Multi Omics Analysis Revealed a Resistance Mechanism of Tibetan Barley (Hordeum vulgare L., Qingke) Infected by Ustilago hordei
Abstract
1. Introduction
2. Results
2.1. Metabolome Profile of Infected Qingke
2.2. Proteome Profile of Infected Qingke
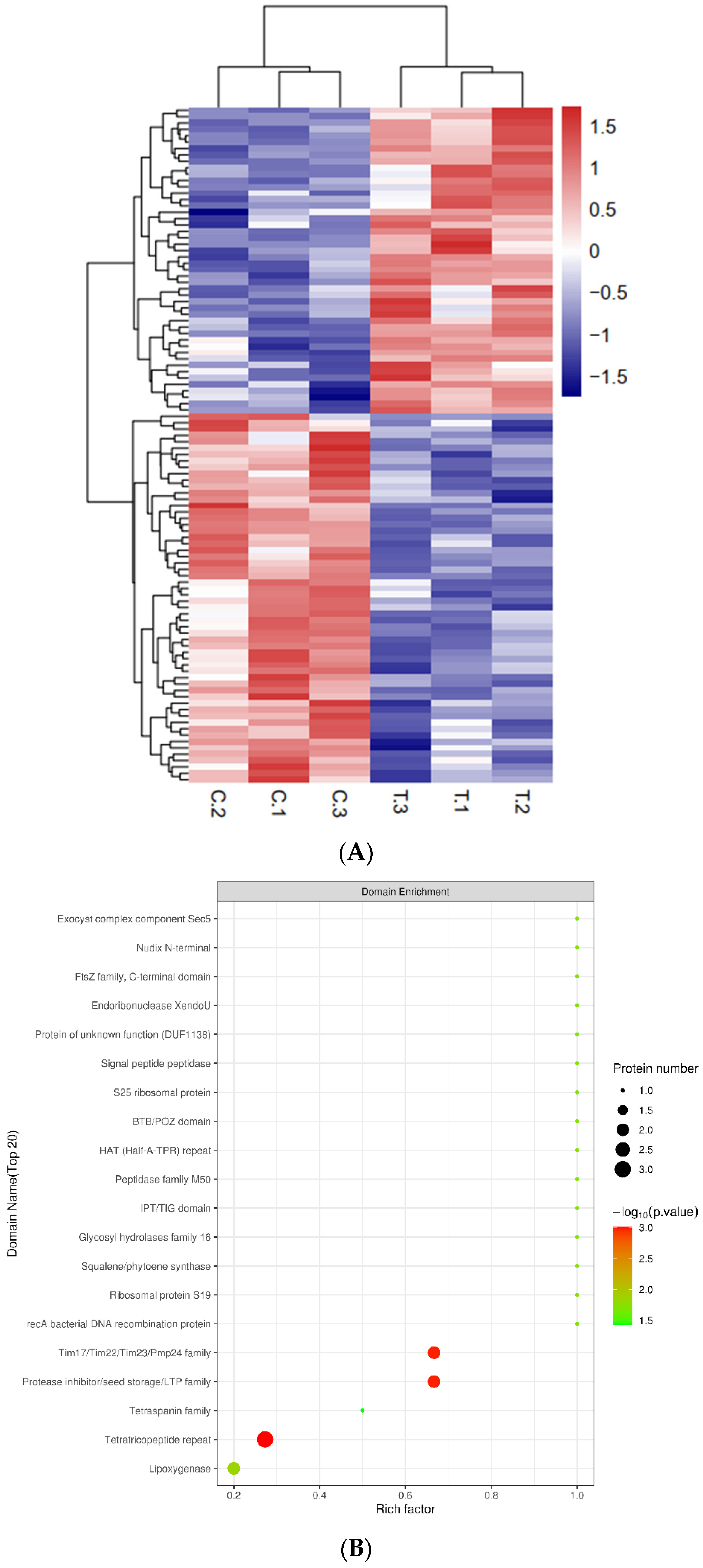
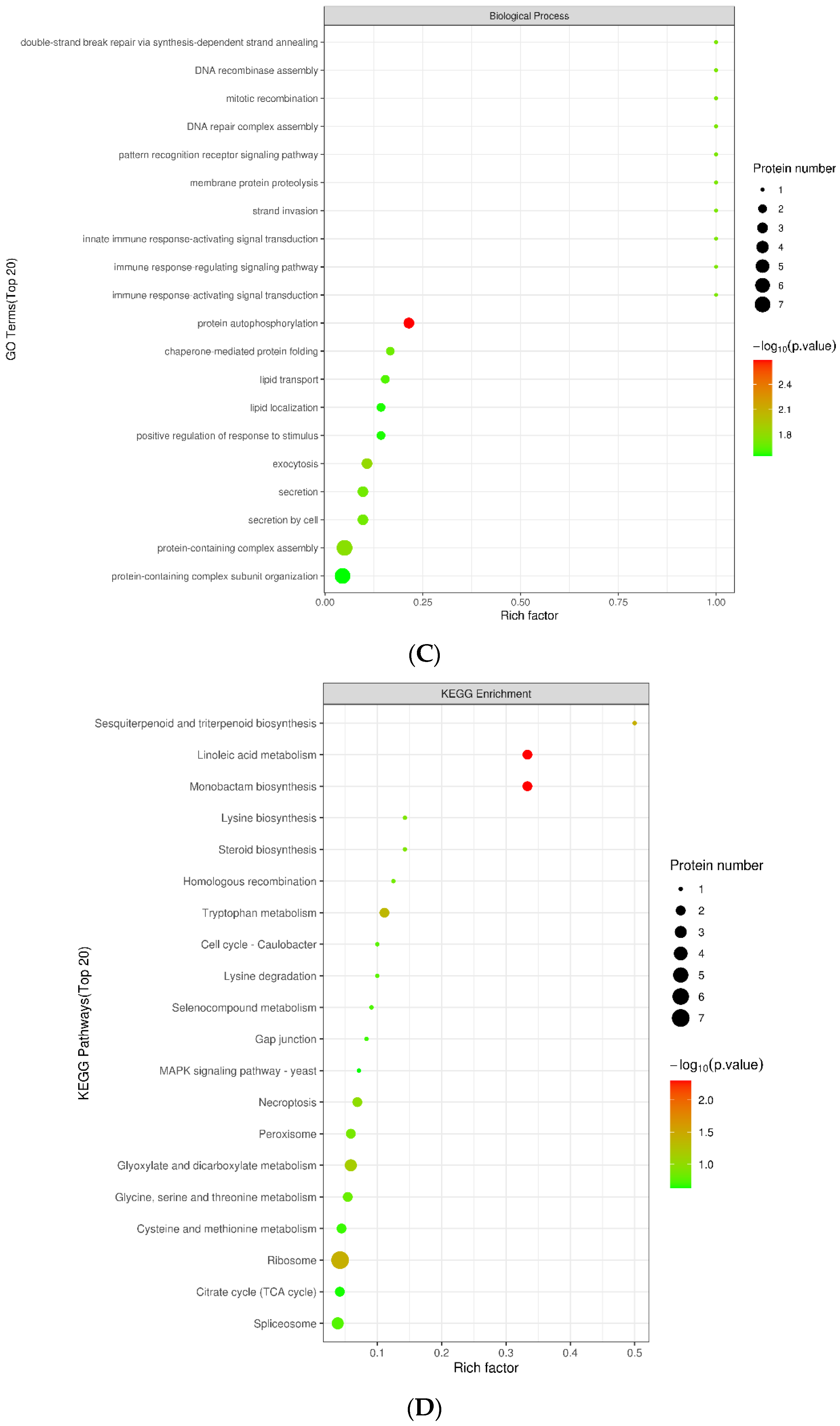
2.3. Transcriptome Analysis of Infected Qingke
2.4. Co-Analysis of Multi Omics
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. RNA Extraction and Sequencing
4.3. Protein Extraction and Digestion
4.4. LC-MS/MS Analysis
4.5. Metabolome Data Processing
4.6. Proteome Data Processing
4.7. Transcriptome Data Processing
4.8. Cluster Analysis
4.9. Subcellular Localization and Function Annotation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, J.; Kaur, A.; Sharma, V.K. Evaluation of barley genotypes for resistance against covered smut disease. Indian Phytopathol. 2020, 73, 359–360. [Google Scholar] [CrossRef]
- Gangwar, O.P.; Bhardwaj, S.C.; Singh, G.; Prasad, P.; Kumar, S. Barley diseases and their management: An Indian perspective. Wheat Barley Res. 2018, 10, 138–150. [Google Scholar] [CrossRef]
- Mysore, K.S.; Ryu, C.-M. Nonhost resistance: How much do we know? Trends Plant Sci. 2004, 9, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Muthappa, S.-K.; Mysore, K. Nonhost Resistance Against Bacterial Pathogens: Retrospectives and Prospects. Annu. Rev. Phytopathol. 2013, 51, 10–1146. [Google Scholar] [CrossRef]
- Boller, T.; He, S.Y. Innate Immunity in Plants: An Arms Race Between Pattern Recognition Receptors in Plants and Effectors in Microbial Pathogens. Science 2009, 324, 742–744. [Google Scholar] [CrossRef]
- Bonardi, V.; Dangl, J. How complex are intracellular immune receptor signaling complexes? Front. Plant Sci. 2012, 3, 237. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.; Muthappa, S.-K.; Tzin, V.; Mysore, K. Regulation of primary metabolism during plant-pathogen interactions and its contribution to plant defense. Front. Plant Sci. 2014, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef] [PubMed]
- De Vleesschauwer, D.; Gheysen, G.; Höfte, M. Hormone defense networking in rice: Tales from a different world. Trends Plant Sci. 2013, 18, 555–565. [Google Scholar] [CrossRef]
