestication and early uses of the emblematic olive tree: Palaeobotanical, historical and molecular evidence from the Middle East. Biol. Rev. Camb. Philos. Soc. 2012, 87, 885–899. [Google Scholar] [CrossRef] [Green Version]
- Zohary, D.; Spiegelroy, P. Beginnings of fruit growing in old world. Science 1975, 187, 319–327. [Google Scholar] [CrossRef]
- Weiss, E. "Beginnings of Fruit Growing in the Old World"—Two generations later. Isr. J. Plant Sci. 2015, 62, 75–85. [Google Scholar] [CrossRef]
- El Ghalayini, Y. Lenguaje Figurado y Cultural: Botanismos Metafóricos en el Léxico y la Fraseo-Paremiología en Español y árabe: El Olivo. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 2012. [Google Scholar]
- Besnard, G.; Terral, J.-F.; Cornille, A. On the origins and domestication of the olive: A review and perspectives. Ann. Bot. 2018, 121, 385–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Ruiz, J.; Ramírez-Tejero, J.A.; Fernández-Pozo, N.; Leyva-Pérez, M.O.; Yan, H.; Rosa, R.; Belaj, A.; Montes, E.; Rodríguez-Ariza, M.O.; Navarro, F.; et al. Transposon activation is a major driver in the genome evolution of cultivated olive trees (Olea europaea L.). Plant Genome 2020, 13, e20010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galili, E.; Langgut, D.; Terral, J.F.; Barazani, O.; Dag, A.; Kolska Horwitz, L.; Ogloblin Ramirez, I.; Rosen, B.; Weinstein-Evron, M.; Chaim, S.; et al. Early production of table olives at a mid-7th millennium BP submerged site off the Carmel coast (Israel). Sci. Rep. 2021, 11, 2218. [Google Scholar] [CrossRef] [PubMed]
- Emanuelli, F.; Lorenzi, S.; Grzeskowiak, L.; Catalano, V.; Stefanini, M.; Troggio, M.; Myles, S.; Martinez-Zapater, J.M.; Zyprian, E.; Moreira, F.M.; et al. Genetic diversity and population structure assessed by SSR and SNP markers in a large germplasm collection of grape. BMC Plant Biol. 2013, 13, 39. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Vega, F.E.; Tan, H.; Lluch, A.E.R.; Meinhardt, L.W.; Fang, W.; Mischke, S.; Irish, B.; Zhang, D. Developing Single Nucleotide Polymorphism (SNP) Markers for the Identification of Coffee Germplasm. Trop. Plant Biol. 2016, 9, 82–95. [Google Scholar] [CrossRef]
- Guichoux, E.; Lagache, L.; Wagner, S.; Chaumeil, P.; Leger, P.; Lepais, O.; Lepoittevin, C.; Malausa, T.; Revardel, E.; Salin, F.; et al. Current trends in microsatellite genotyping. Mol. Ecol. Resour. 2011, 11, 591–611. [Google Scholar] [CrossRef]
- Cabezas, J.A.; Ibanez, J.; Lijavetzky, D.; Velez, D.; Bravo, G.; Rodriguez, V.; Carreno, I.; Jermakow, A.M.; Carreno, J.; Ruiz-Garcia, L.; et al. A 48 SNP set for grapevine cultivar identification. BMC Plant Biol. 2011, 11, 153. [Google Scholar] [CrossRef] [Green Version]
- Mihaljevic, M.Z.; Maletic, E.; Preiner, D.; Zdunic, G.; Bubola, M.; Zyprian, E.; Pejic, I. Genetic Diversity, Population Structure, and Parentage Analysis of Croatian Grapevine Germplasm. Genes 2020, 11, 737. [Google Scholar] [CrossRef]
- Singh, N.; Wu, S.; Raupp, W.J.; Sehgal, S.; Arora, S.; Tiwari, V.; Vikram, P.; Singh, S.; Chhuneja, P.; Gill, B.S.; et al. Efficient curation of genebanks using next generation sequencing reveals substantial duplication of germplasm accessions. Sci. Rep. 2019, 9, 650. [Google Scholar] [CrossRef] [Green Version]
