RNA-Seq Reveals Waterlogging-Triggered Root Plasticity in Mungbean Associated with Ethylene and Jasmonic Acid Signal Integrators for Root Regeneration
Abstract
1. Introduction
2. Results
2.1. H150 Waterlogging Tolerance Associates with Modification of Root Architecture
2.2. Transcriptome Reconfiguration in Mungbean Subjected to Waterlogging
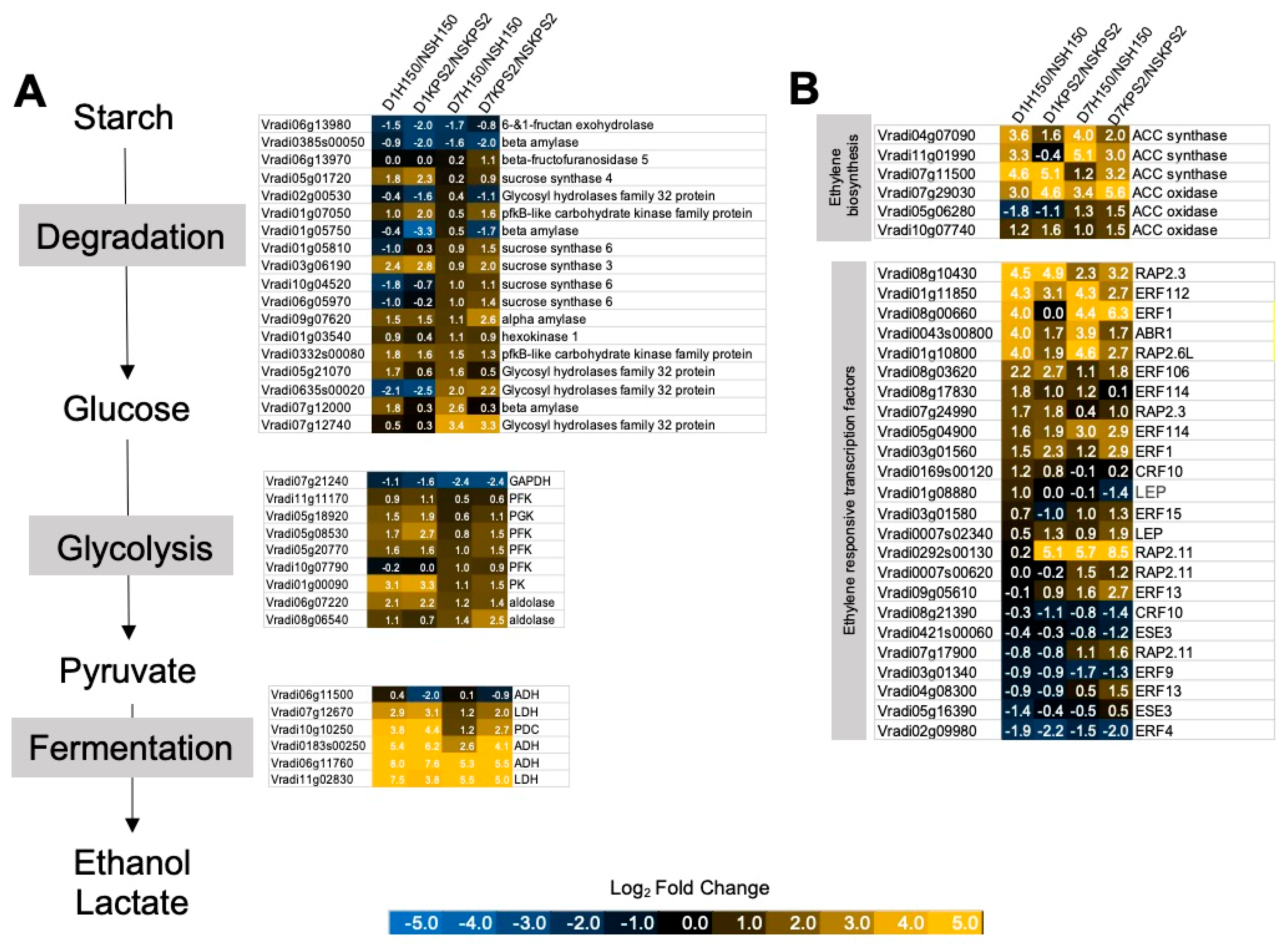
2.3. Differential Regulation of Gene Associated with Anaerobic Fermentation and Glycolysis
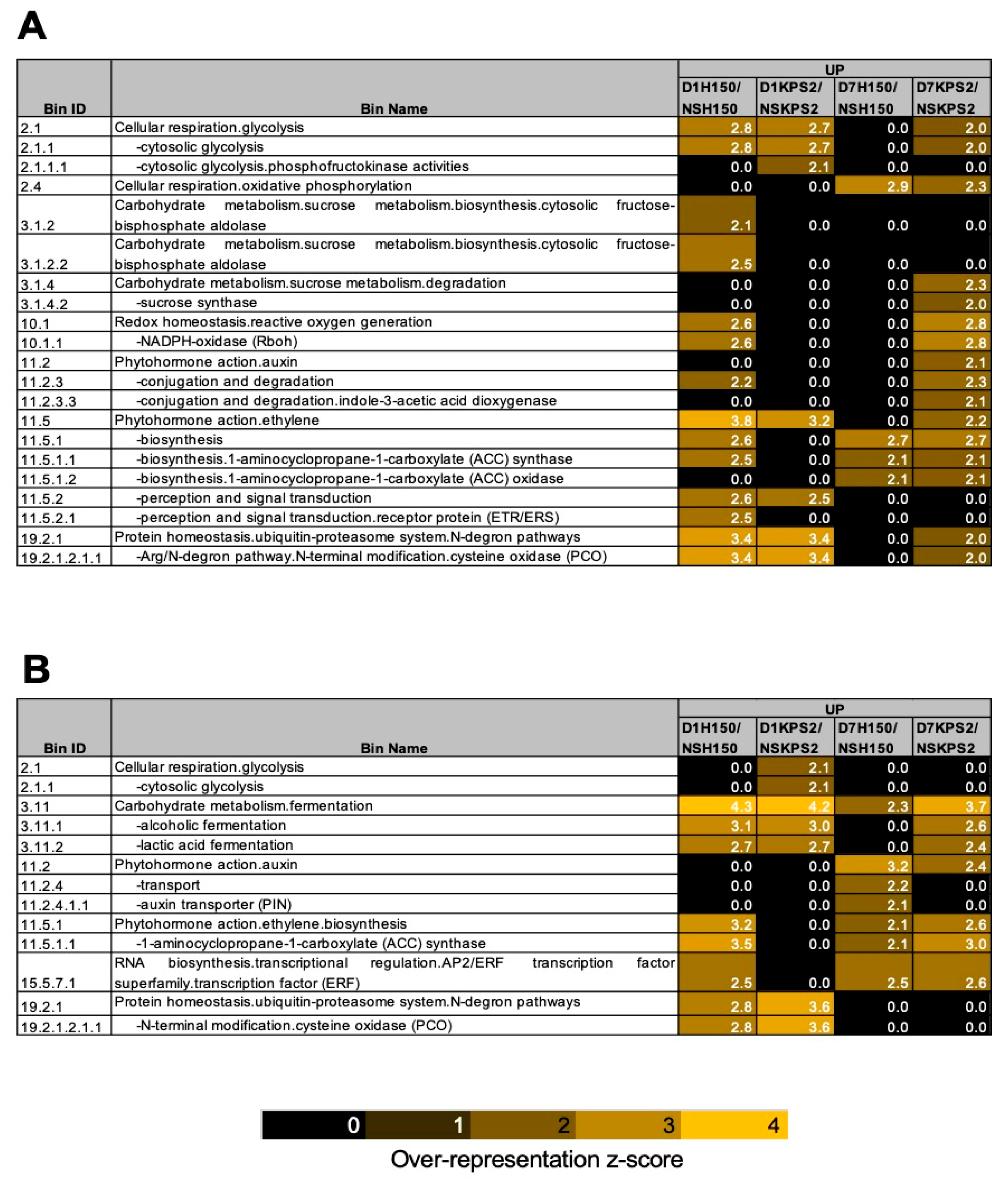
2.4. Differential Regulation of Gene Associated with Ethylene Production and Response
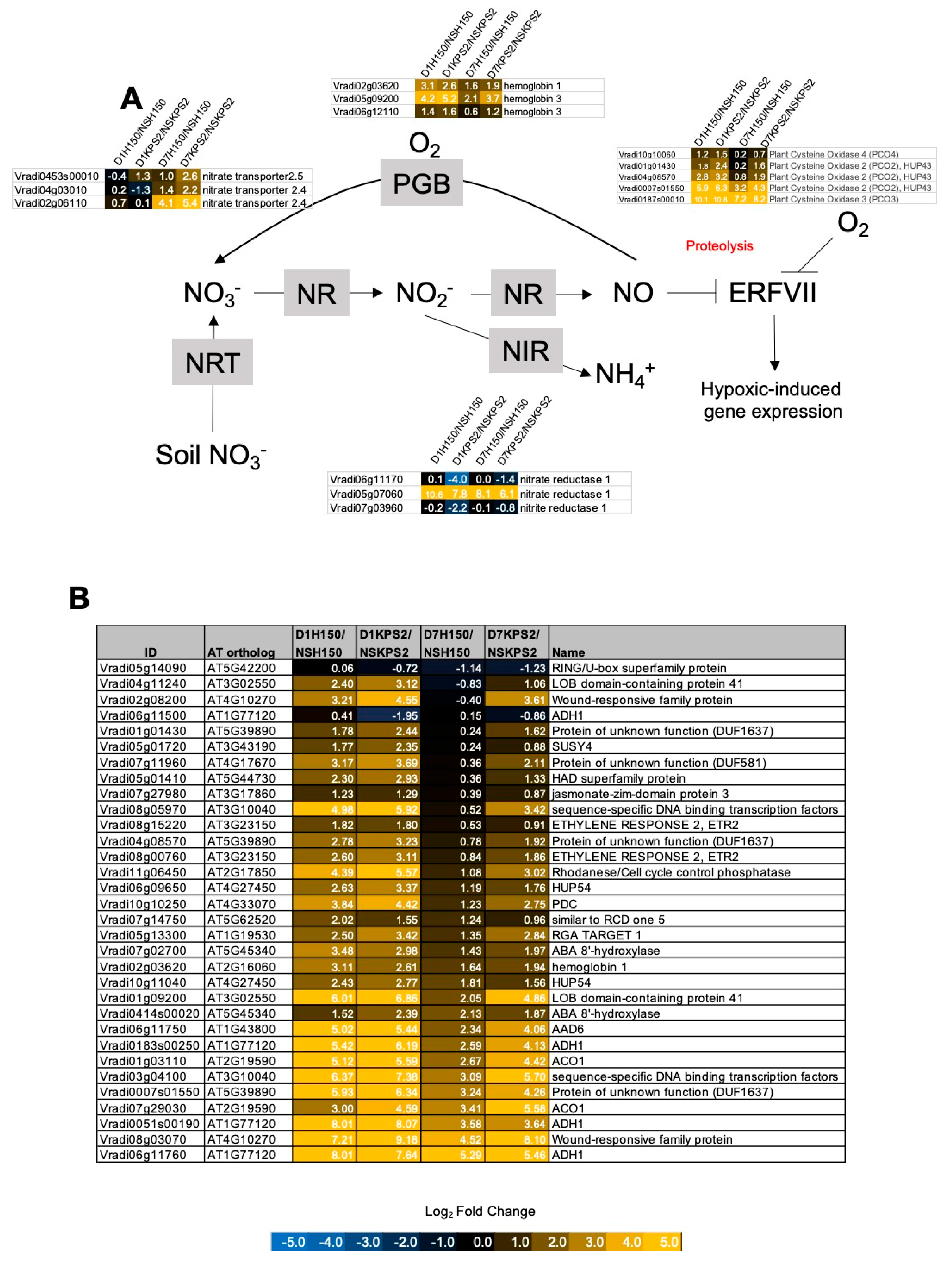
2.5. Differential Regulation of Genes Associated with NO Production, Primary Nitrogen Metabolism, and Hypoxia Sensing and Response
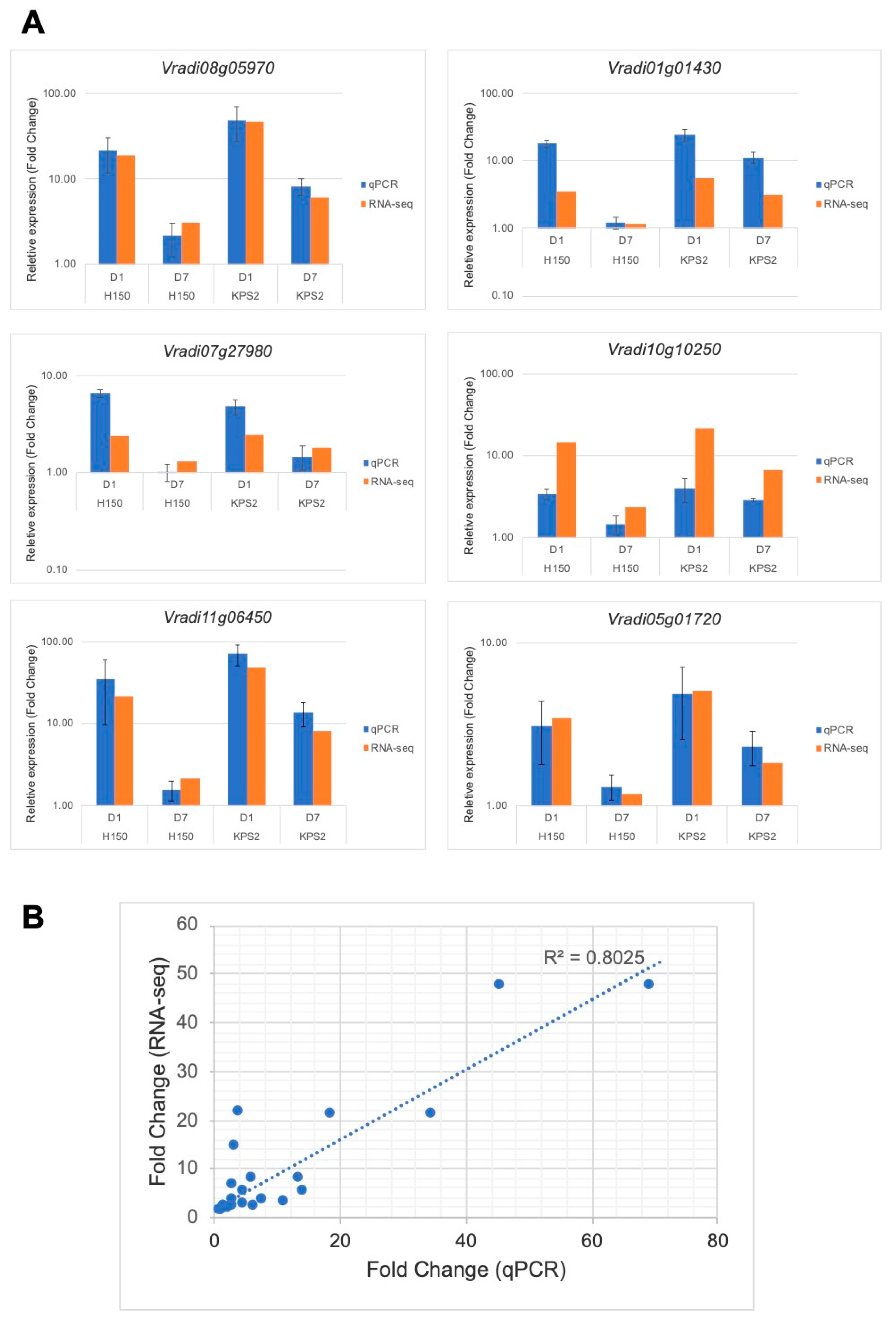
2.6. Validation of RNA-Seq Results
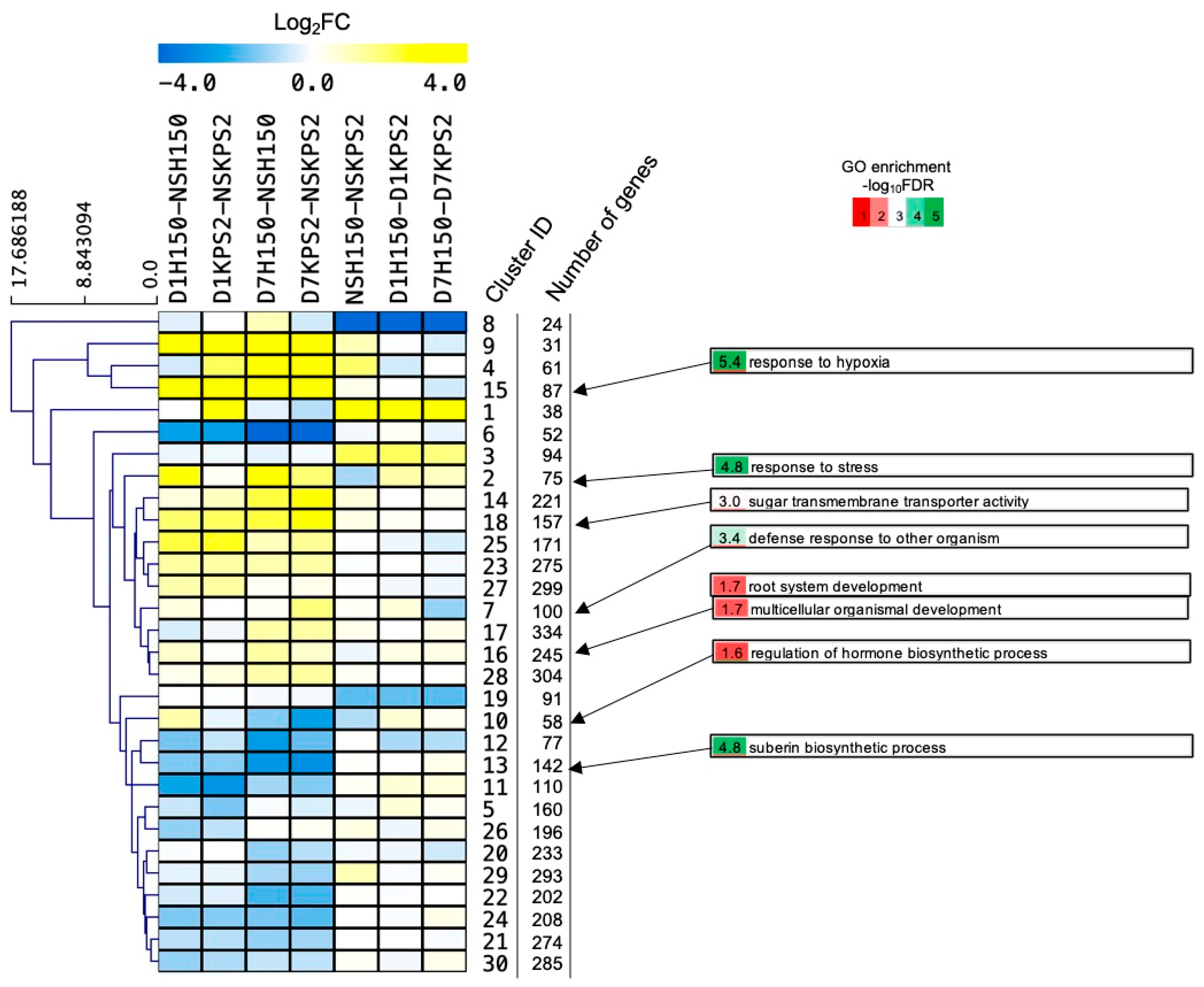
2.7. Cluster Analysis Reveals Early Induction of Stress Responsive Genes and Late Induction of Developmental Genes in the Tolerant Variety
3. Discussion
4. Materials and Methods
4.1. Plant Material and Stress Treatment
4.2. Analysis of Root Phenotypic Plasticity
4.3. RNA Extraction, Library Preparation, and Sequencing
4.4. Differential Gene Expression Analysis
4.5. Ortholog Identification
4.6. Gene Ontology Enrichment Analysis
4.7. Overrepresentation Analysis
4.8. Quantitative-Realtime PCR
4.9. Identification of Co-Regulated Genes by Clustering