- Koornneef, A.; Pieterse, C. Cross Talk in Defense Signaling. Plant Physiol. 2008, 146, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Boubakri, H.; Gargouri, M.; Mliki, A.; Brini, F.; Chong, J.; Jbara, M. Vitamins for enhancing plant resistance. Planta 2016, 244, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Bolton, M.D. Primary metabolism and plant defense—Fuel for the fire. Mol. Plant-Microbe Interact. 2009, 22, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Baldwin, I.T. Fitness costs of induced resistance: Emerging experimental support for a slippery concept. Trends Plant Sci. 2002, 7, 61–67. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Sharif, Y.; Zafar, M.H.; Ali, H.; Khan, K.A. Role of primary metabolites in plant defense against pathogens. Microb. Pathog. 2019, 137, 103728. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ito, M.; Nishida, I.; Watanabe, A. Identification of a novel gene HYS1/CPR5 that has a repressive role in the induction of leaf senescence and pathogen-defence responses in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2002, 29, 427–437. [Google Scholar] [CrossRef]
- Boch, J.; Verbsky, M.; Robertson, T.; Larkin, J.; Kunkel, B. Analysis of Resistance Gene-Mediated Defense Responses in Arabidopsis thaliana Plants Carrying a Mutation in CPR5. Mol. Plant-Microbe Interact. 1998, 11, 1196–1206. [Google Scholar] [CrossRef][Green Version]
- Mur, L.A.J.; Kenton, P.; Atzorn, R.; Miersch, O.; Wasternack, C. The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism and oxidative stress leading to cell death. Plant Physiol. 2006, 140, 249–262. [Google Scholar] [CrossRef]
- Lundquist, P.; Poliakov, A.; Giacomelli, L.; Friso, G.; Appel, M.; McQuinn, R.; Krasnoff, S.; Rowland, E.; Ponnala, L.; Sun, Q.; et al. Loss of Plastoglobule Kinases ABC1K1 and ABC1K3 Causes Conditional Degreening, Modified Prenyl-Lipids, and Recruitment of the Jasmonic Acid Pathway. Plant Cell 2013, 25, 1818–1839. [Google Scholar] [CrossRef]
- Pottinger, S.; Bak, A.; Margets, A.; Helm, M.; Tang, L.; Casteel, C.; Innes, R. Optimizing the PBS1 Decoy System to Confer Resistance to Potyvirus Infection in Arabidopsis and Soybean. Mol. Plant-Microbe Interact. 2020, 33, 932–944. [Google Scholar] [CrossRef]
- Cerveny, L.; Straskova, A.; Dankova, V.; Härtlova, A.; Ceckova, M.; Staud, F.; Stulík, J. Tetratricopeptide Repeat Motifs in the World of Bacterial Pathogens: Role in Virulence Mechanisms. Infect. Immun. 2012, 81, 629–635. [Google Scholar] [CrossRef]