- León, L.; de la Rosa, R.; Arriaza, M. Prioritization of olive breeding objectives in Spain: Analysis of a producers and researchers survey. Span. J. Agric. Res. 2021, 19, e0701. [Google Scholar] [CrossRef]
- Mariotti, R.; Belaj, A.; de La Rosa, R.; Leon, L.; Brizioli, F.; Baldoni, L.; Mousavi, S. EST-SNP Study of Olea europaea L. Uncovers Functional Polymorphisms between Cultivated and Wild Olives. Genes 2020, 11, 916. [Google Scholar] [CrossRef] [PubMed]
- Khadari, B.; El Bakkali, A. Primary Selection and Secondary Diversification: Two Key Processes in the History of Olive Domestication. Int. J. Agron. 2018, 2018, 5607903. [Google Scholar] [CrossRef] [Green Version]
- Julca, I.; Marcet-Houben, M.; Cruz, F.; Gómez-Garrido, J.; Gaut, B.S.; Díez, C.M.; Gut, I.G.; Alioto, T.S.; Vargas, P.; Gabaldón, T. Genomic evidence for recurrent genetic admixture during domestication mediterranean olive trees (Olea europaea L.). BMC Biol. 2020, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Hannachi, H.; Breton, C.; Msallem, M.; El Hadj, S.B.; El Gazzah, M.; Bervillé, A. Differences between native and introduced olive cultivars as revealed by morphology of drupes, oil composition and SSR polymorphisms: A case study in Tunisia. Sci. Hortic. 2008, 116, 280–290. [Google Scholar] [CrossRef]
- Atrouz, K.; Bousba, R.; Marra, F.P.; Marchese, A.; Conforti, F.L.; Perrone, B.; Harkat, H.; Salimonti, A.; Zelasco, S. Algerian Olive Germplasm and Its Relationships with the Central-Western Mediterranean Varieties Contributes to Clarify Cultivated Olive Diversification. Plants 2021, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Terral, J.-F.; Bonhomme, V.; Pagnoux, C.; Ivorra, S.; Newton, C.; Paradis, L.; Ater, M.; Kassout, J.; Limier, B.; Bouby, L.; et al. The Shape Diversity of Olive Stones Resulting from Domestication and Diversification Unveils Traits of the Oldest Known 6500-Years-Old Table Olives from Hishuley Carmel Site (Israel). Agronomy 2021, 11, 2187. [Google Scholar] [CrossRef]
- El Bakkali, A.; Haouane, H.; Moukhli, A.; Costes, E.; van Damme, P.; Khadari, B. Construction of Core Collections Suitable for Association Mapping to Optimize Use of Mediterranean Olive (Olea europaea L.) Genetic Resources. PLoS ONE 2013, 8, e61265. [Google Scholar] [CrossRef] [Green Version]
- De la Rosa, R.; James, C.M.; Tobutt, K.R. Isolation and characterization of polymorphic microsatellites in olive (Olea europaea L.) and their transferability to other genera in the Oleaceae. Mol. Ecol. Notes 2002, 2, 265–267. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [Green Version]
- Marshall, T.C.; Slate, J.; Kruuk, L.E.B.; Pemberton, J.M. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 1998, 7, 639–655. [Google Scholar] [CrossRef] [Green Version]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program cervus accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes—Application to human mitochondrial-DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [Green Version]
- Earl, D.A.; vonHoldt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Kopelman, N.M.; Mayzel, J.; Jakobsson, M.; Rosenberg, N.A.; Mayrose, I. Clumpak: A program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 2015, 15, 1179–1191. [Google Scholar] [CrossRef] [Green Version]
