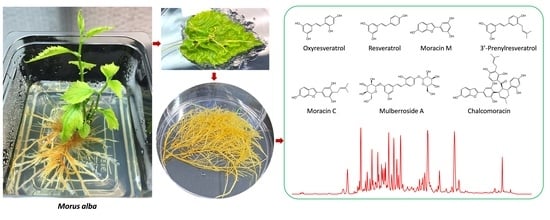
Elicitation of Stilbenes and Benzofuran Derivatives in Hairy Root Cultures of White Mulberry (Morus alba)
Abstract
1. Introduction
2. Results
2.1. Development and Characterization of Hairy Root Cultures of M. alba
2.2. Elicitation of Stilbenes and Moracins in Hairy Root Cultures of M. alba
2.2.1. Phenotype of Hairy Root Line U-D2 upon Multiple Elicitors Treatments
2.2.2. Effect of Different Elicitor Treatments on the Yield of Stilbenes and Benzofurans
3. Discussion
4. Materials and Methods
4.1. Sterilization and Germination of White Mulberry Seeds
4.2. Establishment of Hairy Root Lines of White Mulberry
4.3. Elicitation of Hairy Root Cultures
4.4. Extraction of Phenolics from the Hairy Root Culture Medium and Tissue
4.5. HPLC Analysis
4.6. Liquid Chromatography-Mass Spectrometry Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nam, S.; Jang, H.W.; Shibamoto, T. Antioxidant activities of extracts from teas prepared from medicinal plants, Morus alba L., Camellia sinensis L., and Cudrania tricuspidata, and their volatile components. J. Agric. Food Chem. 2012, 60, 9097–9105. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Rahman, A.A.; Islam, S.; Khandokhar, P.; Parvin, S.; Islam, M.B.; Hossain, M.; Rashid, M.; Sadik, G.; Nasrin, S.; et al. A comparative study on the antioxidant activity of methanolic extracts from different parts of Morus alba L. (Moraceae). BMC Res. Notes 2013, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Sheng, Y.; Zheng, S.; Ma, T.; Zhang, C.; Ou, X.; He, X.; Xu, W.; Huang, K. Mulberry leaf alleviates streptozotocin-induced diabetic rats by attenuating NEFA signaling and modulating intestinal microflora. Sci. Rep. 2017, 7, 12041. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Miyazawa, M.; Kamei, A.; Abe, K.; Kojima, T. Ameliorative effects of mulberry (Morus alba L.) leaves on hyperlipidemia in rats fed a high-fat diet: Induction of fatty acid oxidation, inhibition of lipogenesis, and suppression of oxidative stress. Biosci. Biotechnol. Biochem. 2010, 74, 2385–2395. [Google Scholar] [CrossRef]
- Yang, N.-C.; Jhou, K.-Y.; Tseng, C.-Y. Antihypertensive effect of mulberry leaf aqueous extract containing γ-aminobutyric acid in spontaneously hypertensive rats. Food Chem. 2012, 132, 1796–1801. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, Y.X.; Chen, R.Y.; Kang, J. The latest review on the polyphenols and their bioactivities of Chinese Morus plants. J. Asian Nat. Prod. Res. 2014, 16, 690–702. [Google Scholar] [CrossRef]
- Akinwumi, B.C.; Bordun, K.A.M.; Anderson, H.D. Biological activities of stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef]
- Kim, Y.J.; Chung, S.O.; Kim, J.K.; Park, S.U. Recent studies on resveratrol and its biological and pharmacological activity. EXCLI J. 2017, 16, 602–608. [Google Scholar]
- Lorenz, P.; Roychowdhury, S.; Engelmann, M.; Wolf, G.; Horn, T.F. Oxyresveratrol and resveratrol are potent antioxidants and free radical scavengers: Effect on nitrosative and oxidative stress derived from microglial cells. Nitric Oxide 2003, 9, 64–76. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Tien, Y.-J.; Chen, C.-H.; Beltran, F.N.; Amor, E.C.; Wang, R.-J.; Wu, D.-J.; Mettling, C.; Lin, Y.-L.; Yang, W.-C. Morus alba and active compound oxyresveratrol exert anti-inflammatory activity via inhibition of leukocyte migration involving MEK/ERK signaling. BMC Complement. Altern. Med. 2013, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Jo, H.; Kim, J.-K.; Lim, Y.-H. Oxyresveratrol-containing Ramulus mori ethanol extract attenuates acute colitis by suppressing inflammation and increasing mucin secretion. J. Funct. Foods 2017, 35, 146–158. [Google Scholar] [CrossRef]