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sasidharan, R.; Bailey-Serres, J.; Ashikari, M.; Atwell, B.J.; Colmer, T.D.; Fagerstedt, K.; Fukao, T.; Geigenberger, P.; Hebelstrup, K.H.; Hill, R.D.; et al. Community recommendations on terminology and procedures used in flooding and low oxygen stress research. New Phytol. 2017, 214, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Barrera-Figueroa, B.E.; Juntawong, P.; Peña-Castro, J.M. Submergence and Waterlogging Stress in Plants: A Review Highlighting Research Opportunities and Understudied Aspects. Front. Plant Sci. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, W. Aeration in Higher Plants. Adv. Bot. Res. 1980, 7, 225–332. [Google Scholar] [CrossRef]
- Juntawong, P.; Sirikhachornkit, A.; Pimjan, R.; Sonthirod, C.; Sangsrakru, D.; Yoocha, T.; Tangphatsornruang, S.; Srinives, P. Elucidation of the molecular responses to waterlogging in Jatropha roots by transcriptome profiling. Front. Plant Sci. 2014, 5, 658. [Google Scholar] [CrossRef] [PubMed]
- Somta, P.; Srinives, P. Genome Research in Mungbean [Vigna radiata (L.) Wilczek] and Blackgram [V. mungo (L.) Hepper]. ScienceAsia 2007, 33 (Suppl. 1), 69–74. [Google Scholar] [CrossRef]
- Nair, R.; Schreinemachers, P. Global Status and Economic Importance of Mungbean. In The Mungbean Genome; Nair, R.M., Schafleitner, R., Lee, S.-H., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–8. [Google Scholar] [CrossRef]
- Kyu, K.L.; Malik, A.I.; Colmer, T.D.; Siddique, K.H.M.; Erskine, W. Response of Mungbean (cvs. Celera II-AU and Jade-AU) and Blackgram (cv. Onyx-AU) to Transient Waterlogging. Front. Plant Sci. 2021, 12, 709102. [Google Scholar] [CrossRef]
