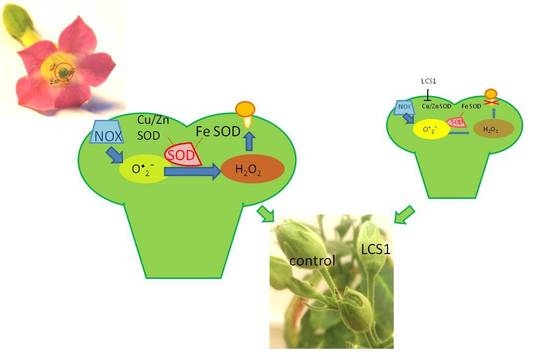
The Balance between Different ROS on Tobacco Stigma during Flowering and Its Role in Pollen Germination
Abstract
:1. Introduction
2. Results
2.1. ROS Dynamics on Stigma
2.2. SOD Activity Dynamics and Isoenzyme Composition
2.3. Significance of ROS Balance for Pollen Germination In Vivo
3. Discussion
4. Materials and Methods
4.1. Plant Cultivation, Stigma Exudate Collection and Pollination In Vivo
4.2. Evaluation of Pollen Germination In Vivo and Fertilization Success (Seed Set)
4.3. EPR Spectroscopy
4.4. Spectrophotometry
4.5. Zymographic Detection of SOD Activity
4.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heslop-Harrison, Y. Control gates and micro-ecology: The pollen-stigma interaction in perspective. Ann. Bot. 2000, 85, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Shivanna, K.R. The Pistil: Structure in Relation to Its Function. In Reproductive Ecology of Flowering Plants: Patterns and Processes; Springer: Singapore, 2020; pp. 41–50. ISBN 9789811542107. [Google Scholar]
- Konar, R.N.; Linskens, H.F. Physiology and biochemistry of the stigmatic fluid of Petunia hybrida. Planta 1966, 71, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Labarca, C.; Loewus, F. The Nutritional Role of Pistil Exudate in Pollen Tube Wall Formation in Lilium longiflorum: I. Utilization of Injected Stigmatic Exudate 1. Plant Physiol. 1972, 50, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rejón, J.D.; Delalande, F.; Schaeffer-Reiss, C.; Carapito, C.; Zienkiewicz, K.; Alché, J.D.; Rodríguez-García, M.I.; Van Dorsselaer, A.; Castro, A.J. The plant stigma exudate: A biochemically active extracellular environment for pollen germination? Plant Signal. Behav. 2014, 9, 5695–5705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rejón, J.D.; Delalande, F.; Schaeffer-Reiss, C.; Carapito, C.; Zienkiewicz, K.; de Dios Alché, J.; Rodríguez-García, M.I.; Van Dorsselaer, A.; Castro, A.J. Proteomics profiling reveals novel proteins and functions of the plant stigma exudate. J. Exp. Bot. 2013, 64, 5695–5705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankaranarayanan, S.; Ju, Y.; Kessler, S.A. Reactive Oxygen Species as Mediators of Gametophyte Development and Double Fertilization in Flowering Plants. Front. Plant Sci. 2020, 11, 1199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Zhang, X.S.; Gao, X.-Q. ROS in the Male–Female Interactions During Pollination: Function and Regulation. Front. Plant Sci. 2020, 11, e00177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.A.; Harper, J.F.; Palanivelu, R. A Fruitful Journey: Pollen Tube Navigation from Germination to Fertilization. Annu. Rev. Plant Biol. 2019, 70, 809–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafra, A.; Rejón, J.D.; Hiscock, S.J.; Alché, J.D.D. Patterns of ROS accumulation in the stigmas of angiosperms and visions into their multi-functionality in plant reproduction. Front. Plant Sci. 2016, 7, 1112–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McInnis, S.M.; Desikan, R.; Hancock, J.T.; Hiscock, S.J. Production of reactive oxygen species and reactive nitrogen species by angiosperm stigmas and pollen: Potential signalling crosstalk? New Phytol. 2006, 172, 221–228. [Google Scholar] [CrossRef]
- Hiscock, S.J.; Bright, J.; McInnis, S.M.; Desikan, R.; Hancock, J.T. Signaling on the stigma. Potential new roles for ROS and NO in plant cell signaling. Plant Signal. Behav. 2007, 2, 23–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bredemeijer, G.M.M. The role of peroxidases in pistil-pollen interactions. Theor. Appl. Genet. 1984, 68, 193–206. [Google Scholar] [CrossRef] [PubMed]