- Sasivimolphan, P.; Lipipun, V.; Likhitwitayawuid, K.; Takemoto, M.; Pramyothin, P.; Hattori, M.; Shiraki, K. Inhibitory activity of oxyresveratrol on wild-type and drug-resistant varicella-zoster virus replication in vitro. Antivir. Res. 2009, 84, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.Y.; Jeon, S.Y.; Nguyen, T.T.; Bae, K.; Song, K.S.; Seong, Y.H. Neuroprotective effect of oxyresveratrol from Smilacis chinae rhizome on amyloid Beta protein (25–35)-induced neurotoxicity in cultured rat cortical neurons. Biol. Pharm. Bull. 2006, 29, 2419–2424. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, K.-W.; Cho, C.-H.; He, Z.; Wang, M. Oxyresveratrol as an antibrowning agent for cloudy apple juices and fresh-cut apples. J. Agric. Food Chem. 2007, 55, 2604–2610. [Google Scholar] [CrossRef]
- Shin, N.H.; Ryu, S.Y.; Choi, E.J.; Kang, S.H.; Chang, I.M.; Min, K.R.; Kim, Y. Oxyresveratrol as the potent inhibitor on dopa oxidase activity of mushroom tyrosinase. Biochem. Biophys. Res. Commun. 1998, 243, 801–803. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, K.T.; Lee, H.S.; Kim, M.; Lim, Y.H. Evaluation of the inhibition of mushroom tyrosinase and cellular tyrosinase activities of oxyresveratrol: Comparison with mulberroside A. J. Enzym. Inhib. Med. Chem. 2012, 27, 495–503. [Google Scholar] [CrossRef]
- Kim, Y.M.; Yun, J.; Lee, C.-K.; Lee, H.; Min, K.R.; Kim, Y. Oxyresveratrol and hydroxystilbene compounds: Inhibitory effect on tyrosinase and mechanism of action. J. Biol. Chem. 2002, 277, 16340–16344. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, M.; Cho, S.G.; Kim, M.K.; Kim, S.W.; Lim, Y.H. Biotransformation of mulberroside A from Morus alba results in enhancement of tyrosinase inhibition. J. Ind. Microbiol. Biotechnol. 2010, 37, 631–637. [Google Scholar] [CrossRef]
- Guo, F.; Zou, Y.; Zheng, Y. Moracin M inhibits lipopolysaccharide-induced inflammatory responses in nucleus pulposus cells via regulating PI3K/Akt/mTOR phosphorylation. Int. Immunopharmacol. 2018, 58, 80–86. [Google Scholar] [CrossRef]
- Chen, S.K.; Zhao, P.; Shao, Y.X.; Li, Z.; Zhang, C.; Liu, P.; He, X.; Luo, H.B.; Hu, X. Moracin M from Morus alba L. is a natural phosphodiesterase-4 inhibitor. Bioorg. Med. Chem. Lett. 2012, 22, 3261–3264. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, M.; Nagao, S.; Ueno, S.; Masamune, T.; Shirata, A.; Takahashi, K. Moracin C and D, new phytoalexins from diseased mulberry. Chem. Lett. 1978, 7, 1239–1240. [Google Scholar] [CrossRef]
- Yao, X.; Wu, D.; Dong, N.; Ouyang, P.; Pu, J.; Hu, Q.; Wang, J.; Lu, W.; Huang, J. Moracin C, a phenolic compound isolated from Artocarpus heterophyllus, suppresses lipopolysaccharide-activated inflammatory responses in murine Raw264.7 macrophages. Int. J. Mol. Sci. 2016, 17, 1199. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Sohn, M.J.; Kim, W.G. Chalcomoracin and moracin C, new inhibitors of Staphylococcus aureus enoyl-acyl carrier protein reductase from Morus alba. Biol. Pharm. Bull. 2012, 35, 791–795. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zelefack, F.; Guilet, D.; Valentin, A.; Fongang, R.C.S.; Kom, B.; Chevalley, S.; Ngouela, S.A.; Tsamo, E.; Fabre, N.; Dijoux-Franca, M.G. Antiplasmodial and cytotoxic activities of flavonoids and arylbenzofuran derivatives from Morus mesozygia. Greener J. Biol. Sci. 2012, 2, 20–24. [Google Scholar]