- Hanumantharao, B.; Nair, R.M.; Nayyar, H. Salinity and High Temperature Tolerance in Mungbean [Vigna radiata (L.) Wilczek] from a Physiological Perspective. Front. Plant Sci. 2016, 7, 957. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Yamauchi, T.; Colmer, T.D.; Pedersen, O.; Nakazono, M. Regulation of Root Traits for Internal Aeration and Tolerance to Soil Waterlogging-Flooding Stress. Plant Physiol. 2018, 176, 1118–1130. [Google Scholar] [CrossRef]
- van Dongen, J.T.; Licausi, F. Oxygen Sensing and Signaling. Annu. Rev. Plant Biol. 2015, 66, 345–367. [Google Scholar] [CrossRef]
- Butsayawarapat, P.; Juntawong, P.; Khamsuk, O.; Somta, P. Comparative Transcriptome Analysis of Waterlogging-Sensitive and Tolerant Zombi Pea (Vigna vexillata) Reveals Energy Conservation and Root Plasticity Controlling Waterlogging Tolerance. Plants 2019, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Song, L.; Chen, H.; Valliyodan, B.; Cheng, P.; Ali, L.; Vuong, T.; Wu, C.; Orlowski, J.; Buckley, B.; et al. A major natural genetic variation associated with root system architecture and plasticity improves waterlogging tolerance and yield in soybean. Plant Cell Environ. 2018, 41, 2169–2182. [Google Scholar] [CrossRef] [PubMed]
- Dawood, T.; Yang, X.; Visser, E.J.; Beek, T.A.T.; Kensche, P.R.; Cristescu, S.M.; Lee, S.; Floková, K.; Nguyen, D.; Mariani, C.; et al. A Co-Opted Hormonal Cascade Activates Dormant Adventitious Root Primordia upon Flooding in Solanum dulcamara. Plant Physiol. 2016, 170, 2351–2364. