Optimal Growth Conditions for Azolla pinnata R. Brown: Impacts of Light Intensity, Nitrogen Addition, pH Control, and Humidity
Abstract
:1. Introduction
- The variation in the media that were used in the studies complicates comparison, especially when wastewater streams were not fully characterised;
- The symbiotic relationship that all Azolla species have with the nitrogen-fixing bacteria Anabaena azollae allows the plants to live in nitrogen-free environments. However, the effects of the presence of nitrogen in the medium on growth rate have not been compared to the growth rate with an absence of nitrogen;
- Light presents many factors that must be considered in order to accurately quantify this variable. The first factor is the type of light: natural versus artificial. The second is the light intensity, which is a variable that has sometimes not been reported. The majority of the studies that are shown in Table 1 used natural light instead of artificial light. The study in [23] varied the intensity of natural light to see the effects on the growth rate of A. pinnata. The effects of varied artificial light conditions on growth rate have not been investigated;
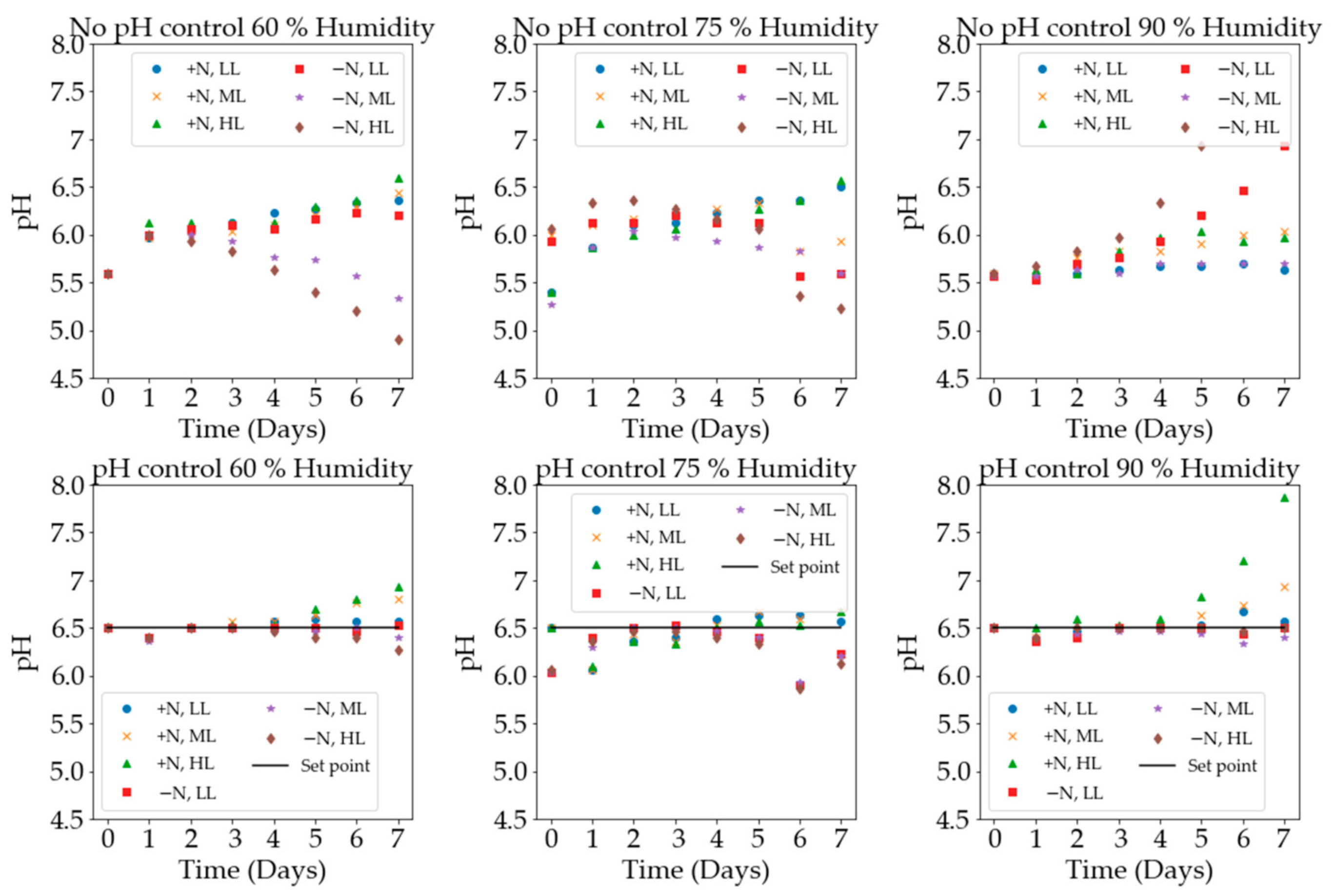
- pH control as a growth condition is a clear gap in the existing literature. The pH of the medium was either adjusted initially, as in [21] and [28], or different pH values were investigated, as in [27]. There has been no comparison of the effects of non-pH-controlled versus pH-controlled conditions on the growth rate of A. pinnata;
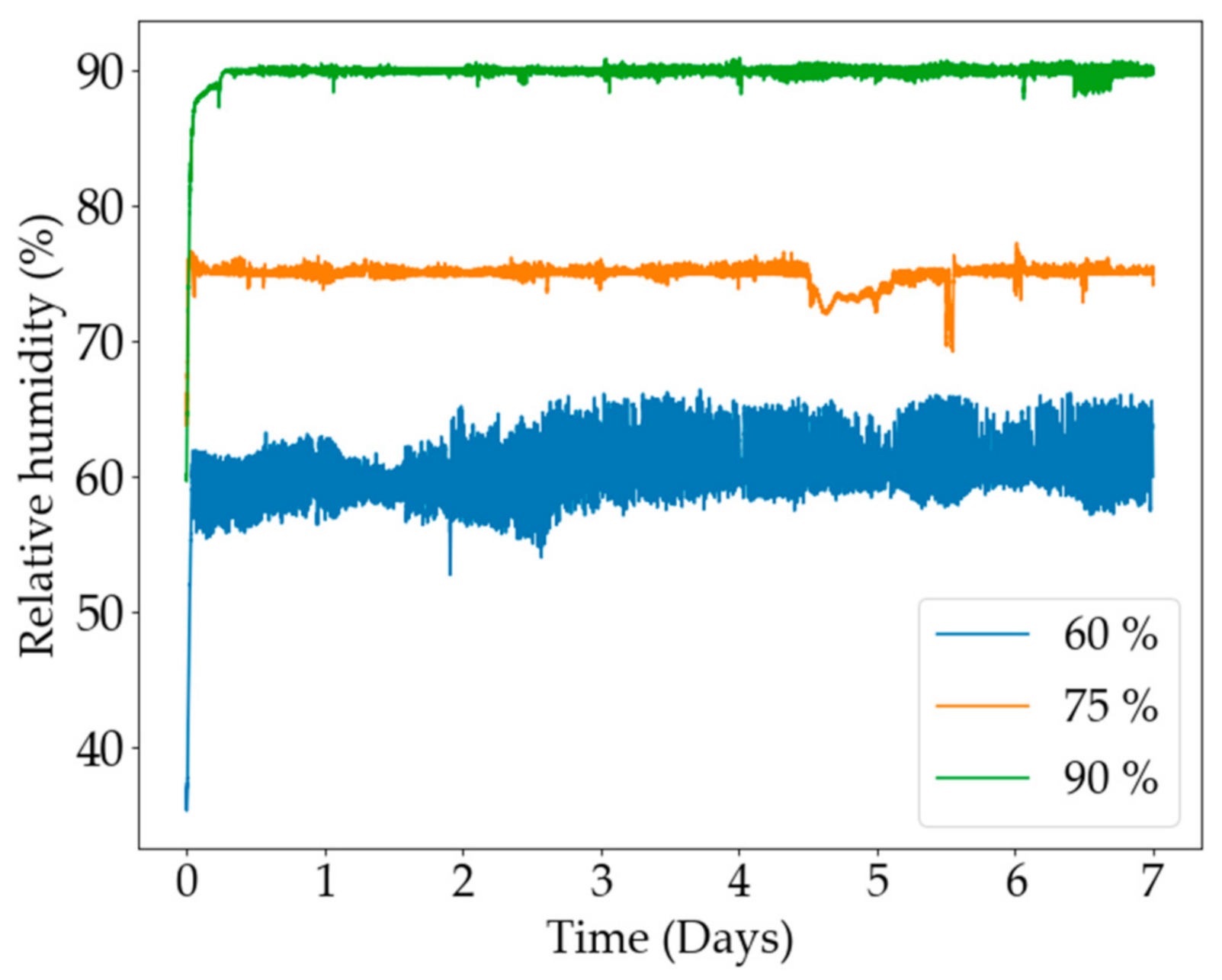
- Humidity has not received serious attention, even though many studies have iterated the importance of a high humidity for the growth of the Azolla species [15]. In the existing A. pinnata studies, either the humidity was not mentioned at all or it was stated that the A. pinnata was grown in a greenhouse, which would increase the humidity. Only [33] and [34] reported humidity values, but these values were not controlled.
