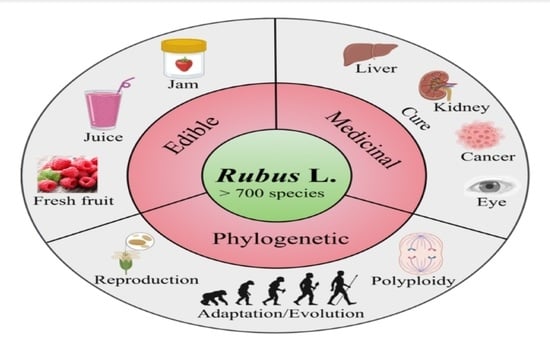
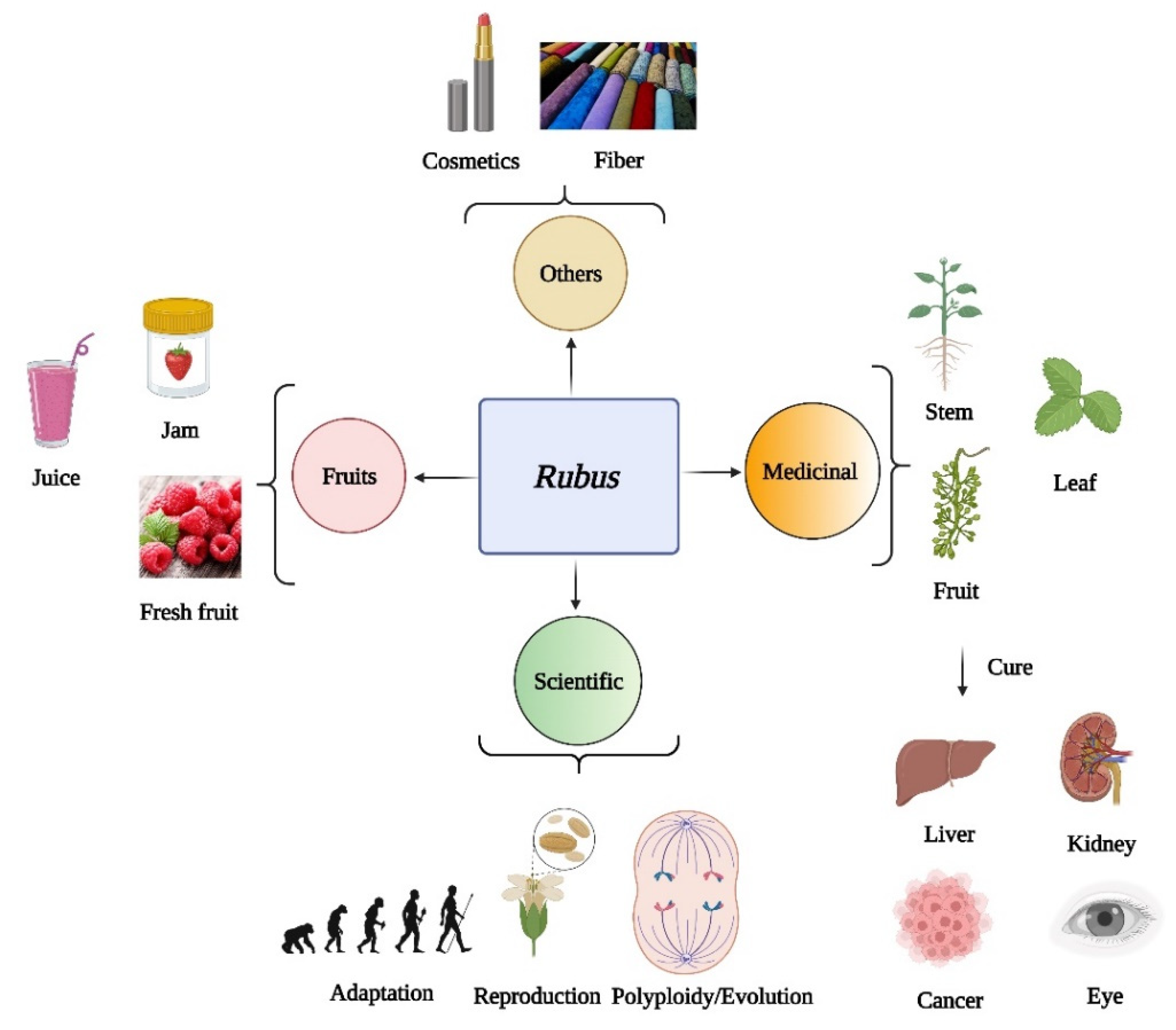
Study on Supergenus Rubus L.: Edible, Medicinal, and Phylogenetic Characterization
Abstract
:1. Introduction
2. Studies of Edible Rubus Species
3. Medicinal Studies of Rubus
| Secondary Metabolite | Species | Concentration * (mg/100 g) | Part | References |
|---|---|---|---|---|
| Anthocyanin | R. chingii | 2.1~326 | Leaf | [20,39,40,105,106] |
| R. fruticosus | Fruit | |||
| R. ideaus | ||||
| R. hirsutus | ||||
| Flavonoid | R. chingii | 2.8~6 | Leaf | [20,34,40,107] |
| R. occidentalis | Fruit | |||
| Phenolic compounds | R. chingii | 13.7~1541 | Root | [19,20,39,40,48,105,106,108] |
| R. occidentalis | Stem | |||
| R. setchuenensis | Leaf | |||
| Flower | ||||
| Fruit | ||||
| Organic acids | R. chingii | 0.2~52.9 | Stem | [20,109] |
| R. coreanus | Leaf | |||
| Fruit | ||||
| Glycoprotein | R. chingii | 14.6~81.4 | Fruit | [63] |
4. Phylogenetic Studies of Rubus
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Focke, W.O. Species Ruborum Monographiae Generis Rubi Prodromus; Bibliotheca Botanica; E. Schweizerbart: Stuttgart, Germany, 1910. [Google Scholar]
- Focke, W.O. Species Ruborum Monographiae Generis Rubi Prodromus; Bibliotheca Botanica; E. Schweizerbart: Stuttgart, Germany, 1911. [Google Scholar]
- Focke, W.O. Species Ruborum Monographiae Generis Rubi Prodromus; Bibliotheca Botanica; E. Schweizerbart: Stuttgart, Germany, 1914. [Google Scholar]
- Jennings, D. Raspberries and Blackberries: Their Breeding, Disease and Growth; Academic Press: New York, NY, USA, 1988. [Google Scholar]
- Janick, J.; Moore, J.N. Fruit Breeding, Vine and Small Fruits; Wiley: New York, NY, USA, 1996; Volume II, pp. 109–190. [Google Scholar]
- Lu, L.T.; Gu, C.Z.; Li, C.L.; Alexander, C.; Bartholomew, B.; Brach, A.R.; Boufford, D.E.; Ikeda, H.; Ohba, H.; Robertson, S.A.; et al. Rubus Linnaeus. Flora China 2003, 9, 195–285. [Google Scholar]
- Alice, L.A.; Campbell, C.S. Phylogeny of Rubus (rosaceae) based on nuclear ribosomal DNA internal transcribed spacer region sequences. Am. J. Bot. 1999, 86, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.A.; Liston, A.; Bassil, N.V.; Alice, L.A.; Bushakra, J.M.; Sutherland, B.L.; Mockler, T.C.; Bryant, D.W.; Hummer, K.E. Target Capture Sequencing Unravels Rubus Evolution. Front. Plant Sci. 2019, 10, 1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.T. A study on the genus Rubus of China. Acta Phytotaxon. Sin. 1983, 21, 13–25. [Google Scholar]
- Kalkman, C. The phylogeny of the Rosaceae. Bot. J. Linn. Soc. 1988, 98, 37–59. [Google Scholar] [CrossRef]
- Gu, Y.; Sun, Z.J.; Cai, J.H.; Huang, Y.S.; He, S.A. Introduction and utilization of small fruits in China, with special refercence to Rubus species. Acta Hortic. 1989, 262, 47–56. [Google Scholar] [CrossRef]
- Foster, T.M.; Bassil, N.V.; Dossett, M.; Leigh Worthington, M.; Graham, J. Genetic and genomic resources for Rubus breeding: A roadmap for the future. Hortic. Res. 2019, 6, 116. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Luo, Z.; Wang, W.; Li, Y.; Zhou, Y.; Shi, Y. Rubus chingii Hu: A Review of the Phytochemistry and Pharmacology. Front. Pharmacol. 2019, 10, 799. [Google Scholar] [CrossRef]
- Zhu, Y.A.; Li, C.; Wang, S.; Lei, L.; Xiao, J. The complete chloroplast genome of Rubus setchuenensis, an edible and medicinal dual-purpose wild plant. Mitochondrial DNA Part B Resour. 2022, 7, 228–230. [Google Scholar] [CrossRef]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, rubus, and ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef]