- Khadari, B.; El Bakkali, A.; Essalouh, L.; Tollon, C.; Pinatel, C.; Besnard, G. Cultivated olive diversification at local and regional scales: Evidence from the genetic characterization of French genetic resources. Front. Plant Sci. 2019, 10, 1593. [Google Scholar] [CrossRef] [Green Version]
- Trujillo, T.; Rallo, L.; Arus, P. Identifying olive cultivars by isozyme analysis. J. Am. Soc. Hortic. Sci. 1995, 120, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Barranco, D.; Trujillo, I.; Rallo, P. Are ‘Oblonga’ and ‘Frantoio’ olives the same cultivar? Hortscience 2000, 35, 1323–1325. [Google Scholar] [CrossRef]
- Besnard, G.; Breton, C.; Baradat, P.; Khadari, B.; Berville, A. Cultivar identification in olive based on RAPD markers. J. Am. Soc. Hortic. Sci. 2001, 126, 668–675. [Google Scholar] [CrossRef] [Green Version]
- Erre, P.; Chessa, I.; Muñoz-Diez, C.; Belaj, A.; Rallo, L.; Trujillo, I. Genetic diversity and relationships between wild and cultivated olives (Olea europaea L.) in Sardinia as assessed by SSR markers. Genet. Resour. Crop Evol. 2010, 57, 41–54. [Google Scholar] [CrossRef]
- Amokrane, K. Identificación del Material de Recepción del Banco de Germoplasma de Olivo de Córdoba Mediante Marcadores SSRs. Ph.D. Thesis, Universidad de Córdoba, Córdoba, Spain, 2010. [Google Scholar]
- Bracci, T.; Sebastiani, L.; Busconi, M.; Fogher, C.; Belaj, A.; Trujillo, I. SSR markers reveal the uniqueness of olive cultivars from the Italian region of Liguria. Sci. Hortic. 2009, 122, 209–215. [Google Scholar] [CrossRef]
- Koehmstedt, A.M.; Aradhya, M.K.; Soleri, D.; Smith, J.L.; Polito, V.S. Molecular characterization of genetic diversity, structure, and differentiation in the olive (Olea europaea L.) germplasm collection of the United States Department of Agriculture. Genet. Resour. Crop Evol. 2011, 58, 519–531. [Google Scholar] [CrossRef] [Green Version]
- Angiolillo, A.; Mencuccini, M.; Baldoni, L. Olive genetic diversity assessed using amplified fragment length polymorphisms. Theor. Appl. Genet. 1999, 98, 411–421. [Google Scholar] [CrossRef]
- Marchionni, C.; Baldoni, L.; Angiolillo, A.; Pannelli, G.; Panara, F. Caratterizzazione genetica delle più importanti cultivar di olivo dell'Umbria. In Proceedings of the V Convegno Nazionale sulla Biodiversità, Caserta, Italy, 9–10 September 1999; pp. 243–247. [Google Scholar]
- Baldoni, L.; Pellegrini, M.; Mencuccini, M.; Angiolillo, A.; Mulas, M. Genetic relationships amon cultivated and wild olives revealed by AFLP markers. Acta Hortic. 2000, 521, 275–284. [Google Scholar] [CrossRef]
- Beghè, D.; Ferrarini, A.; Ganino, T.; Fabbri, A. Molecular characterization and identification of a group of local Olea europaea L. varieties. Tree Genet. Genomes. 2011, 7, 1185–1198. [Google Scholar] [CrossRef]
- Muzzalupo, I.; Stefanizzi, F.; Perri, E. Evaluation of olives cultivated in southern Italy by simple sequence repeat markers. Hortscience 2009, 44, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Haddad, B.; Gristina, A.S.; Mercati, F.; Saadi, A.E.; Aiter, N.; Martorana, A.; Sharaf, A.; Carimi, F. Molecular analysis of the official Algerian olive collection highlighted a hotspot of biodiversity in the central mediterranean basin. Genes 2020, 11, 303. [Google Scholar] [CrossRef] [Green Version]