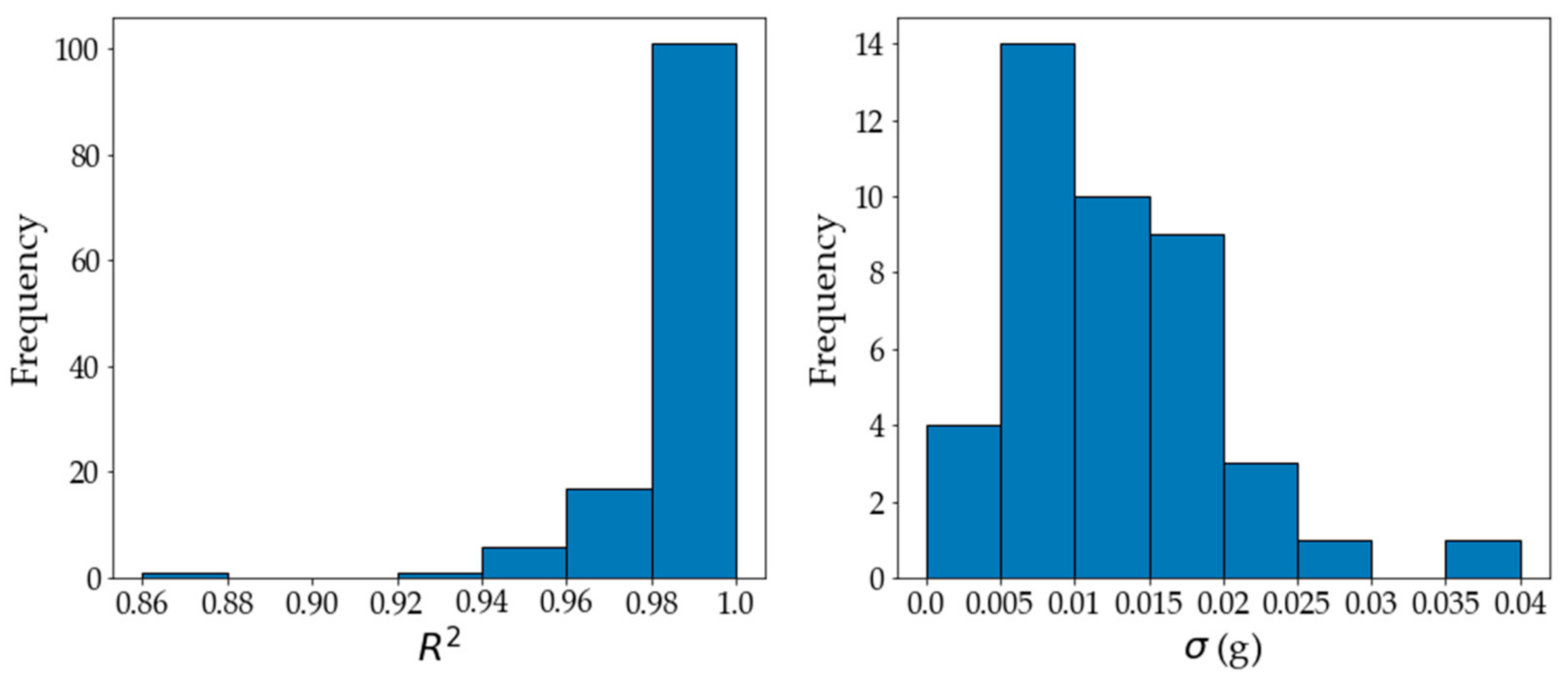
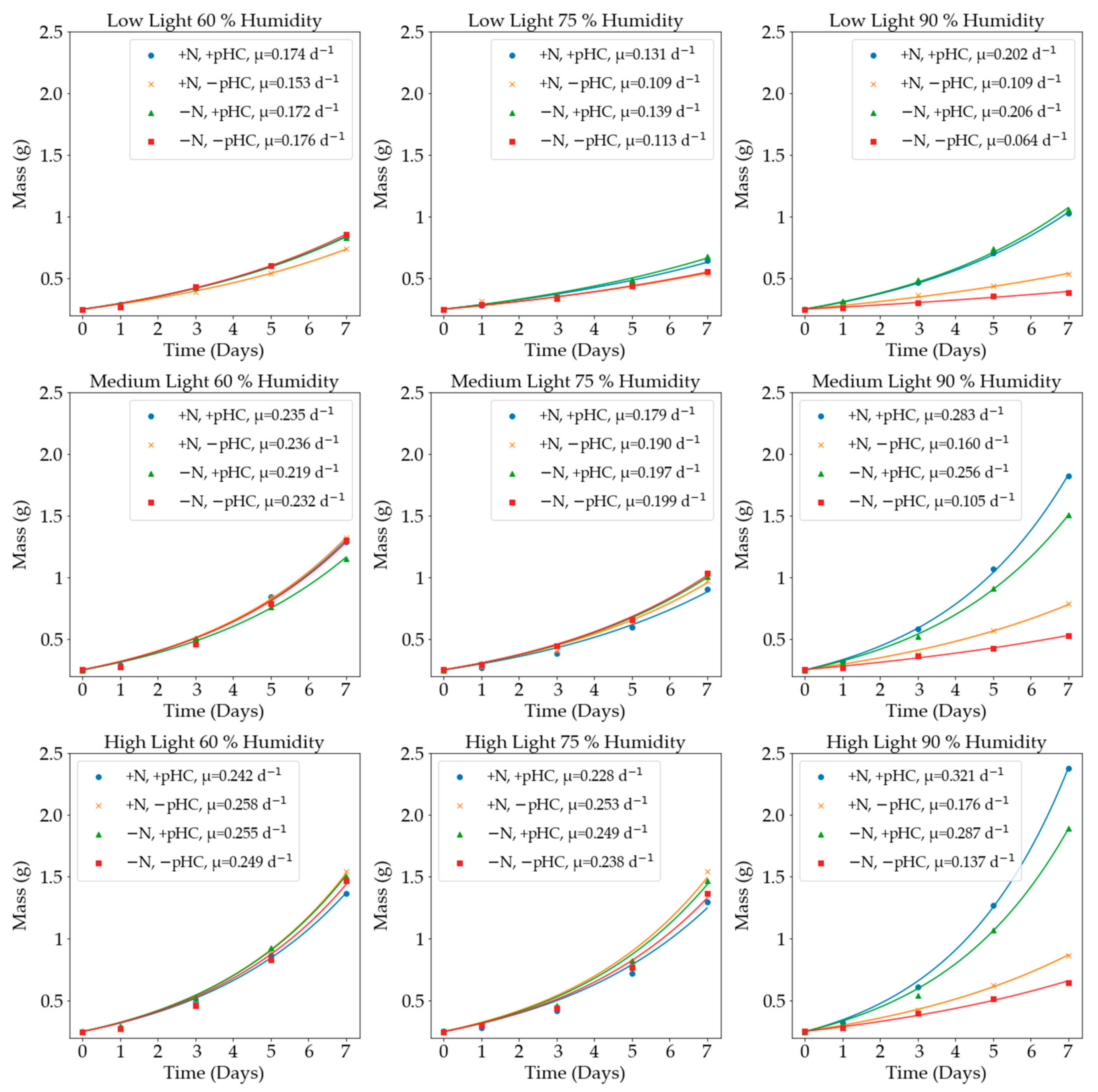
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Analytical Instruments
3.3. Experimental Procedure
3.3.1. Structure
3.3.2. Growth Media
3.3.3. pH Control
3.3.4. Lighting
3.3.5. Humidity
3.3.6. Measurements and Growth Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandratos, N. Countries with rapid population growth and resource constraints: Issues of food, agriculture, and development. Popul. Dev. Rev. 2005, 31, 237–258. [Google Scholar] [CrossRef]
- Gibbs, K.E.; MacKey, R.L.; Currie, D.J. Human land use, agriculture, pesticides and losses of imperiled species. Divers. Distrib. 2009, 15, 242–253. [Google Scholar] [CrossRef]
- Tellez-Rio, A.; Vallejo, A.; García-Marco, S.; Martin-Lammerding, D.; Tenorio, J.L.; Rees, R.M.; Guardia, G. Conservation Agriculture practices reduce the global warming potential of rainfed low N input semi-arid agriculture. Eur. J. Agron. 2017, 84, 95–104. [Google Scholar] [CrossRef]
- Pirard, R.; Belna, K. Agriculture and Deforestation: Is REDD+ Rooted In Evidence? For. Policy Econ. 2012, 21, 62–70. [Google Scholar] [CrossRef]
- Moss, B. Water pollution by agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Forni, C.; Chen, J.; Tancionil, L.; Caiola, M.G. Evaluation of the fern Azolla for growth, nitrogen and phosphorus removal from wastewater. Water Res. 2001, 35, 1592–1598. [Google Scholar] [CrossRef]
- Glibert, P.M.; Maranger, R.; Sobota, D.J.; Bouwman, L. The Haber Bosch-harmful algal bloom (HB-HAB) link. Environ. Res. Lett. 2014, 9, 105001. [Google Scholar] [CrossRef] [Green Version]
