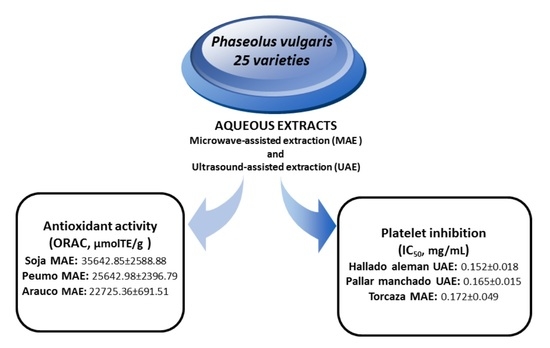
Antioxidant Capacity and Antiplatelet Activity of Aqueous Extracts of Common Bean (Phaseolus vulgaris L.) Obtained with Microwave and Ultrasound Assisted Extraction
Abstract
:1. Introduction
2. Results
2.1. Extraction Yields of Extracts P. vulgaris L.
2.2. Total Phenolic Content of Extracts P. vulgaris L.
2.3. Antioxidant Activity of Extracts P. vulgaris L.
2.4. Cytotoxicity of P. vulgaris L. Extracts on Platelets
2.5. Platelet Antiaggregant Activity of Extracts P. vulgaris L.
2.6. Total Monomeric Anthocyanin Content of Extracts P. vulgaris L.
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Extracts
- Ultrasound-assisted extraction (UAE): An ultrasound bar (QSonica Q125, Newton, CT, USA) was used, at 50% amplitude (10 kHz) with a sonication time of 60 min, to prevent the temperature from rising too high, the Erlenmeyer flask with the mixture of beans and distilled water was placed inside of an ice bath.
- Microwave-assisted extraction (MAE): A 1200 W domestic microwave (WMW606ADWC Whirlpool, Shanghai, China) was used. To carry out the microwave-assisted extraction, the bean mixtures with distilled water in an Erlenmeyer flask, were placed inside them in a 1/40 ratio. The minimum power of 1 was fixed, which is equivalent to 120 W. The extraction time was 60 min.
4.3. Total Soluble Solids and Total Phenolics
4.4. Total Monomeric Anthocyanin Content
4.5. Antioxidant Activity (ORAC)
4.6. Preparation of Human Platelets
4.7. Obtaining Washed Platelets
4.8. Release of Lactate Dehydrogenase (LDH)
4.9. Platelet Aggregation
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Noncommunicable Diseases; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative stress in cardiovascular diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Masselli, E.; Pozzi, G.; Vaccarezza, M.; Mirandola, P.; Galli, D.; Vitale, M.; Carubbi, C.; Gobbi, G. ROS in platelet biology: Functional aspects and methodological insights. Int. J. Mol. Sci. 2020, 21, 4866. [Google Scholar] [CrossRef] [PubMed]
- Calvieri, C.; Tanzilli, G.; Bartimoccia, S.; Cangemi, R.; Arrivi, A.; Dominici, M.; Cammisotto, V.; Viceconte, N.; Mangieri, E.; Frati, G. Interplay between oxidative stress and platelet activation in coronary thrombus of STEMI patients. Antioxidant 2018, 7, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, E.; Malik, V.S.; Hu, F.B. Cardiovascular disease prevention by diet modification: JACC health promotion series. J. Am. Coll. Cardiol. 2018, 72, 914–926. [Google Scholar] [CrossRef]
- Lutz, M.; Fuentes, E.; Ávila, F.; Alarcón, M.; Palomo, I. Roles of phenolic compounds in the reduction of risk factors of cardiovascular diseases. Molecules 2019, 24, 366. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Pérez, L. Antiplatelet effect of Aristotelia chilensis (maqui) extracts through in vitro studies. PLoS ONE 2021, 16, e0250852. [Google Scholar] [CrossRef]
- Rojas-Garbanzo, C.; Rodríguez, L.; Pérez, A.M.; Mayorga-Gross, A.L.; Vásquez-Chaves, V.; Fuentes, E.; Palomo, I. Anti-platelet activity and chemical characterization by UPLC-DAD-ESI-QTOF-MS of the main polyphenols in extracts from Psidium leaves and fruits. Food Res. Int. 2021, 141, 110070. [Google Scholar] [CrossRef]
- Alañón, M.E.; Palomo, I.; Rodríguez, L.; Fuentes, E.; Arráez-Román, D.; Segura-Carretero, A. Antiplatelet activity of natural bioactive extracts from mango (Mangifera indica L.) and its by-products. Antioxidants 2019, 8, 517. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, K.; Xu, B. Polyphenol-rich dry common beans (Phaseolus vulgaris L.) and their health benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef] [Green Version]
- Shang, R.; Wu, H.; Guo, R.; Liu, Q.; Pan, L.; Li, J.; Hu, Z.; Chen, C. The Diversity of Four Anti-nutritional Factors in Common Bean. Hortic. Plant J. 2016, 2, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, L.; Mendez, D.; Montecino, H.; Carrasco, B.; Arevalo, B.; Palomo, I.; Fuentes, E. Role of Phaseolus vulgaris L. in the Prevention of Cardiovascular Diseases–Cardioprotective Potential of Bioactive Compounds. Plants 2022, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Alcázar-Valle, M.; Lugo-Cervantes, E.; Mojica, L.; Morales-Hernández, N.; Reyes-Ramírez, H.; Enríquez-Vara, J.N.; García-Morales, S. Bioactive compounds, antioxidant activity, and antinutritional content of legumes: A comparison between four phaseolus species. Molecules 2020, 25, 3528. [Google Scholar] [CrossRef] [PubMed]