- Karimi Jashni, M.; Mehrabi, R.; Collemare, J.; Mesarich, C.; De Wit, P. The battle in the apoplast: Further insights into the roles of proteases and their inhibitors in plant–pathogen interactions. Front. Plant Sci. 2015, 6, 584. [Google Scholar] [CrossRef]
- Christensen, S.A.; Kolomiets, M.V. The lipid language of plant–fungal interactions. Fungal Genet. Biol. 2011, 48, 4–14. [Google Scholar] [CrossRef]
- Ding, Z. Lipid metabolism disorders contribute to the pathogenesis of Hepatospora eriocheir in the crab Eriocheir sinensis. J. Fish Dis. 2021, 44, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Zeier, J. New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant Cell Environ. 2013, 36, 2085–2103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Mao, B.; Li, Q.; He, Z. Alpha-picolinic acid, a fungal toxin and mammal apoptosis-inducing agent, elicits hypersensitive-like response and enhances disease resistance in rice. Cell Res. 2004, 14, 27–33. [Google Scholar] [CrossRef]
- Ingólfsson, H.; Melo, M.; van Eerden, F.; Arnarez, C.; López, C.; Wassenaar, T.A.; Periole, X.; Vries, A.; Tieleman, D.; Marrink, S. Lipid Organization of the Plasma Membrane. J. Am. Chem. Soc. 2014, 136, 14554–14559. [Google Scholar] [CrossRef]
- Wältermann, M.; Steinbüchel, A. Neutral Lipid Bodies in Prokaryotes: Recent Insights into Structure, Formation, and Relationship to Eukaryotic Lipid Depots. J. Bacteriol. 2005, 187, 3607–3619. [Google Scholar] [CrossRef]
- Shiji, H.; Kenichi, T. Salicylic acid and jasmonic acid crosstalk in plant immunity. Essays Biochem. 2022, 66, 647–656. [Google Scholar] [CrossRef]
- Ku, Y.S.; Sintaha, M.; Cheung, M.Y.; Lam, H.M. Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.J. Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: Do we understand what they are whispering? Int. J. Mol. Sci. 2021, 20, 671. [Google Scholar] [CrossRef] [PubMed]
- Liechti, R.; Farmer, E. The Jasmonate Biochemical Pathway. Sci. STKE Signal Transduct. Knowl. Environ. 2003, 2003, CM18. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, L.; Zhao, R.; Rui, L.; Zhang, S.; Yu, W.; Sheng, J.; Shen, L. Melatonin Induces Disease Resistance to Botrytis cinerea in Tomato Fruit by Activating Jasmonic Acid Signaling Pathway. J. Agric. Food Chem. 2019, 67, 6116–6124. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, Perception, Signal Transduction and Action in Plant Stress Response, Growth and Development. An Update to the 2007 Review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Azami-Sardooei, Z.; França, S.C.; De Vleesschauwer, D.; Höfte, M. Riboflavin induces resistance against Botrytis cinerea in bean, but not in tomato, by priming for a hydrogen peroxide-fueled resistance response. Physiol. Mol. Plant Pathol. 2010, 75, 23–29. [Google Scholar] [CrossRef]