- Stein, L.Y.; Klotz, M.G. Current Biology The nitrogen cycle. Curr. Biol. 2016, 26, R94–R98. [Google Scholar] [CrossRef] [Green Version]
- Stewart, W.D.P. Nitrogen Fixation. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1976, 274, 341–358. [Google Scholar]
- Erlt, G. The Arduous Way to the Haber-Bosch Process. Z. Anorg. Allg. Chem. 2012, 638, 707–709. [Google Scholar] [CrossRef]
- Boerner, L.K. Shrinking the Haber-Bosch process. Sci. Conc. Green Chem. 2019, 97, 5. [Google Scholar]
- Gao, S.; Xu, P.; Zhou, F.; Yang, H.; Zheng, C.; Cao, W.; Tao, S.; Piao, S.; Zhao, Y.; Ji, X.; et al. Quantifying nitrogen leaching response to fertilizer additions in China’s cropland. Environ. Pollut. 2016, 21, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.S.; Carlsson, G.; Hauggaard-Nielsen, H. Intercropping of grain legumes and cereals improves the use of soil N resources and reduces the requirement for synthetic fertilizer N: A global-scale analysis. Agron. Sustain. Dev. 2020, 40, 5. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, C.J. Permaculture: Regenerative—Not merely sustainable. Sci. Prog. 2015, 98, 403–412. [Google Scholar] [CrossRef]
- Wagner, G.M. Azolla: A Review of Its Biology and Utilization. Bot. Rev. 1997, 63, 1–26. [Google Scholar] [CrossRef]
- Ventura, W.; Watanabe, I. Biology and Fertility of Soils Green manure production of Azolla microphylla and Sesbania rostrata and their long-term effects on rice yields and soil fertility. Biol. Fertil. Soils 1993, 15, 241–248. [Google Scholar] [CrossRef]