- Mojica, L.; Meyer, A.; Berhow, M.A.; de Mejía, E.G. Bean cultivars (Phaseolus vulgaris L.) have similar high antioxidant capacity, in vitro inhibition of α-amylase and α-glucosidase while diverse phenolic composition and concentration. Food Res. Int. 2015, 69, 38–48. [Google Scholar] [CrossRef]
- Sutivisedsak, N.; Cheng, H.; Willett, J.; Lesch, W.; Tangsrud, R.; Biswas, A. Microwave-assisted extraction of phenolics from bean (Phaseolus vulgaris L.). Food Res. Int. 2010, 43, 516–519. [Google Scholar] [CrossRef]
- Al Jitan, S.; Alkhoori, S.A.; Yousef, L.F. Phenolic acids from plants: Extraction and application to human health. Stud. Nat. Prod. Chem. 2018, 58, 389–417. [Google Scholar]
- Kumar, M.; Dahuja, A.; Tiwari, S.; Punia, S.; Tak, Y.; Amarowicz, R.; Bhoite, A.G.; Singh, S.; Joshi, S.; Panesar, P.S. Recent trends in extraction of plant bioactives using green technologies: A review. Food Chem. 2021, 353, 129431. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidović, S.; Redovniković, I.R.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Pilařová, V.; Al Hamimi, S.; Cunico, L.P.; Nováková, L.; Turner, C. Extending the design space in solvent extraction–from supercritical fluids to pressurized liquids using carbon dioxide, ethanol, ethyl lactate, and water in a wide range of proportions. Green Chem. 2019, 21, 5427–5436. [Google Scholar] [CrossRef] [Green Version]
- Osorio-Tobón, J.F. Technology. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef]
- Destandau, E.; Michel, T.; Elfakir, C. Microwave-assisted extraction. Nat. Nat. Prod. Extr. Princ. Appl. 2013, 21, 113. [Google Scholar]
- Zurob, E.; Cabezas, R.; Villarroel, E.; Rosas, N.; Merlet, G.; Quijada-Maldonado, E.; Romero, J.; Plaza, A. Design of natural deep eutectic solvents for the ultrasound-assisted extraction of hydroxytyrosol from olive leaves supported by COSMO-RS. Sep. Purif. Technol. 2020, 248, 117054. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Gan, R.-Y.; Ge, Y.-Y.; Zhang, D.; Corke, H. Ultrasonic treatment increases extraction rate of common bean (Phaseolus vulgaris L.) antioxidants. Antioxidants 2019, 8, 83. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Azua, R.; Quinteros, E.F.; Olate-Briones, A.; Moore-Carrasco, R. Phaseolus vulgaris Exerts an Inhibitory Effect on Platelet Aggregation through AKT Dependent Way. Prev. Nutr. Food Sci. 2018, 23, 102–107. [Google Scholar] [CrossRef]
- Gupta, R.B.; Shim, J.-J. Solubility in Supercritical Carbon Dioxide; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Cassol, L.; Rodrigues, E.; Zapata Noreña, C.P. Extracting phenolic compounds from Hibiscus sabdariffa L. calyx using microwave assisted extraction. Ind. Crops Prod. 2019, 133, 168–177. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Sachdev, A.; Kaur, C.; Varghese, E.; Saha, S.; Sairam, K. Evaluation of enzyme and microwave-assisted conditions on extraction of anthocyanins and total phenolics from black soybean (Glycine max L.) seed coat. Int. J. Biol. Macromol. 2019, 135, 1070–1081. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Alejandro, R.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2021, 378, 131918. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Dong, W.; Wei, C.; Hu, R.; Long, Y. Combining integrated ultrasonic-microwave technique with ethanol to maximise extraction of green coffee oil from Arabica coffee beans. Ind. Crops Prod. 2020, 151, 112405. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, B.; Li, H.; Miao, S.; Zheng, B. Optimization of ultrasound-microwave synergistic extraction of prebiotic oligosaccharides from sweet potatoes (Ipomoea batatas L.). Innov. Food Sci. Emerg. Technol. 2019, 54, 51–63. [Google Scholar] [CrossRef]
- Mellinas, A.C.; Jiménez, A.; Garrigós, M.C. Optimization of microwave-assisted extraction of cocoa bean shell waste and evaluation of its antioxidant, physicochemical and functional properties. LWT 2020, 127, 109361. [Google Scholar] [CrossRef]