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, M.; Ji, J.; Xu, Q.; Qi, X.; Chen, X. Comparative RNA-seq based transcriptome profiling of waterlogging response in cucumber hypocotyls reveals novel insights into the de novo adventitious root primordia initiation. BMC Plant Biol. 2017, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, G.; Yan, C.; Cao, N.; Yang, H.; Le, M.; Zhu, F. Depicting the molecular responses of adventitious rooting to waterlogging in melon hypocotyls by transcriptome profiling. 3 Biotech 2021, 11, 351. [Google Scholar] [CrossRef]
- Dawood, T.; Rieu, I.; Wolters-Arts, M.; Derksen, E.B.; Mariani, C.; Visser, E.J.W. Rapid flooding-induced adventitious root development from preformed primordia in Solanum dulcamara. AoB Plants 2014, 6, plt058. [Google Scholar] [CrossRef]
- Mustroph, A. Improving Flooding Tolerance of Crop Plants. Agronomy 2018, 8, 160. [Google Scholar] [CrossRef]
- Ruzicka, K.; Ljung, K.; Vanneste, S.; Podhorská, R.; Beeckman, T.; Friml, J.; Benková, E. Ethylene Regulates Root Growth through Effects on Auxin Biosynthesis and Transport-Dependent Auxin Distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef]
- Pan, R.; Wang, J.; Tian, X. Influence of ethylene on adventitious root formation in mung bean hypocotyl cuttings. Plant Growth Regul. 2002, 36, 135–139. [Google Scholar] [CrossRef]
- Lischweski, S.; Muchow, A.; Guthörl, D.; Hause, B. Jasmonates act positively in adventitious root formation in petunia cuttings. BMC Plant Biol. 2015, 15, 229. [Google Scholar] [CrossRef]