- Bocchi, S.; Malgioglio, A. Azolla-Anabaena as a Biofertilizer for Rice Paddy Fields in the Po Valley, aTemperate Rice Area in Northern Italy. Int. J. Agron. 2010, 2010, 152158. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.L.; Santos, M.C.; Carrapiço, F. Biomass characterization of Azolla filiculoides grown in natural ecosystems and wastewater. Hydrobiologia 1999, 415, 323–327. [Google Scholar] [CrossRef]
- Kaur, M.; Sahoo, P.C.; Kumar, M.; Sachdeva, S.; Puri, S.K. Effect of metal nanoparticles on microbial community shift and syntrophic metabolism during anaerobic digestion of Azolla microphylla. J. Environ. Chem. Eng. 2021, 9, 105841. [Google Scholar] [CrossRef]
- Kösesakal, T. Effects of seasonal changes on pigment composition of Azolla filiculoides lam. Am. Fern J. 2014, 104, 58–66. [Google Scholar] [CrossRef]
- Tung, H.F.; Shen, T.C. Studies of the Azolla pinnata-Anabaena azollae Symbiosis: Growth and Nitrogen Fixation. Source New Phytol. 1981, 87, 743–749. [Google Scholar] [CrossRef]
- Tung, H.F.; Shen, T.C. Growth studies of Azolla pinnata and rice. Aquat. Bot. 1985, 22, 145–152. [Google Scholar] [CrossRef]
- Singh, A.; Srivastava, N. Effect of light intensity on the growth of Azolla pinnata R. Brown at Ranchi, India. Hydrobiologia 1985, 126, 49–52. [Google Scholar] [CrossRef]
- Singh, A.; Srivastava, N. Effect of photoperiod on the growth of Azolla pinnata R. brown. Hydrobiologia 1985, 123, 211–214. [Google Scholar] [CrossRef]
- Chakrabortty, M.; Kushari, D. Influence of Domestic Sewage on Growth and Nitrogen Fixation of Azolla pinnata Tar. Br. Aquat. Bot. 1986, 24, 61–68. [Google Scholar] [CrossRef]