- Guo, Q.L.; Gao, J.Y.; Yang, J.S. Analysis of Bioactive Triterpenes from Rubus chingii by Cyclodextrin-Modified Capillary Electrophoresis. Chromatographia 2005, 62, 145–150. [Google Scholar] [CrossRef]
- Guo, Q.L.; Yang, J.S.; Liu, J.X. Studies on Chemical Constituents in Fruits of Rubus chingii. Chin. Pharm. J. 2007, 42, 1141–1143. [Google Scholar]
- Hummer, K.E.; Janick, J. Rubus Iconography: Antiquity to the Renaissance. Acta Hortic. 2007, 759, 89–106. [Google Scholar] [CrossRef]
- Kaume, L.; Howard, L.R.; Devareddy, L. The blackberry fruit: A review on its composition and chemistry, metabolism and bioavailability, and health benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lei, T.; Han, G.; Yue, J.; Zhang, X.; Yang, Q.; Ruan, H.; Gu, C.; Zhang, Q.; Qian, T.; et al. The chromosome-scale reference genome of Rubus chingii Hu provides insight into the biosynthetic pathway of hydrolyzable tannins. Plant J. 2021, 107, 1466–1477. [Google Scholar] [CrossRef]
- Sun, N.; Wang, Y.; Liu, Y.; Guo, M.-L.; Yin, J. A new ent-labdane diterpene saponin from the fruits of Rubus chingii. Chem. Nat. Compd. 2013, 49, 49–53. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Yang, L.; Jiang, J.-G. Bioactive comparison of main components from unripe fruits of Rubus chingii Hu and identification of the effective component. Food Funct. 2015, 6, 2205–2214. [Google Scholar] [CrossRef]
- Zhong, R.; Guo, Q.; Zhou, G.; Fu, H.; Wan, K. Three new labdane-type diterpene glycosides from fruits of Rubus chingii and their cytotoxic activities against five humor cell lines. Fitoterapia 2015, 102, 23–26. [Google Scholar] [CrossRef]
- Li, K.; Xia, X.X.; Ding, Y.H.; Zhou, W.Y.; Xie, B.S.; Cai, J.B.; Xie, Y.H.; Huang, L.P. Effect of Different Extract Parts From Rubi Fructus on Improving Memory Disorder in Mice. Chin. J. Exp. Tradit. Med. Formulae 2016, 22, 142–147. [Google Scholar] [CrossRef]
- Li, K.Y.; Zeng, M.L.; Li, Q.L.; Zhou, B.H. Identification of polyphenolic composition in the fruits of Rubus chingii Hu and its antioxidant and antiproliferative activity on human bladder cancer T24 cells. J. Food Meas. Charact. 2018, 13, 51–60. [Google Scholar] [CrossRef]
- Rieseberg, L.H.; Willis, J.H. Plant speciation. Science 2007, 317, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.C.; Grabherr, M.G.; Chan, Y.F.; Russell, P.; Mauceli, E.; Johnson, J.; Swofford, R.; Pirun, M.; Zody, M.C.; White, S.; et al. The genomic basis of adaptive evolution in threespine sticklebacks. Nature 2012, 484, 55–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theis, A.; Ronco, F.; Indermaur, A.; Salzburger, W.; Egger, B. Adaptive divergence between lake and stream populations of an East African cichlid fish. Mol. Ecol. 2014, 23, 5304–5322. [Google Scholar] [CrossRef] [PubMed]
- Ferris, K.G.; Barnett, L.L.; Blackman, B.K.; Willis, J.H. The genetic architecture of local adaptation and reproductive isolation in sympatry within the Mimulus guttatus species complex. Mol. Ecol. 2017, 26, 208–224. [Google Scholar] [CrossRef]
- Thompson, M.M. Survey of Chromosome Numbers in Rubus (Rosaceae: Rosoideae). Ann. Mo. Bot. Gard. 1997, 84, 128–164. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Q.; Chen, T.; Tang, H.; Liu, L.; Wang, X. Phylogenetic Insights into Chinese Rubus (Rosaceae) from Multiple Chloroplast and Nuclear DNAs. Front. Plant Sci. 2016, 7, 968. [Google Scholar] [CrossRef] [Green Version]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Rubus. Flora Europaea; Cambridge University Press: Cambridge, UK, 1980; Volume 5, 452p. [Google Scholar]