- Aware, C.B.; Patil, R.R.; Vyavahare, G.D.; Gurme, S.T.; Jadhav, J.P. Ultrasound-assisted aqueous extraction of phenolic, flavonoid compounds and antioxidant activity of Mucuna macrocarpa beans: Response surface methodology optimization. J. Am. Coll. Nutr. 2019, 38, 364–372. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R.; et al. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, X.-Y.; Gan, R.-Y.; Zheng, J.; Li, Y.; Zhang, J.-J.; Xu, D.-P.; Li, H.-B. Optimization of ultrasound-assisted extraction of antioxidant polyphenols from the seed coats of red sword bean (Canavalia gladiate (Jacq.) DC.). Antioxidants 2019, 8, 200. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez Madrera, R.; Campa Negrillo, A.; Suárez Valles, B.; Ferreira Fernández, J. Phenolic Content and Antioxidant Activity in Seeds of Common Bean (Phaseolus vulgaris L.). Foods 2021, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Fernandez, X.; Yousef, G.G.; Loarca-Pina, G.; De Mejia, E.; Lila, M.A. Characterization of polyphenolics in the seed coat of Black Jamapa bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 2005, 53, 4615–4622. [Google Scholar] [CrossRef]
- Nakashima, S.; Koike, T.; Nozawa, Y. Genistein, a protein tyrosine kinase inhibitor, inhibits thromboxane A2-mediated human platelet responses. Mol. Pharmacol. 1991, 39, 475–480. [Google Scholar]
- Luceri, C.; Giannini, L.; Lodovici, M.; Antonucci, E.; Abbate, R.; Masini, E.; Dolara, P. p-Coumaric acid, a common dietary phenol, inhibits platelet activity in vitro and in vivo. Br. J. Nutr. 2007, 97, 458–463. [Google Scholar] [CrossRef] [Green Version]
- Ostertag, L.M.; O’Kennedy, N.; Horgan, G.W.; Kroon, P.A.; Duthie, G.G.; de Roos, B. In vitro anti-platelet effects of simple plant-derived phenolic compounds are only found at high, non-physiological concentrations. Mol. Nutr. Food Res. 2011, 55, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Caballero, J.; Alarcón, M.; Rojas, A.; Palomo, I. Chlorogenic acid inhibits human platelet activation and thrombus formation. PLoS ONE 2014, 9, e90699. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.-J.; Kang, H.-J.; Kim, Y.-J.; Lee, D.-H.; Kwon, H.-W.; Kim, Y.-Y.; Park, H.-J. Inhibition of platelet aggregation by chlorogenic acid via cAMP and cGMP-dependent manner. Blood Coagul. Fibrinolysis 2012, 23, 629–635. [Google Scholar] [CrossRef]
- Hong, Q.; Ma, Z.-C.; Huang, H.; Wang, Y.-G.; Tan, H.-L.; Xiao, C.-R.; Liang, Q.-D.; Zhang, H.-T.; Gao, Y. Antithrombotic activities of ferulic acid via intracellular cyclic nucleotide signaling. Eur. J. Pharmacol. 2016, 777, 1–8. [Google Scholar] [CrossRef]
- Prince, P.S.M.; Roy, A.J. p-Coumaric acid attenuates apoptosis in isoproterenol-induced myocardial infarcted rats by inhibiting oxidative stress. Int. J. Cardiol. 2013, 168, 3259–3266. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, M.; El-Mahdy, M.; Abd-Ellah, M.; Helal, G.; Khalifa, F.; Hamada, F. Influence of p-coumaric acid on doxorubicin-induced oxidative stress in rat’s heart. Pharmacol. Res. 2003, 48, 461–465. [Google Scholar] [CrossRef]
- Wan, Y.J.; Zhuang, P.W.; Zhang, Y.J. Antithrombotic activity of Formononetin sodium and its mechanism. Chin. J. New Drugs 2016, 25, 1355–1358. [Google Scholar]
- Landolfi, R.; Mower, R.L.; Steiner, M. Modification of platelet function and arachidonic acid metabolism by bioflavonoids: Structure-activity relations. Biochem. Pharmacol. 1984, 33, 1525–1530. [Google Scholar] [CrossRef]
- Zang, B.; Jin, M.; Wu, W.; Chen, W.; Piao, Y.; Li, J. Antagonistic effect of myricetin on platelet activing factor. Cancer Metastasis Rev. 2003, 38, 831–833. [Google Scholar]
- Gottstein, N.; Ewins, B.A.; Eccleston, C.; Hubbard, G.P.; Kavanagh, I.C.; Minihane, A.-M.; Weinberg, P.D.; Rimbach, G. Effect of genistein and daidzein on platelet aggregation and monocyte and endothelial function. Br. J. Nutr. 2003, 89, 607–615. [Google Scholar] [CrossRef]
- Zhu, H.; Zou, L.; Tian, J.; Lin, F.; He, J.; Hou, J. Protective effects of sulphonated formononetin in a rat model of cerebral ischemia and reperfusion injury. Plant Med. 2014, 80, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, Y.; Yatomi, Y.; Jinnai, Y.; Kume, S. Effects of genistein, a tyrosine kinase inhibitor, on platelet functions: Genistein attenuates thrombin-induced Ca2+ mobilization in human platelets by affecting polyphosphoinositide turnover. Biochem. Pharmacol. 1993, 46, 395–403. [Google Scholar] [CrossRef]
- Dhar, A.; Paul, A.; Shukla, S. Platelet-activating factor stimulation of tyrosine kinase and its relationship to phospholipase C in rabbit platelets: Studies with genistein and monoclonal antibody to phosphotyrosine. Mol. Pharmacol. 1990, 37, 519–525. [Google Scholar]