- Fendri, M.; Trujillo, I.; Trigui, A.; Rodriguez-Garcia, M.I.; Ramirez, J.D.A. Simple sequence repeat identification and endocarp characterization of olive tree accessions in a Tunisian germplasm collection. Hortscience 2010, 45, 1429–1436. [Google Scholar] [CrossRef] [Green Version]
- Ben Mohamed, M.; Zelasco, S.; Ben Ali, S.; Guasmi, F.; Triki, T.; Conforti, F.L.; Naziha, G.K. Exploring olive trees genetic variability in the South East of Tunisia. Genet. and Mol. Res. 2017, 16, gmr16039850. [Google Scholar] [CrossRef]
- Viñuales-Andreu, J. Variedades de olivo del Somontano; Área de Desarrollo de la Diputación de Huesca e Instituto de Estudios Altoaragoneses: Huesca, Spain, 2007; pp. 1–162. [Google Scholar]
- Emmanouilidou, M.G.; Kyriacou, M.C.; Trujillo, I. Characterization and identification of indigenous olive germplasm from Cyprus using morphological and simple sequence repeat markers. Hortscience 2018, 53, 1306–1313. [Google Scholar] [CrossRef] [Green Version]
- Chalak, L.; Haouane, H.; Essalouh, L.; Santoni, S.; Besnard, G.; Khadari, B. Extent of the genetic diversity in Lebanese olive (Olea europaea L.) trees: A mixture of an ancient germplasm with recently introduced varieties. Genet. Resour. Crop Evol. 2015, 62, 621–633. [Google Scholar] [CrossRef]
- Rahmani, S.M.; Debbabi, O.S.; Ben Naceur, M. Molecular markers: An important tool to analyze the genetic diversity of local Tunisian olive varieties. EuroMediterr. J. Environ. Integr. 2019, 4, 29. [Google Scholar] [CrossRef]
- Isik, N.; Doganlar, S.; Frary, A. Genetic diversity of Turkish olive varieties assessed by simple sequence repeat and sequence-related amplified polymorphism markers. Crop Sci. 2011, 51, 1646–1654. [Google Scholar] [CrossRef] [Green Version]
- Anestiadou, K.; Nikoloudakis, N.; Hagidimitriou, M.; Katsiotis, A. Monumental olive trees of Cyprus contributed to the establishment of the contemporary olive germplasm. PLoS ONE 2017, 12, e0187697. [Google Scholar] [CrossRef] [Green Version]
- Íñiguez, A.; Paz, S.; Illa, F.J. Variedades de Olivo Cultivadas en la Comunidad Valenciana; Conselleria d'Agricultura, Peixca i Alimentació, Generalitat Valenciana: Valencia, Spain, 2001; pp. 1–273. [Google Scholar]
- Belaj, A.; Caballero, J.M.; Barranco, D.; Rallo, L.; Trujillo, I. Genetic characterization and identification of new accessions from Syria in an olive germplasm bank by means of RAPD markers. Euphytica 2003, 134, 261–268. [Google Scholar] [CrossRef]
- Lazović, B.; Klepo, T.; Adakalić, M.; Šatović, Z.; Arbeiter, A.B.; Hladnik, M.; Strikić, F.; Liber, Z.; Bandelj, D. Intra-varietal variability and genetic relationships among the homonymic East Adriatic olive (Olea europaea L.) varieties. Sci. Hortic. 2018, 236, 175–185. [Google Scholar] [CrossRef]
- Susamci, E.; Romero, C.; Tuncay, Ö; Brenes, M. An explanation for the natural de-bittering of Hurma olives during ripening on the tree. Grasas y Aceites 2017, 68, e182. [Google Scholar] [CrossRef] [Green Version]
- Belaj, A.; Satovic, Z.; Ismaili, H.; Panajoti, D.; Rallo, L.; Trujillo, I. RAPD genetic diversity of Albanian olive germplasm and its relationships with other Mediterranean countries. Euphytica 2003, 130, 387–395. [Google Scholar] [CrossRef]