- Thompson, M.M.; Zhao, C.M. Chromosome numbers of Rubus Species in southwest China. Acta Hortic. 1993, 352, 493–502. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Wang, M.; Yang, L.; Jiang, J.-G.; Zhao, J.-W.; Zhu, W. Flavonoid glycosides from Rubus chingii Hu fruits display anti-inflammatory activity through suppressing MAPKs activation in macrophages. J. Funct. Foods 2015, 18, 235–243. [Google Scholar] [CrossRef]
- Alice, L.A.; Eriksson, T.; Eriksen, B.; Campbell, C.S. Hybridization and Gene Flow Between Distantly Related Species of Rubus (Rosaceae): Evidence from Nuclear Ribosomal DNA Internal Transcribed Spacer Region Sequences. Syst. Bot. 2001, 26, 769–778. [Google Scholar] [CrossRef]
- Sochor, M.; Vašut, R.J.; Sharbel, T.F.; Trávníček, B. How just a few makes a lot: Speciation via reticulation and apomixis on example of European brambles (Rubus subgen. Rubus, Rosaceae). Mol. Phylogenet. Evol. 2015, 89, 13–27. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, C.-H.; Hu, Y.; Wen, J.; Li, S.; Yi, T.; Chen, H.; Xiang, J.; Ma, H. Evolution of Rosaceae Fruit Types Based on Nuclear Phylogeny in the Context of Geological Times and Genome Duplication. Mol. Biol. Evol. 2017, 34, 262–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Sutton, K.L.; Harris, G.K. Raspberries and Related Fruits. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 586–591. [Google Scholar]
- Siriwoharn, T.; Wrolstad, R.E.; Finn, C.E.; Pereira, C.B. Influence of cultivar, maturity, and sampling on blackberry (Rubus, L. Hybrids) anthocyanins, polyphenolics, and antioxidant properties. J. Agric. Food Chem. 2004, 52, 8021–8030. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Croge, C.P.; Cuquel, F.L.; Pintro, P.; Biasi, L.A.; Bona, C. Antioxidant Capacity and Polyphenolic Compounds of Blackberries Produced in Different Climates. HortScience A Publ. Am. Soc. Hortic. Sci. 2019, 54, 2209–2213. [Google Scholar] [CrossRef]
- Ancos, B.d.; González, E.M.; Cano, M.P. Ellagic acid, vitamin C, and total phenolic contents and radical scavenging capacity affected by freezing and frozen storage in raspberry fruit. J. Agric. Food Chem. 2000, 48, 4565–4570. [Google Scholar] [CrossRef] [Green Version]
- Haffner, K.; Rosenfeld, H.J.; Skrede, G.; Wang, L. Quality of red raspberry Rubus idaeus L. cultivars after storage in controlled and normal atmospheres. Postharvest Biol. Technol. 2002, 24, 279–289. [Google Scholar] [CrossRef]
- Mazur, S.P.; Nes, A.; Wold, A.B.; Remberg, S.F.; Aaby, K. Quality and chemical composition of ten red raspberry (Rubus idaeus L.) genotypes during three harvest seasons. Food Chem. 2014, 160, 233–240. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.-T.; Wang, C.Y. The influence of light and maturity on fruit quality and flavonoid content of red raspberries. Food Chem. 2009, 112, 676–684. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [Green Version]