- Ye, B.-B.; Shang, G.-D.; Pan, Y.; Xu, Z.-G.; Zhou, C.-M.; Mao, Y.-B.; Bao, N.; Sun, L.; Xu, T.; Wang, J.-W. AP2/ERF Transcription Factors Integrate Age and Wound Signals for Root Regeneration. Plant Cell 2020, 32, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.-L.; Miao, Z.-Q.; Wang, Z.; Yu, L.-H.; Cai, X.-T.; Xiang, C.-B. Arabidopsis ERF1 Mediates Cross-Talk between Ethylene and Auxin Biosynthesis during Primary Root Elongation by Regulating ASA1 Expression. PLoS Genet. 2016, 12, e1005760. [Google Scholar] [CrossRef] [PubMed]
- Dob, A.; Lakehal, A.; Novak, O.; Bellini, C. Jasmonate inhibits adventitious root initiation through repression of CKX1 and activation of RAP2.6L transcription factor in Arabidopsis. J. Exp. Bot. 2021, 72, 7107–7118. [Google Scholar] [CrossRef] [PubMed]
- Lakehal, A.; Dob, A.; Rahneshan, Z.; Novák, O.; Escamez, S.; Alallaq, S.; Strnad, M.; Tuominen, H.; Bellini, C. ETHYLENE RESPONSE FACTOR 115integrates jasmonate and cytokinin signaling machineries to repress adventitious rooting inArabidopsis. New Phytol. 2020, 228, 1611–1626. [Google Scholar] [CrossRef] [PubMed]
- Heyman, J.; Canher, B.; Bisht, A.; Christiaens, F.; De Veylder, L. Emerging role of the plant ERF transcription factors in coordinating wound defense responses and repair. J. Cell Sci. 2018, 131, jcs208215. [Google Scholar] [CrossRef]
- Jin, Q.; Xu, Y.; Mattson, N.; Li, X.; Wang, B.; Zhang, X.; Jiang, H.; Liu, X.; Wang, Y.; Yao, D. Identification of Submergence-Responsive MicroRNAs and Their Targets Reveals Complex MiRNA-Mediated Regulatory Networks in Lotus (Nelumbo nucifera Gaertn). Front. Plant Sci. 2017, 8, 6. [Google Scholar] [CrossRef]