- Chtourou, Y.; Kamoun, Z.; Zarrouk, W.; Kebieche, M.; Kallel, C.; Gdoura, R.; Fetoui, H. Naringenin ameliorates renal and platelet purinergic signalling alterations in high-cholesterol fed rats through the suppression of ROS and NF-κB signaling pathways. Food Funct. 2016, 7, 183–193. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, Z.; Reheman, A.; Jin, J.W.; Li, C.; Wang, Y.; Andrews, M.C.; Chen, P.; Zhu, G.; Ling, W. Plant food delphinidin-3-glucoside significantly inhibits platelet activation and thrombosis: Novel protective roles against cardiovascular diseases. PLoS ONE 2012, 7, e37323. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Chen, Y.; Adili, R.; McKeown, T.; Chen, P.; Zhu, G.; Li, D.; Ling, W.; Ni, H.; Yang, Y. Plant-based food cyanidin-3-glucoside modulates human platelet glycoprotein VI signaling and inhibits platelet activation and thrombus formation. J. Nutr. 2017, 147, 1917–1925. [Google Scholar] [CrossRef] [Green Version]
- Song, F.; Zhu, Y.; Shi, Z.; Tian, J.; Deng, X.; Ren, J.; Andrews, M.C.; Ni, H.; Ling, W.; Yang, Y. Plant food anthocyanins inhibit platelet granule secretion in hypercholesterolaemia: Involving the signalling pathway of PI3K–Akt. Thromb. Haemost. 2014, 112, 981–991. [Google Scholar] [CrossRef]
- Zang, L.-Y.; Cosma, G.; Gardner, H.; Shi, X.; Castranova, V.; Vallyathan, V. Effect of antioxidant protection by p-coumaric acid on low-density lipoprotein cholesterol oxidation. Am. J. Physiol.-Cell Physiol. 2000, 279, C954–C960. [Google Scholar] [CrossRef] [Green Version]
- Aboonabi, A.; Meyer, R.R.; Gaiz, A.; Singh, I. Anthocyanins in berries exhibited anti-atherogenicity and antiplatelet activities in a metabolic syndrome population. Nutr. Res. 2020, 76, 82–93. [Google Scholar] [CrossRef]
- Fuentes, E.; Wehinger, S.; Trostchansky, A. Regulation of Key Antiplatelet Pathways by Bioactive Compounds with Minimal Bleeding Risk. Int. J. Mol. Sci. 2021, 22, 12380. [Google Scholar] [CrossRef]
- Méndez, D.; Arauna, D.; Fuentes, F.; Araya-Maturana, R.; Palomo, I.; Alarcón, M.; Sebastián, D.; Zorzano, A.; Fuentes, E. Mitoquinone (MitoQ) inhibits platelet activation steps by reducing ROS levels. Int. J. Mol. Sci. 2020, 21, 6192. [Google Scholar] [CrossRef]
- Fuentes, E.; Palomo, I. Relationship between platelet PPARs, cAMP Levels, and P-selectin expression: Antiplatelet activity of natural products. Evid.-Based Complement. Altern. Med. 2013, 2013, 861786. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, E.; Palomo, I. Antiplatelet effects of natural bioactive compounds by multiple targets: Food and drug interactions. J. Funct. Foods 2014, 6, 73–81. [Google Scholar] [CrossRef]
- González, B.; Vogel, H.; Razmilic, I.; Wolfram, E. Polyphenol, anthocyanin and antioxidant content in different parts of maqui fruits (Aristotelia chilensis) during ripening and conservation treatments after harvest. Ind. Crops Prod. 2015, 76, 158–165. [Google Scholar] [CrossRef]
- Céspedes, C.L.; El-Hafidi, M.; Pavon, N.; Alarcon, J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), Maqui. Food Chem. 2008, 107, 820–829. [Google Scholar] [CrossRef]
- Martino, H.S.D.; Bigonha, S.M.; Cardoso, L.D.M.; Rosa, C.D.O.B.; Costa, N.M.B.; Cárdenas, L.D.L.Á.R.; Ribeiro, S.M.R. Nutritional and bioactive compounds of bean: Benefits to human health. In Hispanic Foods: Chemistry and Bioactive Compounds; ACS Publications: Washington, DC, USA, 2012; pp. 233–258. [Google Scholar]
- Llompart, M.; Celeiro, M.; Dagnac, T. Microwave-assisted extraction of pharmaceuticals, personal care products and industrial contaminants in the environment. TrAC Trends Anal. Chem. 2019, 116, 136–150. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Cánovas, J.; Barrajón-Catalán, E.; Micol, V.; Cárcel, J.A.; García-Pérez, J.V. Kinetic and compositional study of phenolic extraction from olive leaves (var. Serrana) by using power ultrasound. Innov. Food Sci. Emerg. Technol. 2013, 17, 120–129. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, S.; Lee, H.S.; Kim, B.-K.; Ohuchi, K.; Shin, K.H. Inhibitory effects of isorhamnetin-3-O-β-D-glucoside from Salicornia herbacea on rat lens aldose reductase and sorbitol accumulation in streptozotocin-induced diabetic rat tissues. Biol. Pharm. Bull. 2005, 28, 916–918. [Google Scholar] [CrossRef] [Green Version]
- Zapata Díez, C.D.; Zapata Ocampo, P.A. Estandarización del Método ORAC Como Herramienta Básica de Análisis de la Capacidad Antioxidante de Diversas Sustancias. 2019. Available online: repository.ces.edu.co (accessed on 5 April 2022).