- Gülçin, İ.; Topal, F.; Çakmakçı, R.; Bilsel, M.; Gören, A.C.; Erdogan, U. Pomological Features, Nutritional Quality, Polyphenol Content Analysis, and Antioxidant Properties of Domesticated and 3 Wild Ecotype Forms of Raspberries (Rubus idaeus L.). J. Food Sci. 2011, 76, C585–C593. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Viškelis, P.; Venskutonis, P.R. Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various raspberry (Rubus spp.) cultivars. Food Chem. 2012, 132, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Gangrade, T.; Punasiya, R.; Ghulaxe, C. Rubus fruticosus (blackberry) use as an herbal medicine. Pharmacogn. Rev. 2014, 8, 101–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strik, B.; Clark, J.; Finn, C.; Banados, M. Worldwide Blackberry Production. HortTechnology 2007, 17, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Hassani, S.; Shariatpanahi, M.; Tavakoli, F.; Nili-Ahmadabadi, A.; Abdollahi, M. The changes of bioactive ingredients and antioxidant properties in various berries during jam processing International Journal of Biosciences|IJB. Int. J. Biosci. IJB 2015, 6, 172–179. [Google Scholar] [CrossRef]
- Tanksley, S.D.; McCouch, S.R. Seed banks and molecular maps: Unlocking genetic potential from the wild. Science 1997, 277, 1063–1066. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.M.; Linton, E.; Messing, J.; Doebley, J.F. Pattern of diversity in the genomic region near the maize domestication gene tb1. Proc. Natl. Acad. Sci. USA 2004, 101, 700–707. [Google Scholar] [CrossRef] [Green Version]
- Flint-Garcia, S.A. Genetics and consequences of crop domestication. J. Agric. Food Chem. 2013, 61, 8267–8276. [Google Scholar] [CrossRef]
- Li, J.; Yuan, D.; Wang, P.; Wang, Q.; Sun, M.; Liu, Z.; Si, H.; Xu, Z.; Ma, Y.; Zhang, B.; et al. Cotton pan-genome retrieves the lost sequences and genes during domestication and selection. Genome Biol. 2021, 22, 119. [Google Scholar] [CrossRef]
- Graham, J.; Smith, K.; MacKenzie, K.; Jorgenson, L.; Hackett, C.; Powell, W. The construction of a genetic linkage map of red raspberry (Rubus idaeus subsp. idaeus) based on AFLPs, genomic-SSR and EST-SSR markers. Theor. Appl. Genet. Theor. Angew. Genet. 2004, 109, 740–749. [Google Scholar] [CrossRef]
- Bushakra, J.M.; Stephens, M.J.; Atmadjaja, A.N.; Lewers, K.S.; Symonds, V.V.; Udall, J.A.; Chagné, D.; Buck, E.J.; Gardiner, S.E. Construction of black (Rubus occidentalis) and red (R. idaeus) raspberry linkage maps and their comparison to the genomes of strawberry, apple, and peach. Theor. Appl. Genet. Theor. Angew. Genet. 2012, 125, 311–327. [Google Scholar] [CrossRef]
- Randell, R.; Howarth, D.; Morden, C. Genetic analysis of natural hybrids between endemic and alien Rubus (Rosaceae) species in Hawai‘i. Conserv. Genet. 2004, 5, 217–230. [Google Scholar] [CrossRef]
- Castillo, N.; Reed, B.M.; Graham, J.; Fernández-Fernández, F.; Bassil, N. Microsatellite Markers for Raspberry and Blackberry. J. Am. Soc. Hortic. Sci. 2010, 135, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Bushakra, J.M.; Lewers, K.S.; Staton, M.E.; Zhebentyayeva, T.; Saski, C.A. Developing expressed sequence tag libraries and the discovery of simple sequence repeat markers for two species of raspberry (Rubus L.). BMC Plant Biol. 2015, 15, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Jiang, J.; Shu, L.; Li, X.; Huang, J.; Qian, B.; Wang, X.; Li, X.; Chen, J.; Xu, H. Combined transcriptomic and metabolic analyses reveal potential mechanism for fruit development and quality control of Chinese raspberry (Rubus chingii Hu). Plant Cell Rep. 2021, 40, 1923–1946. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Yan, D.M.; Wang, Y.K.; Zhang, T.; Xiao, X.R.; Dai, M.Y.; Zhang, S.W.; Liu, H.N.; Li, F. Discovery of quality markers in Rubus chingii Hu using UPLC-ESI-QTOF-MS. J. Pharm. Biomed. Anal. 2021, 203, 114200. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.J.; Liu, Z.; Wang, Y.P.; Yang, D.; Yang, R.; Qu, L.B. Studies on the anti-aging activity of a glycoprotein isolated from Fupenzi (Rubus chingii Hu.) and its regulation on klotho gene expression in mice kidney. Int. J. Biol. Macromol. 2018, 119, 470–476. [Google Scholar] [CrossRef]