- Franke, K.R.; Schmidt, S.A.; Park, S.; Jeong, D.-H.; Accerbi, M.; Green, P.J. Analysis of Brachypodium miRNA targets: Evidence for diverse control during stress and conservation in bioenergy crops. BMC Genom. 2018, 19, 547. [Google Scholar] [CrossRef]
- Moldovan, D.; Spriggs, A.; Yang, J.; Pogson, B.J.; Dennis, E.S.; Wilson, I.W. Hypoxia-responsive microRNAs and trans-acting small interfering RNAs in Arabidopsis. J. Exp. Bot. 2010, 61, 165–177. [Google Scholar] [CrossRef]
- Horchani, F.; Aschi-Smiti, S.; Brouquisse, R. Involvement of nitrate reduction in the tolerance of tomato (Solanum lycopersicum L.) plants to prolonged root hypoxia. Acta Physiol. Plant. 2010, 32, 1113–1123. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Salgado, I.; Sodek, L. Involvement of nitrite in the nitrate-mediated modulation of fermentative metabolism and nitric oxide production of soybean roots during hypoxia. Planta 2013, 237, 255–264. [Google Scholar] [CrossRef]
- Chen, T.; Yuan, F.; Song, J.; Wang, B. Nitric oxide participates in waterlogging tolerance through enhanced adventitious root formation in the euhalophyte Suaeda salsa. Funct. Plant Biol. 2016, 43, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liao, W.-B.; Yu, J.-H.; Ren, P.-J.; Dawuda, M.M.; Wang, M.; Niu, L.-J.; Li, X.-P.; Xu, X.-T. Nitric Oxide Is Involved in Ethylene-Induced Adventitious Rooting in Marigold (Tagetes erecta L.). Can. J. Plant Sci. 2017, 97, 620–631. [Google Scholar] [CrossRef][Green Version]