- Bowling, S.A.; Clarke, J.D.; Liu, Y.; Klessig, D.F.; Dong, X. The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell 1997, 9, 1573–1584. [Google Scholar]
- Zhang, S.; Yang, X.; Sun, M.; Sun, F.; Deng, S.; Dong, H. Riboflavin-induced Priming for Pathogen Defense in Arabidopsis thaliana. J. Integr. Plant Biol. 2009, 51, 167–174. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, A.; Xing, D. Modulation of cellular redox status by thiamine-activated NADPH oxidase confers Arabidopsis resistance to Sclerotinia sclerotiorum. J. Exp. Bot. 2013, 64, 3261–3272. [Google Scholar] [CrossRef]
- Turner, J.G.; Ellis, C.; Devoto, A. The jasmonate signal pathway. Plant Cell 2002, 14, S153–S164. [Google Scholar] [CrossRef]
- Wolucka, B.; Goossens, A.; Inze, D. Methyl jasmonate stimulates the de novo biosynthesis of vitamin C in plant cell suspensions. J. Exp. Bot. 2005, 56, 2527–2538. [Google Scholar] [CrossRef]
- Dias, C.V.; Mendes, J.S.; dos Santos, A.C.; Pirovani, C.P.; da Silva Gesteira, A.; Micheli, F.; Gramacho, K.P.; Hammerstone, J.; Mazzafera, P.; de Mattos Cascardo, J.C. Hydrogen peroxide formation in cacao tissues infected by the hemibiotrophic fungus Moniliophthora perniciosa. Plant Physiol. Biochem. 2011, 49, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Han, J.; Kim, Y.-J.; Song, M.; Yang, Z.; He, Y.; Fu, R.; Luo, Z.; Hu, J.; Liang, W.; et al. Two rice receptor-like kinases maintain male fertility under changing temperatures. Proc. Natl. Acad. Sci. USA 2017, 114, 12327–12332. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Kumar, I.S.; Nadarajah, K. Elicitor and Receptor Molecules: Orchestrators of Plant Defense and Immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef] [PubMed]
- Felix, G.; Regenass, M.; Boller, T. Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: Induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant J. 2002, 4, 307–316. [Google Scholar] [CrossRef]
- Kwon, S.I.; Kim, S.H.; Bhattacharjee, S.; Noh, J.-J.; Gassmann, W. SRFR1, a suppressor of effector-triggered immunity, encodes a conserved tetratricopeptide repeat protein with similarity to transcriptional repressors. Plant J. Cell Mol. Biol. 2009, 57, 109–119. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Park, S.-C.; Hwang, I.; Cheong, H.; Nah, J.-W.; Hahm, K.-S.; Park, Y. Protease Inhibitors from Plants with Antimicrobial Activity. Int. J. Mol. Sci. 2009, 10, 2860–2872. [Google Scholar] [CrossRef]