- McInnis, S.M.; Costa, L.M.; Gutiérrez-Marcos, J.F.; Henderson, C.A.; Hiscock, S.J. Isolation and characterization of a polymorphic stigma-specific class III peroxidase gene from Senecio squalidus L. (Asteraceae). Plant Mol. Biol. 2005, 57, 659–677. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Bhatla, S.C. Accumulation and scavenging of reactive oxygen species and nitric oxide correlate with stigma maturation and pollen–stigma interaction in sunflower. Acta Physiol. Plant. 2013, 35, 2777–2787. [Google Scholar] [CrossRef]
- Smirnova, A.V.; Timofeyev, K.N.; Breygina, M.A.; Matveyeva, N.P.; Yermakov, I.P. Antioxidant properties of the pollen exine polymer matrix. Biophysics 2012, 57, 174–178. [Google Scholar] [CrossRef]
- Breygina, M.; Klimenko, E.; Shilov, E.; Podolyan, A.; Mamaeva, A.; Zgoda, V.; Fesenko, I. Hydrogen peroxide in tobacco stigma exudate affects pollen proteome and membrane potential in pollen tubes. Plant Biol. 2021, 23, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Breygina, M.; Klimenko, E. ROS and ions in cell signaling during sexual plant reproduction. Int. J. Mol. Sci. 2020, 21, 9476. [Google Scholar] [CrossRef]
- Podolyan, A.; Maksimov, N.; Breygina, M. Redox-regulation of ion homeostasis in growing lily pollen tubes. J. Plant Physiol. 2019, 243, 153050. [Google Scholar] [CrossRef]
- Breygina, M.A.; Abramochkin, D.V.; Maksimov, N.M.; Yermakov, I.P. Hydrogen peroxide affects ion channels in lily pollen grain protoplasts. Plant Biol. 2016, 18, 761–767. [Google Scholar] [CrossRef]
- Podolyan, A.; Luneva, O.; Klimenko, E.; Breygina, M. Oxygen radicals and cytoplasm zoning in growing lily pollen tubes. Plant Reprod. 2021, 34, 103–115. [Google Scholar] [CrossRef]
- Segal, A.W. NADPH oxidases as electrochemical generators to produce ion fluxes and turgor in fungi, plants and humans. Open Biol. 2016, 6, 160028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiscock, S.J.; Allen, A.M. Diverse cell signalling pathways regulate pollen-stigma interactions: The search for consensus. New Phytol. 2008, 179, 286–317. [Google Scholar] [CrossRef] [PubMed]
- Serrano, I.; Romero-Puertas, M.C.; Sandalio, L.M.; Olmedilla, A. The role of reactive oxygen species and nitric oxide in programmed cell death associated with self-incompatibility. J. Exp. Bot. 2015, 66, 2869–2876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhlemann, J.K.; Younts, T.L.B.; Muday, G.K. Flavonols control pollen tube growth and integrity by regulating ROS homeostasis during high-temperature stress. Proc. Natl. Acad. Sci. USA 2018, 115, E11188–E11197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djanaguiraman, M.; Perumal, R.; Jagadish, S.V.K.; Ciampitti, I.A.; Welti, R.; Prasad, P.V. V Sensitivity of sorghum pollen and pistil to high-temperature stress. Plant. Cell Environ. 2018, 41, 1065–1082. [Google Scholar] [CrossRef]
- Zafra, A.; Rodríguez-García, M.I.; Alché, J.D.D. Cellular localization of ROS and NO in olive reproductive tissues during flower development. BMC Plant Biol. 2010, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Lan, X.; Yang, J.; Abhinandan, K.; Nie, Y.; Li, X.; Li, Y.; Samuel, M.A. Flavonoids and ROS Play Opposing Roles in Mediating Pollination in Ornamental Kale (Brassica oleracea var. acephala). Mol. Plant 2017, 10, 1361–1364. [Google Scholar] [CrossRef] [Green Version]
- Bhatla, S.C.; Gogna, M.; Jain, P.; Singh, N.; Mukherjee, S.; Kalra, G. Signaling mechanisms and biochemical pathways regulating pollen-stigma interaction, seed development and seedling growth in sunflower under salt stress. Plant Signal. Behav. 2021, 16, 1958129. [Google Scholar] [CrossRef]
- Santiago, J.P.; Sharkey, T.D. Pollen development at high temperature and role of carbon and nitrogen metabolites. Plant. Cell Environ. 2019, 42, 2759–2775. [Google Scholar] [CrossRef] [Green Version]
- Xie, D.-L.; Zheng, X.-L.; Zhou, C.-Y.; Kanwar, M.K.; Zhou, J. Functions of Redox Signaling in Pollen Development and Stress Response. Antioxidants 2022, 11, 287. [Google Scholar] [CrossRef]