- Mustroph, A.; Zanetti, M.E.; Jang, C.J.H.; Holtan, H.E.; Repetti, P.P.; Galbraith, D.W.; Girke, T.; Bailey-Serres, J. Profiling translatomes of discrete cell populations resolves altered cellular priorities during hypoxia in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 18843–18848. [Google Scholar] [CrossRef]
- Mustroph, A.; Lee, S.C.; Oosumi, T.; Zanetti, M.E.; Yang, H.; Ma, K.; Yaghoubi-Masihi, A.; Fukao, T.; Bailey-Serres, J. Cross-Kingdom Comparison of Transcriptomic Adjustments to Low-Oxygen Stress Highlights Conserved and Plant-Specific Responses. Plant Physiol. 2010, 152, 1484–1500. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Kim, S.K.; Kim, M.Y.; Lestari, P.; Kim, K.H.; Ha, B.-K.; Jun, T.H.; Hwang, W.J.; Lee, T.; Lee, J.; et al. Genome sequence of mungbean and insights into evolution within Vigna species. Nat. Commun. 2014, 5, 5443. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Xu, L.; Dong, Z.; Fang, L.; Luo, Y.; Wei, Z.; Guo, H.; Zhang, G.; Gu, Y.Q.; Coleman-Derr, D.; Xia, Q.; et al. OrthoVenn2: A web server for whole-genome comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res. 2019, 47, W52–W58. [Google Scholar] [CrossRef]
- Horan, K.; Jang, C.; Bailey-Serres, J.; Mittler, R.; Shelton, C.; Harper, J.F.; Zhu, J.-K.; Cushman, J.C.; Gollery, M.; Girke, T. Annotating Genes of Known and Unknown Function by Large-Scale Coexpression Analysis. Plant Physiol. 2008, 147, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Usadel, B.; Nagel, A.; Steinhauser, D.; Gibon, Y.; Bläsing, O.E.; Redestig, H.; Sreenivasulu, N.; Krall, L.; Hannah, M.A.; Poree, F.; et al. PageMan: An interactive ontology tool to generate, display, and annotate overview graphs for profiling experiments. BMC Bioinform. 2006, 7, 535. [Google Scholar] [CrossRef] [PubMed]