- Sheng, J.Y.; Wang, S.Q.; Liu, K.H.; Zhu, B.; Zhang, Q.Y.; Qin, L.P.; Wu, J.J. Rubus chingii Hu: An overview of botany, traditional uses, phytochemistry, and pharmacology. Chin. J. Nat. Med. 2020, 18, 401–416. [Google Scholar] [CrossRef]
- Xie, Y.H.; Lian, B.; Gong, J.H.; Tu, L.D.; Zhang, Y.T.; Huang, L.P. Preparation of Magnetic Chitosan Hyamine Microspheres and Separation of Phenolic Acids from Rubus Chingii Hu. Adv. Mater. Res. 2013, 634–638, 1347–1351. [Google Scholar] [CrossRef]
- Cai, Y.Q.; Hu, J.H.; Qin, J.; Sun, T.; Li, X.L. Rhododendron Molle (Ericaceae): Phytochemistry, pharmacology, and toxicology. Chin. J. Nat. Med. 2018, 16, 0401–0410. [Google Scholar] [CrossRef]
- Li, X.; Sun, J.; Chen, Z.; Jiang, J.; Jackson, A. Characterization of carotenoids and phenolics during fruit ripening of Chinese raspberry (Rubus chingii Hu). RSC Adv. 2021, 11, 10804–10813. [Google Scholar] [CrossRef]
- Ding, H.-Y. Extracts and constituents of Rubus chingii with 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity. Int. J. Mol. Sci. 2011, 12, 3941–3949. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhu, W.R.; Huang, H.L.; Zeng, Y.R.; Yu, W.W. Effective medicinal ingredients and screening of excellent germplasm in Rubus chingii. China J. Chin. Mater. Med. 2021, 46, 575–581. [Google Scholar] [CrossRef]
- Madrigal-Gamboa, V.; Jiménez-Arias, J.; Hidalgo, O.; Quesada, S.; Pérez, A.M.; Azofeifa, G. Membrane processing effect of blackberry (Rubus adenotrichos) on cytotoxic and pro-apoptotic activities against cancer cell lines. J. Food Process. Preserv. 2021, 45, e15575. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, M.; Fu, X.; Hou, J.; Wang, Y.; Shi, F.; Hu, S. Molecular mechanisms underlying macrophage immunomodulatory activity of Rubus chingii Hu polysaccharides. Int. J. Biol. Macromol. 2021, 185, 907–916. [Google Scholar] [CrossRef]
- Tolentino, F.; Araújo, P.A.d.; Marques, E.d.S.; Petreanu, M.; Andrade, S.F.d.; Niero, R.; Perazzo, F.F.; Rosa, P.C.P.; Maistro, E.L. In vivo evaluation of the genetic toxicity of Rubus niveus Thunb. (Rosaceae) extract and initial screening of its potential chemoprevention against doxorubicin-induced DNA damage. J. Ethnopharmacol. 2015, 164, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Kanegusuku, M.; Benassi, J.C.; Pedrosa, R.C.; Yunes, R.A.; Filho, V.C.; Maia, A.A.; de Souza, M.M.; Delle Monache, F.; Niero, R. Cytotoxic, hypoglycemic activity and phytochemical analysis of Rubus imperialis (Rosaceae). Z. Naturforschung. C J. Biosci. 2002, 57, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Om, A.-S.; Song, Y.-N.; Noh, G.; Kim, H.; Choe, J. Nutrition Composition and Single, 14-Day and 13-Week Repeated Oral Dose Toxicity Studies of the Leaves and Stems of Rubus coreanus Miquel. Molecules 2016, 21, 65. [Google Scholar] [CrossRef] [Green Version]
- AlQahtani, F.S.; AlShebly, M.M.; Govindarajan, M.; Senthilmurugan, S.; Vijayan, P.; Benelli, G. Green and facile biosynthesis of silver nanocomposites using the aqueous extract of Rubus ellipticus leaves: Toxicity and oviposition deterrent activity against Zika virus, malaria and filariasis mosquito vectors. J. Asia-Pac. Entomol. 2017, 20, 157–164. [Google Scholar] [CrossRef]