- Sarkar, A. Effects of light intensity on growth of Azolla pinnata ta cultured in effluent collected from the banka stream in West Bengal, India. Aquat. Bot. 1986, 26, 189–194. [Google Scholar] [CrossRef]
- Cary, P.R.; Weerts, P.G.J. Growth and nutrient composition of Azolla pinnata R. Brown and Azolla filiculoides Lamarck as affected by water temperature, nitrogen and phosphorus supply, light intensity and pH. Aquat. Bot. 1992, 43, 163–180. [Google Scholar] [CrossRef]
- Maejima, K.; Uheda, E.; Kitoh, S.; Shiomi, N. Differences in growth rate, N2 fixation in different Azolla pinnata strains. Environ. Exp. Bot. 2002, 47, 143–147. [Google Scholar] [CrossRef]
- Rai, V.; Sharma, N.K.; Rai, A.K. Growth and cellular ion content of a salt-sensitive symbiotic system Azolla pinnata-Anabaena azollae under NaCl stress. J. Plant Physiol. 2006, 163, 937–944. [Google Scholar] [CrossRef]
- Ambio, P.K. Wastewater Management through Biomass of Azolla pinnata: An Eco-sustainable Approach. Ambio 2007, 36, 426–428. [Google Scholar]
- Prasad, S.M.; Singh, A.; Singh, P. Physiological, biochemical and growth responses of Azolla pinnata to chlorpyrifos and cypermethrin pesticides exposure: A comparative study. Chem. Ecol. 2015, 31, 285–298. [Google Scholar] [CrossRef]
- Gupta, S.K.; Chandra, R.; Shinde, K.P. Study of chemical composition and mineral content of sun dried Azolla pinnata. J. Pharmacogn. Phytochem. 2018, 7, 1214. Available online: http://www.iajavs.com/currentissue.php (accessed on 11 August 2021).