- Ou, B.; Chang, T.; Huang, D.; Prior, R.L. Determination of total antioxidant capacity by oxygen radical absorbance capacity (ORAC) using fluorescein as the fluorescence probe: First action 2012.23. J. AOAC Int. 2013, 96, 1372–1376. [Google Scholar] [CrossRef]
- Rodriguez, L.; Badimon, L.; Mendez, D.; Padro, T.; Vilahur, G.; Pena, E.; Carrasco, B.; Vogel, H.; Palomo, I.; Fuentes, E. Antiplatelet Activity of Isorhamnetin via Mitochondrial Regulation. Antioxidants 2021, 10, 666. [Google Scholar] [CrossRef]
- Mendez, D.; Urra, F.A.; Millas-Vargas, J.P.; Alarcon, M.; Rodriguez-Lavado, J.; Palomo, I.; Trostchansky, A.; Araya-Maturana, R.; Fuentes, E. Synthesis of antiplatelet ortho-carbonyl hydroquinones with differential action on platelet aggregation stimulated by collagen or TRAP-6. Eur. J. Med. Chem. 2020, 192, 112187. [Google Scholar] [CrossRef]
- Mendez, D.; Donoso-Bustamante, V.; Pablo Millas-Vargas, J.; Pessoa-Mahana, H.; Araya-Maturana, R.; Fuentes, E. Synthesis and pharmacological evaluation of acylhydroquinone derivatives as potent antiplatelet agents. Biochem. Pharmacol. 2021, 183, 114341. [Google Scholar] [CrossRef]
- Rojas-Garbanzo, C. Psidium Fruits: Endemic fruits of latin America with a wide variety of phytochemicals. Ann. Nutr. Food Sci. 2018, 2, 1016. [Google Scholar]
- Yang, Q.Q.; Gan, R.Y.; Ge, Y.Y.; Zhang, D.; Corke, H. Polyphenols in common beans (Phaseolus vulgaris L.): Chemistry, analysis, and factors affecting composition. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1518–1539. [Google Scholar] [CrossRef] [Green Version]
- Ombra, M.N.; d’Acierno, A.; Nazzaro, F.; Riccardi, R.; Spigno, P.; Zaccardelli, M.; Pane, C.; Maione, M.; Fratianni, F. Phenolic composition and antioxidant and antiproliferative activities of the extracts of twelve common bean (Phaseolus vulgaris L.) endemic ecotypes of Southern Italy before and after cooking. Oxidative Med. Cell. Longev. 2016, 2016, 1398298. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Singh, N.; Thakur, S.; Kaur, A. Ultrasound assisted extraction of polyphenols and their distribution in whole mung bean, hull and cotyledon. J. Food Sci. Technol. 2017, 54, 921–932. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Cai, W.; Wu, T.; Xu, B. Phytochemical distribution in hull and cotyledon of adzuki bean (Vigna angularis L.) and mung bean (Vigna radiate L.), and their contribution to antioxidant, anti-inflammatory and anti-diabetic activities. Food Chem. 2016, 201, 350–360. [Google Scholar] [CrossRef]


| Landraces | Extraction Technique | Soluble Solid Content | TPC (mg GAE/g) | ORAC μmolTE/g | TRAP-6 10 µM PA (%) | ADP 4 µM PA (%) |
|---|---|---|---|---|---|---|
| Arauco | MAE | 1.50 ± 0.00 a | 572.58 ± 10.21 a | 22,725.36 ± 691.51 a | 26.8 ± 1.2 a | 71.3 ± 1.5 a |
| UAE | 1.33 ± 0.06 a | 464.94 ± 10.36 a | 9048.36 ± 289.97 b | 49.1 ± 3.5 b | 71.0 ± 2.5 a | |