- Ali, N.; Shaoib, M.; Shah, S.W.A.; Shah, I.; Shuaib, M. Pharmacological profile of the aerial parts of Rubus ulmifolius Schott. BMC Complement. Altern. Med. 2017, 17, 59. [Google Scholar] [CrossRef] [Green Version]
- Ke, H.; Bao, T.; Chen, W. New function of polysaccharide from Rubus chingii Hu: Protective effect against ethyl carbamate induced cytotoxicity. J. Sci. Food Agric. 2021, 101, 3156–3164. [Google Scholar] [CrossRef]
- Kawahara, Y.; de la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.; Zhou, S.; et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Peluso, P.; Shi, J.; Liang, T.; Stitzer, M.C.; Wang, B.; Campbell, M.S.; Stein, J.C.; Wei, X.; Chin, C.-S.; et al. Improved maize reference genome with single-molecule technologies. Nature 2017, 546, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tu, L.; Yuan, D.; Zhu, D.; Shen, C.; Li, J.; Liu, F.; Pei, L.; Wang, P.; Zhao, G.; et al. Reference genome sequences of two cultivated allotetraploid cottons, Gossypium hirsutum and Gossypium barbadense. Nat. Genet. 2019, 51, 224–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The genome of the domesticated apple (Malus × domestica Borkh.). Nat. Genet. 2010, 42, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Z.; Shi, Z.; Zhang, S.; Ming, R.; Zhu, S.; Khan, M.A.; Tao, S.; Korban, S.S.; Wang, H.; et al. The genome of the pear (Pyrus bretschneideri Rehd.). Genome Res. 2013, 23, 396–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benz, B.F. Archaeological evidence of teosinte domestication from Guilá Naquitz, Oaxaca. Proc. Natl. Acad. Sci. USA 2001, 98, 2104–2106. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Yang, S.; Gong, J.; Zhao, Q.; Feng, Q.; Zhan, Q.; Zhao, Y.; Li, W.; Cheng, B.; Xia, J.; et al. Genomic architecture of heterosis for yield traits in rice. Nature 2016, 537, 629–633. [Google Scholar] [CrossRef]
- Chen, P.; Li, Z.; Zhang, D.; Shen, W.; Xie, Y.; Zhang, J.; Jiang, L.; Li, X.; Shen, X.; Geng, D.; et al. Insights into the effect of human civilization on Malus evolution and domestication. Plant Biotechnol. J. 2021, 19, 2206–2220. [Google Scholar] [CrossRef]
- Varshney, R.K.; Nayak, S.N.; May, G.D.; Jackson, S.A. Next-generation sequencing technologies and their implications for crop genetics and breeding. Trends Biotechnol. 2009, 27, 522–530. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.; Satya, P. Next generation sequencing technologies for next generation plant breeding. Front. Plant Sci. 2014, 5, 367. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Gonzalez, R.H.; Segovia, V.; Bird, N.; Fenwick, P.; Holdgate, S.; Berry, S.; Jack, P.; Caccamo, M.; Uauy, C. RNA-Seq bulked segregant analysis enables the identification of high-resolution genetic markers for breeding in hexaploid wheat. Plant Biotechnol. J. 2015, 13, 613–624. [Google Scholar] [CrossRef]
- Julio, E.; Malpica, A.; Cotucheau, J.; Bachet, S.; Volpatti, R.; Decorps, C.; Dorlhac de Borne, F. RNA-Seq analysis of Orobanche resistance in Nicotiana tabacum: Development of molecular markers for breeding recessive tolerance from ‘Wika’ tobacco variety. Euphytica 2019, 216, 6. [Google Scholar] [CrossRef]
- Hyun, T.K.; Lee, S.; Kumar, D.; Rim, Y.; Kumar, R.; Lee, S.Y.; Lee, C.H.; Kim, J.Y. RNA-seq analysis of Rubus idaeus cv. Nova: Transcriptome sequencing and de novo assembly for subsequent functional genomics approaches. Plant Cell Rep. 2014, 33, 1617–1628. [Google Scholar] [CrossRef]