- Juntawong, P.; Sorenson, R.; Bailey-Serres, J. Cold shock protein 1 chaperones mRNAs during translation inArabidopsis thaliana. Plant J. 2013, 74, 1016–1028. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sreeratree, J.; Butsayawarapat, P.; Chaisan, T.; Somta, P.; Juntawong, P. RNA-Seq Reveals Waterlogging-Triggered Root Plasticity in Mungbean Associated with Ethylene and Jasmonic Acid Signal Integrators for Root Regeneration. Plants 2022, 11, 930. https://doi.org/10.3390/plants11070930
Sreeratree J, Butsayawarapat P, Chaisan T, Somta P, Juntawong P. RNA-Seq Reveals Waterlogging-Triggered Root Plasticity in Mungbean Associated with Ethylene and Jasmonic Acid Signal Integrators for Root Regeneration. Plants. 2022; 11(7):930. https://doi.org/10.3390/plants11070930
Chicago/Turabian StyleSreeratree, Jaruwan, Pimprapai Butsayawarapat, Tanapon Chaisan, Prakit Somta, and Piyada Juntawong. 2022. "RNA-Seq Reveals Waterlogging-Triggered Root Plasticity in Mungbean Associated with Ethylene and Jasmonic Acid Signal Integrators for Root Regeneration" Plants 11, no. 7: 930. https://doi.org/10.3390/plants11070930
APA StyleSreeratree, J., Butsayawarapat, P., Chaisan, T., Somta, P., & Juntawong, P. (2022). RNA-Seq Reveals Waterlogging-Triggered Root Plasticity in Mungbean Associated with Ethylene and Jasmonic Acid Signal Integrators for Root Regeneration. Plants, 11(7), 930. https://doi.org/10.3390/plants11070930