- Muzzalupo, I.; Muto, A.; Badolati, G.; Veizi, A.; Chiappetta, A. Genotyping of Albania olive (Olea europaea) germplasm by SSR molecular marker. Emir. J. Food Agric. 2018, 30, 573–580. [Google Scholar] [CrossRef]
- Tous, J.; Romero-Aroca, A. Variedades del Olivo; Fundación "La Caixa"-AEDOS S.A.: Barcelona, Spain, 1993; pp. 1–172. [Google Scholar]
- Noormohammadi, Z.; Hosseini-Mazinani, M.; Trujillo, I.; Ratio, L.; Belaj, A.; Sadeghizadeh, M. Identification and classification of main Iranian olive cultivars using Microsatellite markers. Hortscience 2007, 42, 1545–1550. [Google Scholar] [CrossRef] [Green Version]

| Countries | Field Accessions | New Accessions at Different Propagation Facilities | No. of Accessions | Total No. Trees/Plants | No. of Different Genotypes/Country * |
|---|---|---|---|---|---|
| Albania | 20 | 5 | 25 | 51 | 20 |
| Algeria | 51 | 51 | 112 | 32 | |
| Argentina | 6 | 6 | 13 | 4 | |
| Bosnia and Herzegovina | 0 | 2 | 2 | 2 | 1 |
| Chile | 13 | 13 | 35 | 7 | |
| Croatia | 24 | 3 | 27 | 71 | 22 |
| Cyprus | 11 | 1 | 12 | 22 | 3 |
| Egypt | 26 | 3 | 29 | 66 | 20 |
| France | 14 | 18 | 32 | 92 | 30 |
| Greece | 27 | 13 | 40 | 105 | 30 |
| Iran | 10 | 10 | 28 | 9 | |
| Iraq | 0 | 3 | 3 | 8 | 3 |
| Israel | 14 | 14 | 37 | 11 | |
| Italy | 170 | 1 | 171 | 408 | 83 |
| Jordan | 5 | 10 | 15 | 32 | 8 |
| Lebanon | 18 | 22 | 40 | 119 | 12 |
| Mexico | 8 | 1 | 9 | 20 | 8 |
| Montenegro | 8 | 1 | 9 | 22 | 8 |
| Morocco | 22 | 22 | 49 | 10 | |
| Pakistan | 1 | 1 | 2 | 1 | |
| Peru | 1 | 2 | 3 | 9 | 2 |
| Portugal | 11 | 11 | 22 | 9 | |
| Slovenia | 0 | 4 | 4 | 6 | 4 |
| Spain | 330 | 71 | 401 | 991 | 254 |
| Syria | 81 | 37 | 118 | 301 | 71 |
| Tunisia | 114 | 114 | 297 | 45 | |
| Turkey | 18 | 66 | 84 | 169 | 51 |
| Uruguay | 1 | 1 | 2 | 5 | 1 |
| USA | 5 | 5 | 11 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belaj, A.; Ninot, A.; Gómez-Gálvez, F.J.; El Riachy, M.; Gurbuz-Veral, M.; Torres, M.; Lazaj, A.; Klepo, T.; Paz, S.; Ugarte, J.; et al. Utility of EST-SNP Markers for Improving Management and Use of Olive Genetic Resources: A Case Study at the Worldwide Olive Germplasm Bank of Córdoba. Plants 2022, 11, 921. https://doi.org/10.3390/plants11070921
Belaj A, Ninot A, Gómez-Gálvez FJ, El Riachy M, Gurbuz-Veral M, Torres M, Lazaj A, Klepo T, Paz S, Ugarte J, et al. Utility of EST-SNP Markers for Improving Management and Use of Olive Genetic Resources: A Case Study at the Worldwide Olive Germplasm Bank of Córdoba. Plants. 2022; 11(7):921. https://doi.org/10.3390/plants11070921
Chicago/Turabian StyleBelaj, Angjelina, Antònia Ninot, Francisco J. Gómez-Gálvez, Milad El Riachy, Melek Gurbuz-Veral, Mariela Torres, Adhurim Lazaj, Tatjana Klepo, Sergio Paz, Javier Ugarte, and et al. 2022. "Utility of EST-SNP Markers for Improving Management and Use of Olive Genetic Resources: A Case Study at the Worldwide Olive Germplasm Bank of Córdoba" Plants 11, no. 7: 921. https://doi.org/10.3390/plants11070921
APA StyleBelaj, A., Ninot, A., Gómez-Gálvez, F. J., El Riachy, M., Gurbuz-Veral, M., Torres, M., Lazaj, A., Klepo, T., Paz, S., Ugarte, J., Baldoni, L., Lorite, I. J., Šatović, Z., & de la Rosa, R. (2022). Utility of EST-SNP Markers for Improving Management and Use of Olive Genetic Resources: A Case Study at the Worldwide Olive Germplasm Bank of Córdoba. Plants, 11(7), 921. https://doi.org/10.3390/plants11070921