- VanBuren, R.; Bryant, D.; Bushakra, J.M.; Vining, K.J.; Edger, P.P.; Rowley, E.R.; Priest, H.D.; Michael, T.P.; Lyons, E.; Filichkin, S.A.; et al. The genome of black raspberry (Rubus occidentalis). Plant J. 2016, 87, 535–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VanBuren, R.; Wai, C.M.; Colle, M.; Wang, J.; Sullivan, S.; Bushakra, J.M.; Liachko, I.; Vining, K.J.; Dossett, M.; Finn, C.E.; et al. A near complete, chromosome-scale assembly of the black raspberry (Rubus occidentalis) genome. GigaScience 2018, 7, giy094. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Tian, Y.; Liang, W. The complete chloroplast genome sequence of Rubus eucalyptus (Rosaceae). Mitochondrial DNA Part B Resour. 2019, 4, 2976–2977. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Liu, Y.; Hai, P. The complete chloroplast genome of Tibetan medicinal plant Rubus phoenicolasius Maxim. Mitochondrial DNA Part B Resour. 2021, 6, 886–887. [Google Scholar] [CrossRef]
- Davik, J.; Røen, D.; Lysøe, E.; Buti, M.; Rossman, S.; Alsheikh, M.; Aiden, E.L.; Dudchenko, O.; Sargent, D.J. A chromosome-level genome sequence assembly of the red raspberry (Rubus idaeus L.). PLoS ONE 2022, 17, e0265096. [Google Scholar] [CrossRef]
- Zhu, J.K. The Future of Gene-Edited Crops in China. Natl. Sci. Rev. 2022, nwac063. [Google Scholar] [CrossRef]
- Li, X.; Jiang, J.; Chen, Z.; Jackson, A. Transcriptomic, Proteomic and Metabolomic Analysis of Flavonoid Biosynthesis During Fruit Maturation in Rubus chingii Hu. Front. Plant Sci. 2021, 12, 706667. [Google Scholar] [CrossRef]
- Li, X.; Sun, J.; Chen, Z.; Jiang, J.; Jackson, A. Metabolite profile and genes/proteins expression in β-citraturin biosynthesis during fruit ripening in Chinese raspberry (Rubus chingii Hu). Plant Physiol. Biochem. 2021, 163, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Qiao, F.; Guo, W.; Wu, W. Characterization of the complete chloroplast genome sequence of Rubus rufus Focke (Rosaceae). Mitochondrial DNA Part B Resour. 2021, 6, 3093–3094. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Park, J.Y.; Kang, J.H.; Lee, W.H.; Yang, T.J. Diversity and authentication of Rubus accessions revealed by complete plastid genome and rDNA sequences. Mitochondrial DNA Part B Resour. 2021, 6, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, Z.; Gao, C.; Ge, Y.; Cheng, R. The complete chloroplast genome sequence of Rubus hirsutus Thunb. and a comparative analysis within Rubus species. Genetica 2021, 149, 299–311. [Google Scholar] [CrossRef]
- Su, X.H.; Duan, R.; Sun, Y.Y.; Wen, J.F.; Kang, D.G.; Lee, H.S.; Cho, K.W.; Jin, S.N. Cardiovascular effects of ethanol extract of Rubus chingii Hu (Rosaceae) in rats: An in vivo and in vitro approach. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2014, 65, 417–424. [Google Scholar]
- He, Y.; Jin, S.; Ma, Z.; Zhao, J.; Yang, Q.; Zhang, Q.; Zhao, Y.; Yao, B. The antioxidant compounds isolated from the fruits of chinese wild raspberry Rubus Chingii Hu. Nat. Prod. Res. 2018, 34, 872–875. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.; Guo, Q.; Gao, X.; Ma, Q.; Xue, Z.; Ferri, N.; Zhang, M.; Chen, H. Identification of Ellagitannins in the Unripe Fruit of Rubus Chingii Hu and Evaluation of its Potential Antidiabetic Activity. J. Agric. Food Chem. 2019, 67, 7025–7039. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Fu, Y.; Zhou, X.; Chen, S.; Sun, Y.; Shen, Y.; Ye, X. Chemical composition and antioxidant activity of Chinese wild raspberry (Rubus hirsutus Thunb.). LWT Food Sci. Technol. 2015, 60, 1262–1268. [Google Scholar] [CrossRef]