- Kösesakal, T.; Yildiz, M. Growth performance and biochemical profile of Azolla pinnata and Azolla caroliniana grown under greenhouse conditions. Arch. Biol. Sci. 2019, 71, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Goala, M.; Yadav, K.K.; Alam, J.; Adelodun, B.; Choi, K.S.; Cabral-Pinto, M.M.S.; Hamid, A.A.; Alhoshanc, M.; Ali, F.A.A.; Shukla, A.K. Phytoremediation of dairy wastewater using Azolla pinnata: Application of image processing technique for leaflet growth simulation. J. Water Process Eng. 2021, 42, 102152. [Google Scholar] [CrossRef]
- Sang, H.W.F.; Van, V.V.; Kijne, J.W.; Tam, V.T.; Planque, K. Use of Azolla as a Test Organism in a Growth Chamber of Simple Design; Martinus Nijhoff Publishers: Leiden, The Netherlands, 1987. [Google Scholar]
- Shiomi, N.; Kitoh, S. Culture of Azolla in a pond, nutrient composition, and use as fish feed. Soil Sci. Plant Nutr. 2001, 47, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Janes, R. Growth and survival of Azolla filiculoides in Britain: I. Vegetative reproduction. New Phytol. 1998, 138, 367–375. [Google Scholar] [CrossRef]


| Summary | Growth Medium | pH Control | Nitrogen Presence/Absence | Light Intensity Comparison 1 | Humidity Control | μ (Day−1) | Reference |
|---|---|---|---|---|---|---|---|
| A. pinnata growth and nitrogen fixation symbiosis | Fertiliser and floodwater | Not controlled | Present | Varied natural light; 30,000 lx to 120,000 lx | Not measured | 0.126 | [21] |
| A. pinnata influence on rice crops | Fertiliser and floodwater | Not controlled | Present | Natural light; not quantified | Greenhouse conditions | 0.110 | [22] |
| Effects of light intensity on A. pinnata | Fertiliser with floodwater | Not controlled | Present | Varied natural light; 15,000 lx to 80,000 lx | Not measured | 0.085 | [23] |
| Effects of photoperiods on the growth of A. pinnata | Fertiliser with tap water | Not controlled | Present | Artificial light; 7500 lx | Not measured | 0.090 | [24] |
| Water remediation of sewage using A. pinnata | Sewage and tap water | Not controlled | Present | Natural light; not quantified | Greenhouse conditions | 0.025 | [25] |
| Effects of light intensity on A. pinnata grown in effluent | Sewage and tap water | Not controlled | Present | Varied natural light; 64,800 lx to 40,500 lx | Greenhouse conditions | 0.056 | [26] |
| Growth of A. pinnata under different growth conditions | Nutrient enriched water | Different initial pH values investigated; not controlled | Varied presence | Varied natural light; not quantified | Greenhouse conditions | 0.283 | [27] |
| Growth comparison of A. pinnata strains | Standard medium [16] | pH controlled at 6.1; no uncontrolled data | Present | Artificial light; 10,800 lx | Not measured | 0.300 | [28] |
| Effects of varying salinity on A. pinnata growth | Hoagland solution with 10 mM of NaCl added | Not controlled | Absent | Artificial light; 5130 lx | Not measured | 0.037 | [29] |
| Water remediation of sewage using A. pinnata | Sewage and tap water | Not controlled | Present | Natural light; not quantified | Not measured | 0.251 | [30] |
| Growth comparison of A. pinnata in the presence of pesticides | Standard medium [16] with pesticide | Not controlled | Present | Artificial light; 10,800 lx | Not measured | 0.0650 | [31] |
| Chemical composition of sun-dried A. pinnata | Fertiliser with floodwater | Not controlled | Present | Natural light; not quantified | Not measured | 0.139 | [32] |
| Growth analysis of A. pinnata in greenhouse conditions | Hoagland solution | Not controlled | Absent | Natural light; not quantified | Greenhouse conditions; 60% to 70% | 0.124 | [33] |
| Phytoremediation of dairy wastewater using A. pinnata | Dairy wastewater | Not controlled | Present | Artificial light; 2000 lx | Laboratory conditions; 55% to 70% | 0.100 | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, M.E.J.; Mathe, L.O.J.; van Rooyen, I.L.; Brink, H.G.; Nicol, W. Optimal Growth Conditions for Azolla pinnata R. Brown: Impacts of Light Intensity, Nitrogen Addition, pH Control, and Humidity. Plants 2022, 11, 1048. https://doi.org/10.3390/plants11081048
da Silva MEJ, Mathe LOJ, van Rooyen IL, Brink HG, Nicol W. Optimal Growth Conditions for Azolla pinnata R. Brown: Impacts of Light Intensity, Nitrogen Addition, pH Control, and Humidity. Plants. 2022; 11(8):1048. https://doi.org/10.3390/plants11081048
Chicago/Turabian Styleda Silva, Maria Emelia Jesus, Lebani Oarabile Joy Mathe, Ignatius Leopoldus van Rooyen, Hendrik Gideon Brink, and Willie Nicol. 2022. "Optimal Growth Conditions for Azolla pinnata R. Brown: Impacts of Light Intensity, Nitrogen Addition, pH Control, and Humidity" Plants 11, no. 8: 1048. https://doi.org/10.3390/plants11081048