- Valueva, T.; Mosolov, V. Role of inhibitors of proteolytic enzymes in plant defense against phytopathogenic microorganisms. Biochemistry 2004, 69, 1305–1309. [Google Scholar] [CrossRef]
- Laluk, K.; Mengiste, T. The Arabidopsis extracellular UNUSUAL SERINE PROTEASE INHIBITOR functions in resistance to necrotrophic fungi and insect herbivory. Plant J. Cell Mol. Biol. 2011, 68, 480–494. [Google Scholar] [CrossRef]
- van der Linde, K.; Hemetsberger, C.; Kastner, C.; Kaschani, F.; Hoorn, R.; Kumlehn, J.; Doehlemann, G. A Maize Cystatin Suppresses Host Immunity by Inhibiting Apoplastic Cysteine Proteases. Plant Cell 2012, 24, 1285–1300. [Google Scholar] [CrossRef]
- Hopkins, M.; Lampi, Y.; Wang, T.-W.; Liu, Z.; Thompson, J. Eukaryotic Translation Initiation Factor 5A Is Involved in Pathogen-Induced Cell Death and Development of Disease Symptoms in Arabidopsis. Plant Physiol. 2008, 148, 479–489. [Google Scholar] [CrossRef]
- Xiao, W.Y.; Sheen, J.; Jang, J. The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol. Biol. 2000, 44, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lim, J.-H.; Ahn, C.; Park, K.; Kim, G.; Kim, W.T.; Pai, H.-S. Mitochondria-Associated Hexokinases Play a Role in the Control of Programmed Cell Death in Nicotiana benthamiana. Plant Cell 2006, 18, 2341–2355. [Google Scholar] [CrossRef] [PubMed]
- Tadege, M.; Bucher, M.; Stähli, W.; Suter, M.; Dupuis, I.; Kuhlemeier, C. Activation of plant defense responses and sugar efflux by expression of pyruvate decarboxylase in potato leaves. Plant J. 1998, 16, 661–671. [Google Scholar] [CrossRef]
- Scheideler, M.; Schlaich, N.L.; Fellenberg, K.; Beissbarth, T.; Hauser, N.C.; Vingron, M.; Slusarenko, A.J.; Hoheisel, J.D. Monitoring the Switch from Housekeeping to Pathogen Defense Metabolism in Arabidopsis thaliana Using cDNA Arrays. J. Biol. Chem. 2002, 277, 10555–10561. [Google Scholar] [CrossRef]
- Liu, G.; Ji, Y.; Bhuiyan, N.; Pilot, G.; Selvaraj, G.; Zou, J.; Wei, Y. Amino Acid Homeostasis Modulates Salicylic Acid-Associated Redox Status and Defense Responses in Arabidopsis. Plant Cell 2010, 22, 3845–3863. [Google Scholar] [CrossRef]