- Han, N.; Gu, Y.; Ye, C.; Cao, Y.; Liu, Z.; Yin, J. Antithrombotic activity of fractions and components obtained from raspberry leaves (Rubus chingii). Food Chem. 2012, 132, 181–185. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Nam, J.H.; Jeong, J.H.; Rho, I.R. Effect of plant part, extraction method, and harvest time over antioxidant yield of rubus coreanus. Pharmacogn. Mag. 2020, 16, 455–461. [Google Scholar]
- Howarth, D.G.; Gardner, D.E.; Morden, C.W. Phylogeny of Rubus subgenus Idaeobatus (Rosaceae) and its implications toward colonization of the Hawaiian Islands. Syst. Bot. 1997, 22, 433–441. [Google Scholar] [CrossRef]
- Morden, C.W.; Gardner, D.E.; Weniger, D.A. Phylogeny and biogeography of pacific Rubus subgenus Idaeobatus (Rosaceae) species: Investigating the origin of the endemic Hawaiian raspberry R. macraei. Pac. Sci. 2003, 57, 181–197. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Q.; Chen, T.; Zhang, J.; He, W.; Liu, L.; Luo, Y.; Sun, B.; Zhang, Y.; Tang, H.-r.; et al. Allopolyploid origin in Rubus (Rosaceae) inferred from nuclear granule-bound starch synthase I (GBSSI) sequences. BMC Plant Biol. 2019, 19, 303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Q.; Tang, H.; Wang, X. Phylogeny of Chinese Rubus (Rosaceae) based on nuclear ribosomal internal transcribed spacer (ITS) sequences. Acta Hortic. 2018, 1208, 13–24. [Google Scholar] [CrossRef]
- Alice, L.A.; Dodson, T.M.; Sutherland, B.L. Diversity and relationships of Bhutanese Rubus (Rosaceae). Acta Hortic. 2008, 777, 63–70. [Google Scholar] [CrossRef]
- Yang, J.Y.; Pak, J.-H. Phylogeny of korean rubus (rosaceae) based on its (nrDNA) and trnL/F intergenic region (cpdna). J. Plant Biol. 2006, 49, 44–54. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Chen, Q.; Luo, Y.; Zhang, Y.; Tang, H.R.; Wang, X.R. Phylogenetic Utility of Chinese Rubus (Rosaceae) Based on ndhF Sequence. Acta Hortic. Sin. 2015, 42, 19–30. [Google Scholar] [CrossRef]
- Imanishi, H.; Nakahara, K.; Tsuyuzaki, H. Genetic relationships among native and introduced Rubus species in Japan based on rbcL sequence. Acta Hortic. 2008, 769, 195–199. [Google Scholar] [CrossRef]

| Subgenus | Code | Species in Subgenus | Ploidy Level (x = 7) | References |
|---|---|---|---|---|
| Anoplobatus | An | 9 | 2x | [8,30,111,112] |
| Chamaebatus | Cb | 6 (5) | 2x, 6x | [8,30,31] |
| Chamaemorus | Cm | 1 (1) | 6x, 8x | [8,30] |
| Comaropsis | Co | 2 | 4x | [8,30] |
| Cylactis | Cy | 18 (8) | 2x−4x | [8,30,31] |
| Dalibarda | Da | 5 | 2x | [8,30] |
| Dalibardastrum | Ds | 15 (10) | 4x, 6x | [8,30,31,111,112,113] |
| Idaeobatus | Id | 125 (83) | 2x, 3x, 4x, 13x, 18x | [8,20,30,31,111,112,113] |
| Lampobatus | La | 10 (1) | 4x | [8,30] |
| Malachobatus | Ma | 104 (85) | 4x, 6x, 8x, 14x | [30,31,111,112,113] |
| Orobatus | Or | 16 | 6x | [8,30] |
| Rubus | Ru | 444 (1) | 2x−12x | [8,30,111,112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Q.; Manghwar, H.; Hu, W. Study on Supergenus Rubus L.: Edible, Medicinal, and Phylogenetic Characterization. Plants 2022, 11, 1211. https://doi.org/10.3390/plants11091211
Meng Q, Manghwar H, Hu W. Study on Supergenus Rubus L.: Edible, Medicinal, and Phylogenetic Characterization. Plants. 2022; 11(9):1211. https://doi.org/10.3390/plants11091211
Chicago/Turabian StyleMeng, Qinglin, Hakim Manghwar, and Weiming Hu. 2022. "Study on Supergenus Rubus L.: Edible, Medicinal, and Phylogenetic Characterization" Plants 11, no. 9: 1211. https://doi.org/10.3390/plants11091211