| Blanco Español | MAE | 0.93 ± 0.06 b | 241.94 ± 18.17 b | 4709.13 ± 1233.93 c | 23.8 ± 2.6 a | 75.7 ± 2.2 ab |
| UAE | 0.97 ± 0.06 b | 280.27 ± 46.02 b | 2721.33 ± 257.49 c | 36.2 ± 3.0 ab | 71.5 ± 3.0 a | |
| Bombero | MAE | 1.00 ± 0.17 b | 311.73 ± 4.42 ba | 3775.75 ± 966.34 c | 77.5 ± 3.1 c | 92.3 ± 0.8 cb |
| UAE | 0.90 ± 0.06 b | 227 ± 22.95 b | 2821.35 ± 70.83 c | 17.8 ± 2.4 a | 71.5 ± 2.4 a | |
| Cabrita | MAE | 1.00 ± 0.10 b | 264.66 ± 11.62 ba | 8045.22 ± 600.05 b | 36.3 ± 3.1 ab | 84.7 ± 0.8 b |
| UAE | 1.27 ± 0.06 ab | 437.89 ± 36.74 a | 14,489.49 ± 344.15 db | 65.0 ± 3.2 cb | 87.9 ± 1.4 b | |
| Cimarrón | MAE | 2.03 ± 0.06 c | 520.47 ± 31.28 a | 2722.64 ± 932.78 c | 79.1 ± 3.4 c | 73.8 ± 3.8 a |
| UAE | 2.27 ± 0.06 c | 352.27 ± 34.03 ba | 9612.22 ± 534.08 b | 78.6 ± 2.4 c | 84.2 ± 1.3 b | |
| Cisne | MAE | 1.07 ± 0.06 ab | 220.08 ± 11.98 b | 3657.96 ± 386.98 c | 60.1 ± 4.1 cb | 80.8 ± 2.5 ba |
| UAE | 0.87 ± 0.06 b | 246.85 ± 17.66 ba | 3310.31 ± 301.25 c | 72.4 ± 3.7 c | 86.5 ± 1.2 b | |
| Coscorrón | MAE | 1.27 ± 0.06 ab | 280.06 ± 38.53 ba | 2704.07 ± 482.92 c | 7.9 ± 0.4 d | 73.8 ± 1.9 a |
| UAE | 0.97 ± 0.06 b | 225.19 ± 11.30 ba | Nd | 54.9 ± 1.0 b | 84.8 ± 0.8 b | |
| Frutilla | MAE | 1.10 ± 0.00 ba | 414.45 ± 29.54 a | 21,630.05 ± 260.90 a | 21.3 ± 1.1 a | 82.1 ± 1.8 b |
| UAE | 1.07 ± 0.06 b | 383.32 ± 39.47 ba | 14,238.93 ± 910.50 db | 50.3 ± 1.7 b | 85.4 ± 0.6 b | |
| Ganso | MAE | 1.70 ± 0.10 a | 431.83 ± 34.86 a | 9375.30 ± 1722.49 b | 17.1 ± 2.7 a | 74.1 ± 1.2 a |
| UAE | 1.47 ± 0.06 a | 318.96 ± 27.29 ba | 3313.60 ± 351.80 c | 69.8 ± 3.9 c | 82.3 ± 0.7 b | |
| Hallado Alemán | MAE | 1.00 ± 0.00 b | 541.85 ± 33.37 a | 17,682.97 ± 248.60 d | 85.8 ± 0.7 c | 81.8 ± 1.2 b |
| UAE | 0.93 ± 0.15 b | 337.91 ± 19.47 ba | 11,321.46 ± 987.80 bd | 11.0 ± 0.9 ad | 73.3 ± 2.9 a | |
| Lunatus | MAE | 1.53 ± 0.06 a | 283.09 ± 69.11 ba | 5857.60 ± 2350.40 b | 86.8 ± 0.8 c | 90.8 ± 0.4 cb |
| UAE | 1.47 ± 0.12 a | 298.27 ± 26.91 ba | 797.13 ± 158.30 c | 7.8 ± 1.4 d | 72.6 ± 1.6 a | |
| Manteca | MAE | 1.53 ± 0.06 a | 603.89 ± 6.83 ac | 12,561.54 ± 1096.83 bd | 55.2 ± 1.3 b | 79.8 ± 1.4 ab |
| UAE | 1.00 ± 0.00 b | 321.96 ± 5.92 ba | 2867.47 ± 214.95 c | 78.5 ± 2.9 c | 87.9 ± 1.2 bc | |
| Mantequilla | MAE | 1.50 ± 0.00 a | 308.89 ± 9.40 ba | 13,721.58 ± 3113.94 db | 22.3 ± 1.8 a | 76.7 ± 2.4 ab |
| UAE | 0.93 ± 0.06 b | 340.85 ± 22.87 ba | 3976.00 ± 463.28 c | 56.3 ± 1.3 b | 76.4 ± 1.8 ab | |
| Negro | MAE | 1.30 ± 0.00 a | 806.90 ± 6.66 c | 16,659.61 ± 158.45 d | 7.8 ± 1.2 d | 73.6 ± 2.0 a |
| UAE | 1.00 ± 0.10 b | 543.40 ± 12.02 a | 7243.07 ± 964.23 b | 25.3 ± 1.4 a | 84.0 ± 0.9 bc | |
| Negro Arauco | MAE | 1.27 ± 0.02 a | 345.89 ± 47.58 ba | 8955.42 ± 516.75 b | 28.5 ± 2.8 a | 72.8 ± 2.0 a |
| UAE | 1.17 ± 0.02 ba | 323.62 ± 77.94 ba | 3481.76 ± 759.27 c | 47.0 ± 1.7 b | 78.0 ± 1.4 ab | |
| Pajarito | MAE | 1.33 ± 0.06 a | 594.36 ± 8.80 a | 18,723.60 ± 1700.55 ad | 56.7 ± 0.9 b | 84.1 ± 1.1 bc |