- Mandal, M.; Chandra-Shekara, A.C.; Jeong, R.-D.; Yu, K.; Zhu, S.; Chanda, B.; Navarre, R.; Kachroo, A.; Kachroo, P. Oleic acid-dependent modulation of NITRIC OXIDE ASSOCIATED1 protein levels regulates nitric oxide-mediated defense signaling in Arabidopsis. Plant Cell 2012, 24, 1654–1674. [Google Scholar] [CrossRef]
- Yaeno, T.; Matsuda, O.; Iba, K. Role of chloroplast trienoic fatty acids in plant disease defense responses. Plant J. Cell Mol. Biol. 2005, 40, 931–941. [Google Scholar] [CrossRef]
- Ebeed, H.; Stevenson, S.; Cuming, A.; Baker, A. Conserved and differential transcriptional responses of peroxisome associated pathways to drought, dehydration and ABA. J. Exp. Bot. 2018, 69, 4971–4985. [Google Scholar] [CrossRef]
- Yan, Y.; Stolz, S.; Chételat, A.; Reymond, P.; Pagni, M.; Dubugnon, L.; Farmer, E. A Downstream Mediator in the Growth Repression Limb of the Jasmonate Pathway. Plant Cell 2007, 19, 2470–2483. [Google Scholar] [CrossRef]
- Vörös, K.; Feussner, I.; Kühn, H.; Lee, J.; Graner, A.; Löbler, M.; Parthier, B.; Wasternack, C. Characterization of a methyljasmonate-inducible lipoxygenase from barley (Hordeum vulgare cv. Salome) leaves. Eur. J. Biochem. 2001, 251, 36–44. [Google Scholar] [CrossRef]
- Denisov, I.; Makris, T.; Sligar, S.; Schlichting, I. Structure and Chemistry of Cytochrome P450. Chem. Rev. 2005, 105, 2253–2277. [Google Scholar] [CrossRef] [PubMed]
- Pandian, B.A.; Sathishraj, R.; Maduraimuthu, D.; Prasad, P.V.V.; Jugulam, M. Role of Cytochrome P450 Enzymes in Plant Stress Response. Antioxidants 2020, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Boccard, J.; Rutledge, D.N. A consensus orthogonal partial least squares discriminant analysis (OPLS-DA) strategy for multiblock Omics data fusion. Anal. Chim. Acta 2013, 769, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Love, M.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, A. Java Treeview—Extensible visualization of microarray data. Bioinformatics 2004, 20, 3246–3248. [Google Scholar] [CrossRef]
- Yu, C.-S.; Lin, C.-J.; Hwang, J.-K. Predicting subcellular localization of proteins for Gram-negative bacteria by support vector machines based on n-peptide compositions. Protein Sci. 2004, 13, 1402–1406. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.; Wang, J.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]




| GO ID | Term | Category |
|---|---|---|
| GO: 0022607 | cellular component assembly | BP |
| GO: 0006887 | exocytosis | BP |
| GO: 0010876 | lipid localization | BP |