- Fábián, A.; Sáfrán, E.; Szabó-Eitel, G.; Barnabás, B.; Jäger, K. Stigma Functionality and Fertility Are Reduced by Heat and Drought Co-stress in Wheat. Front. Plant Sci. 2019, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Dafni, A.; Maués, M.M. A rapid and simple procedure to determine stigma receptivity. Sex. Plant Reprod. 1998, 11, 177–180. [Google Scholar] [CrossRef]
- Tsukagoshi, H. Control of root growth and development by reactive oxygen species. Curr. Opin. Plant Biol. 2016, 29, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Carol, R.J.; Dolan, L. The role of reactive oxygen species in cell growth: Lessons from root hairs. J. Exp. Bot. 2006, 57, 1829–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cresti, M.; Keijzer, C.J.; Tiezzi, A.; Ciampolini, F.; Focardi, S. Stigma of Nicotiana: Ultrastructural and biochemical studies. Am. J. Bot. 1986, 73, 1713–1722. [Google Scholar] [CrossRef]
- Kiyono, H.; Katano, K.; Suzuki, N. Links between Regulatory Systems of ROS and Carbohydrates in Reproductive Development. Plants 2021, 10, 1652. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lianping, S.; Yu, X.; David, V.; Chao, P.; Xiang, S.; Zhiwen, L.; Lijun, C.; Hua, Z.; Zhifu, H.; et al. Pollen PCP-B peptides unlock a stigma peptide–receptor kinase gating mechanism for pollination. Science 2021, 372, 171–175. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Yu, G. Identification of superoxide dismutase isoenzymes in tobacco pollen. Front. Biol. China 2009, 4, 442–445. [Google Scholar] [CrossRef]
- Wu, J.; Shang, Z.; Wu, J.; Jiang, X.; Moschou, P.N.; Sun, W.; Roubelakis-Angelakis, K.A.; Zhang, S. Spermidine oxidase-derived H2O2 regulates pollen plasma membrane hyperpolarization-activated Ca2+-permeable channels and pollen tube growth. Plant J. 2010, 63, 1042–1053. [Google Scholar] [CrossRef]
- Benkő, P.; Jee, S.; Kaszler, N.; Fehér, A.; Gémes, K. Polyamines treatment during pollen germination and pollen tube elongation in tobacco modulate reactive oxygen species and nitric oxide homeostasis. J. Plant Physiol. 2020, 244, 153085. [Google Scholar] [CrossRef]
- Parre, E.; Geitmann, A. More than a leak sealant. The mechanical properties of callose in pollen tubes. Plant Physiol. 2005, 137, 274–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senesi, N.; Senesi, G.S. Electron-spin resonance spectroscopy. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 426–437. ISBN 9780123485304. [Google Scholar]
- Haywood, R. Spin-Trapping: Theory and Applications. In Encyclopedia of Biophysics; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2447–2453. [Google Scholar]
- Li, Z.-G. Measurement of signaling molecules calcium ion, reactive sulfur species, reactive carbonyl species, reactive nitrogen species, and reactive oxygen species in plants. In Plant Signaling Molecules Role and Regulation Under Stressful Environments; Khan, M.I., Reddy, P.S., Ferrante, A., Khan, N., Eds.; Woodhead Publishing: Sawston, UK, 2019; Chapter 5; pp. 83–103. ISBN 978-0-12-816451-8. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breygina, M.; Schekaleva, O.; Klimenko, E.; Luneva, O. The Balance between Different ROS on Tobacco Stigma during Flowering and Its Role in Pollen Germination. Plants 2022, 11, 993. https://doi.org/10.3390/plants11070993
Breygina M, Schekaleva O, Klimenko E, Luneva O. The Balance between Different ROS on Tobacco Stigma during Flowering and Its Role in Pollen Germination. Plants. 2022; 11(7):993. https://doi.org/10.3390/plants11070993
Chicago/Turabian StyleBreygina, Maria, Olga Schekaleva, Ekaterina Klimenko, and Oksana Luneva. 2022. "The Balance between Different ROS on Tobacco Stigma during Flowering and Its Role in Pollen Germination" Plants 11, no. 7: 993. https://doi.org/10.3390/plants11070993
APA StyleBreygina, M., Schekaleva, O., Klimenko, E., & Luneva, O. (2022). The Balance between Different ROS on Tobacco Stigma during Flowering and Its Role in Pollen Germination. Plants, 11(7), 993. https://doi.org/10.3390/plants11070993