| UAE | 1.23 ± 0.06 a | 485.70 ± 18.84 a | 12,904.29 ± 968.70 bd | 78.0 ± 2.8 c | 87.7 ± 1.3 bc | |
| Palo | MAE | 1.30 ± 0.10 a | 423.06 ± 27.19 a | 17,828.49 ± 826.24 d | 85.4 ± 0.3 c | 81.0 ± 3.3 bc |
| UAE | 1.33 ± 0.06 a | 391.23 ± 50.86 ba | 16,179.95 ± 2142.43 d | 23.5 ± 1.5 a | 71.1 ± 3.4 a | |
| Pallar Manchado | MAE | 0.93 ± 0.06 b | 316.52 ± 93.50 ba | 15,983.88 ± 674.45 d | 20.9 ± 1.0 a | 88.0 ± 1.3 bc |
| UAE | 0.90 ± 0.00 b | 374.63 ± 53.69 ba | 9331.82 ± 891.31 b | 18.7 ± 1.1 a | 74.0 ± 0.6 a | |
| Pallar Morado | MAE | 1.17 ± 0.06 ab | 795.95 ± 29.97 c | 11,233.61 ± 1702.32 b | 31.9 ± 0.6 ab | 73.5 ± 2.6 a |
| UAE | 1.03 ± 0.06 b | 495.63 ± 39.09 a | 10,993.16 ± 476.40 b | 20.8 ± 1.0 a | 68.0 ± 1.6 a | |
| Peumo | MAE | 1.40 ± 0.10 a | 866.17 ± 30.28 c | 25,642.98 ± 2396.79 a | 46.1 ± 6.2 b | 81.8 ± 1.9 b |
| UAE | 0.90 ± 0.00 b | 720.52 ± 18.43 c | 22,596.47 ± 3452.67 a | 81.7 ± 1.7 c | 82.9 ± 0.5 b | |
| Rojo | MAE | 1.00 ± 0.00 b | 590.86 ± 24.25 a | 12,809.11 ± 976.94 b | 35.5 ± 2.7 ab | 75.0 ± 2.0 ba |
| UAE | 0.90 ± 0.10 b | 518.22 ± 11.90 a | 6535.65 ± 1872.82 b | 57.1 ± 7.5 bc | 69.0 ± 7.0 a | |
| Sapito | MAE | 1.17 ± 0.06 ab | 265.30 ± 12.93 ba | 5184.58 ± 359.74 b | 69.6 ± 4.7 c | 69.0 ± 4.8 a |
| UAE | 1.13 ± 0.06 ab | 232.68 ± 4.14 ba | 4345.33 ± 26.73 c | 79.4 ± 3.0 c | 87.8 ± 0.9 bc | |
| Soja | MAE | 1.83 ± 0.06 a | 893.45 ± 87.30 c | 35,642.85 ± 2588.88 e | 22.4 ± 2.8 a | 75.2 ± 2.2 ab |
| UAE | 1.63 ± 0.06 a | 696.85 ± 21.06 ca | 19,820.36 ± 1474.80 a | 28.1 ± 2.9 a | 74.0 ± 1.9 a | |
| Torcaza | MAE | 1.57 ± 0.06 a | 349.19 ± 16.56 ab | 5720.66 ± 145.28 b | 27.5 ± 2.0 a | 78.7 ± 3.4 ab |
| UAE | 1.63 ± 0.12 a | 302.92 ± 15.42 ba | 3337.06 ± 888.64 c | 48.0 ± 0.7 b | 66.2 ± 3.9 a | |
| Tórtola | MAE | 1.90 ± 0.00 c | 327.65 ± 24.89 ba | 13,617.59 ± 1519.75 bd | 12.0 ± 1.3 da | 78.2 ± 1.7 ab |
| UAE | 1.00 ± 0.10 b | 284.80 ± 6.42 ba | 3561.15 ± 880.60 c | 75.06 ± 2.9 c | 80.8 ± 1.2 b |
| Extracts | Cyanidin-3-glucoside (mg/100 g of Sample) |
|---|---|
| MAE Pallar Morado | 25.81 ± 0.02 |
| MAE Ganso | 24.86 ± 0.04 |
| UAE Bombero | 23.58 ± 0.03 |
| MAE Tortola | 12.72 ± 0.02 |
| MAE Soja | 12.33 ± 0.03 |
| UAE Palo | 10.58 ± 0.01 |
| MAE Pallar Manchado | 9.83 ± 0.01 |
| MAE Negro Arauco | 9.27 ± 0.01 |
| UAE negro | 4.42 ± 0.01 |
| UAE Blanco Español | 0.86 ± 0.00 |
| Bioactive Compound | Cardioprotective Effect and/or Mechanisms | Reference | |
|---|---|---|---|
| Phenolic acids (coumaric, chlorogenic, ferulic, cinnamic, sinapic, protocatechuic, vanillic, syringic acid) | Inhibit platelet adhesion and/or aggregation | [48,49,50,51,52] | |
| Inhibit LDL oxidation | [66] | ||
| Protects the heart against oxidative stress | [53,54] | ||
| Inhibits platelet inflammatory mediators | [50] | ||
| Increases cAMP levels | [50,51,52] | ||
| Inhibit P-selectin expression | [49,53] | ||
| activation of cAMP and cGMP signaling pathways | [50,51,52] | ||