| GO: 0006869 | lipid transport | BP |
| GO: 0048584 | positive regulation of response to stimulus | BP |
| GO: 0006468 | protein phosphorylation | BP |
| GO: 0065003 | protein-containing complex assembly | BP |
| GO: 0043933 | protein-containing complex subunit organization | BP |
| GO: 0080134 | regulation of response to stress | BP |
| GO: 1901700 | response to oxygen-containing compound | BP |
| GO: 0046903 | secretion | BP |
| GO: 0032940 | secretion by cell | BP |
| GO: 0071944 | cell periphery | CC |
| GO: 0005515 | protein binding | MF |
| Pathway | KEGG ID | Metabolomic | Proteomic | Transcriptome |
|---|---|---|---|---|
| Amino acid metabolism | ||||
| Cysteine and methionine metabolism | ko00270 | Y | Y | Y |
| Glycine, serine and threonine metabolism | ko00260 | Y | Y | Y |
| Tryptophan metabolism | ko00380 | Y | Y | Y |
| Valine, leucine and isoleucine degradation | ko00280 | Y | Y | Y |
| Lysine degradation | ko00310 | Y | Y | |
| Alanine, aspartate and glutamate metabolism | ko00250 | Y | Y | |
| Arginine biosynthesis | ko00220 | Y | Y | |
| Histidine metabolism | ko00340 | Y | Y | |
| Tyrosine metabolism | ko00350 | Y | Y | |
| Lysine biosynthesis | ko00300 | Y | Y | |
| Biosynthesis of other secondary metabolites | ||||
| Monobactam biosynthesis | ko00261 | Y | Y | |
| Phenylpropanoid biosynthesis | ko00940 | Y | Y | |
| Carbohydrate metabolism | ||||
| Amino sugar and nucleotide sugar metabolism | ko00520 | Y | Y | |
| Ascorbate and aldarate metabolism | ko00053 | Y | Y | |
| Galactose metabolism | ko00052 | Y | Y | |
| Glycolysis/Gluconeogenesis | ko00010 | Y | Y | |
| Glyoxylate and dicarboxylate metabolism | ko00630 | Y | Y | |
| Pentose and glucuronate interconversions | ko00040 | Y | Y | |
| Propanoate metabolism | ko00640 | Y | Y | |
| Pyruvate metabolism | ko00620 | Y | Y | |
| Starch and sucrose metabolism | ko00500 | Y | Y | |
| Cell growth and death | ||||
| Ferroptosis | ko04216 | Y | Y | |
| Energy metabolism | ||||
| Carbon fixation in photosynthetic organisms | ko00710 | Y | Y | |
| Methane metabolism | ko00680 | Y | Y | |
| Environmental adaptation | ||||
| Thermogenesis | ko04714 | Y | Y | |
| Global and overview maps | ||||
| Biosynthesis of amino acids | ko01230 | Y | Y | |
| Biosynthesis of secondary metabolites | ko01110 | Y | Y | |
| Carbon metabolism | ko01200 | Y | Y | |
| Degradation of aromatic compounds | ko01220 | Y | Y | |
| Metabolic pathways | ko01100 | Y | Y | |