| Flavonoids (Myricerin, Genistein, Glycitein, Formononetin, Naringenin, Hesperetin, Daidzein, Catechin, Kaemferol-3-glucoside, Taxifolin, Apigenin-7 Glycoside, Luteolin-7 Glycoside) | Inhibit platelet aggregation induced by several agonists | [55,56,57,58] | |
| Inhibit PI3 production | [59,60,61] | ||
| Protect against cerebral ischemia and reperfusion injury in rats and enhances cerebrovascular angiogenesis in human umbilical vein endothelial cells | [59] | ||
| Inhibits levels of inflammatory markers | [62] | ||
| Increases cAMP levels | [56] | ||
| Anthocyanins (Delphinidin, Petunidin, Malvidin, Cyanidin-3-O-glucoside, Pelargonidin-3-O-glucoside) | Inhibit platelet aggregation induced by several agonists | [63,67] | |
| Inhibit the secretion of alpha and dense granules | [65] | ||
| Inhibit PI3K/Akt activation, eNOS phosphorylation and cGMP production | [63] | ||
| Inhibit P-selectin expression | [63,64,65] | ||
| Chilean Phaseolus vulgaris L. Landraces | Country | Region | Village | GPS Coordinate |
|---|---|---|---|---|
| Bombero, Cimarrón, Cisne, Ganso, Tórtola, Lunatus | Chile | Maule | Curepto, Maule | −34°.99′09″34, −72°.01′56″31 |
| Arauco, Palo, Pallar Manchado, Pallar Morado, Negro Arauco, Hallado Alemán, Manteca | Chile | Maule | Hualañe | −35°.01′44″19, −71°.73′94″57 |
| Cabrita, Coscorrón, Pajarito, Frutilla, Peumo, Rojo, Sapito | Chile | Ñuble | Chanco, Cobquecura | −36°.28′66″29, −72°.71′92″36 |
| Blanco Español, Torcaza, Negro, Mantequilla | Chile | Maule | Longaví | −35°.96′80″49, −71°.71′36″48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, L.; Plaza, A.; Méndez, D.; Carrasco, B.; Tellería, F.; Palomo, I.; Fuentes, E. Antioxidant Capacity and Antiplatelet Activity of Aqueous Extracts of Common Bean (Phaseolus vulgaris L.) Obtained with Microwave and Ultrasound Assisted Extraction. Plants 2022, 11, 1179. https://doi.org/10.3390/plants11091179
Rodríguez L, Plaza A, Méndez D, Carrasco B, Tellería F, Palomo I, Fuentes E. Antioxidant Capacity and Antiplatelet Activity of Aqueous Extracts of Common Bean (Phaseolus vulgaris L.) Obtained with Microwave and Ultrasound Assisted Extraction. Plants. 2022; 11(9):1179. https://doi.org/10.3390/plants11091179
Chicago/Turabian StyleRodríguez, Lyanne, Andrea Plaza, Diego Méndez, Basilio Carrasco, Francisca Tellería, Iván Palomo, and Eduardo Fuentes. 2022. "Antioxidant Capacity and Antiplatelet Activity of Aqueous Extracts of Common Bean (Phaseolus vulgaris L.) Obtained with Microwave and Ultrasound Assisted Extraction" Plants 11, no. 9: 1179. https://doi.org/10.3390/plants11091179
APA StyleRodríguez, L., Plaza, A., Méndez, D., Carrasco, B., Tellería, F., Palomo, I., & Fuentes, E. (2022). Antioxidant Capacity and Antiplatelet Activity of Aqueous Extracts of Common Bean (Phaseolus vulgaris L.) Obtained with Microwave and Ultrasound Assisted Extraction. Plants, 11(9), 1179. https://doi.org/10.3390/plants11091179