| Microbial metabolism in diverse environments | Y | Y | ||
| Lipid metabolism | ||||
| alpha-Linolenic acid metabolism | ko01120 | Y | Y | Y |
| Biosynthesis of unsaturated fatty acids | ko01040 | Y | Y | |
| Fatty acid biosynthesis | ko00592 | Y | Y | |
| Steroid biosynthesis | ko01040 | Y | Y | |
| Metabolism of cofactors and vitamins | ||||
| Pantothenate and CoA biosynthesis | ko00770 | Y | Y | |
| Metabolism of other amino acids | ||||
| beta-Alanine metabolism | ko00410 | Y | Y | |
| Glutathione metabolism | ko00480 | Y | Y | Y |
| Metabolism of terpenoids and polyketides | ||||
| Limonene and pinene degradation | ko00903 | Y | Y | |
| Nucleotide metabolism | ||||
| Purine metabolism | ko00230 | Y | Y | |
| Pyrimidine metabolism | ko00240 | Y | Y | |
| Signal transduction | ||||
| cAMP signaling pathway | ko04024 | Y | Y | |
| cGMP-PKG signaling pathway | ko04022 | Y | Y | |
| HIF-1 signaling pathway | ko04066 | Y | Y | |
| Plant hormone signal transduction | ko04075 | Y | Y | |
| Transport and catabolism | ||||
| Peroxisome | ko04146 | Y | Y | |
| Xenobiotics biodegradation and metabolism | ||||
| Drug metabolism—cytochrome P450 | ko00982 | Y | Y | |
| Drug metabolism—other enzymes | ko00983 | Y | Y | Y |
| Metabolism of xenobiotics by cytochrome P450 | ko00980 | Y | Y |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, J.; Wu, T.; Liu, P.; Li, P.; Yao, X.; Liu, H.; Ciren, Y. Multi Omics Analysis Revealed a Resistance Mechanism of Tibetan Barley (Hordeum vulgare L., Qingke) Infected by Ustilago hordei. Plants 2023, 12, 157. https://doi.org/10.3390/plants12010157
Li J, Zhang J, Wu T, Liu P, Li P, Yao X, Liu H, Ciren Y. Multi Omics Analysis Revealed a Resistance Mechanism of Tibetan Barley (Hordeum vulgare L., Qingke) Infected by Ustilago hordei. Plants. 2023; 12(1):157. https://doi.org/10.3390/plants12010157
Chicago/Turabian StyleLi, Juan, Jixiang Zhang, Tao Wu, Pei Liu, Pu Li, Xiaobo Yao, Hechun Liu, and Yangla Ciren. 2023. "Multi Omics Analysis Revealed a Resistance Mechanism of Tibetan Barley (Hordeum vulgare L., Qingke) Infected by Ustilago hordei" Plants 12, no. 1: 157. https://doi.org/10.3390/plants12010157
APA StyleLi, J., Zhang, J., Wu, T., Liu, P., Li, P., Yao, X., Liu, H., & Ciren, Y. (2023). Multi Omics Analysis Revealed a Resistance Mechanism of Tibetan Barley (Hordeum vulgare L., Qingke) Infected by Ustilago hordei. Plants, 12(1), 157. https://doi.org/10.3390/plants12010157