- Ferlinahayati; Syah, Y.M.; Juliawaty, L.D.; Achmad, S.A.; Hakim, E.H.; Takayama, H.; Said, I.M.; Latip, J. Phenolic constituents from the wood of Morus australis with cytotoxic activity. Z. Naturforsch. C Biosci. 2008, 63, 35–39. [Google Scholar] [CrossRef]
- Li, X.; Xie, H.; Zhan, R.; Chen, D. Effect of double bond position on 2-phenyl-benzofuran antioxidants: A comparative study of moracin C and iso-moracin C. Molecules 2018, 23, 754. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Wang, Y.; Zhang, Y. Bioassay-guided screening and isolation of α-glucosidase and tyrosinase inhibitors from leaves of Morus alba. Food Chem. 2012, 131, 617–625. [Google Scholar] [CrossRef]
- Takasugi, M.; Nagao, S.; Masamune, T.; Shirata, A.; Takahashi, K. Chalcomoracin, a natural Diels-Alder adduct from diseased mulberry. Chem. Lett. 1980, 9, 1573–1576. [Google Scholar] [CrossRef]
- Fukai, T.; Oku, Y.; Hano, Y.; Terada, S. Antimicrobial activities of hydrophobic 2-arylbenzofurans and an isoflavone against vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. Planta Med. 2004, 70, 685–687. [Google Scholar] [CrossRef]
- Fukai, T.; Kaitou, K.; Terada, S. Antimicrobial activity of 2-arylbenzofurans from Morus species against methicillin-resistant Staphylococcus aureus. Fitoterapia 2005, 76, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.J.; Tang, Y.B.; Chen, R.Y.; Yu, D.Q. Three new cytotoxic Diels-Alder-type adducts from Morus australis. Chem. Biodivers. 2007, 4, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Chou, C.-C.; Li, R.; Liu, J.; Zhang, L.; Zhu, W.; Hu, J.; Yang, B.; Tian, J. Chalcomoracin is a potent anticancer agent acting through triggering oxidative stress via a mitophagy- and paraptosis-dependent mechanism. Sci. Rep. 2018, 8, 9566. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.Y.; Wan, Y.; Yao, X.H.; Zhao, W.G.; Hu, R.Z.; Chen, C.; Li, L.; Zhang, D.Y.; Wu, G.H. Effect of different planting areas on the chemical compositions and hypoglycemic and antioxidant activities of mulberry leaf extracts in Southern China. PLoS ONE 2018, 13, e0198072. [Google Scholar] [CrossRef]
- Lou, D.S.; Zou, F.M.; Yan, H.; Gui, Z.Z. Factors influencing the biosynthesis of 1-deoxynojirimycin in Morus alba L. Afr. J. Agric. Res. 2011, 6, 2998–3006. [Google Scholar]
- Wang, R.; Chen, R.; Li, J.; Liu, X.; Xie, K.; Chen, D.; Yin, Y.; Tao, X.; Xie, D.; Zou, J.; et al. Molecular characterization and phylogenetic analysis of two novel regio-specific flavonoid prenyltransferases from Morus alba and Cudrania tricuspidata. J. Biol. Chem. 2014, 289, 35815–35825. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhu, W.; Liu, S.; Guan, Q.; Chen, X.; Huang, W.; Wang, T.; Yang, B.; Tian, J. Molecular characterization of a geranyl diphosphate-specific prenyltransferase catalyzing stilbenoid prenylation from Morus alba. Plant Cell Physiol. 2018, 59, 2214–2227. [Google Scholar] [CrossRef]
- Guillon, S.; Trémouillaux-Guiller, J.; Pati, P.K.; Rideau, M.; Gantet, P. Hairy root research: Recent scenario and exciting prospects. Curr. Opin. Plant Biol. 2006, 9, 341–346. [Google Scholar] [CrossRef]
- Gutierrez-Valdes, N.; Häkkinen, S.T.; Lemasson, C.; Guillet, M.; Oksman-Caldentey, K.-M.; Ritala, A.; Cardon, F. Hairy root cultures-A versatile tool with multiple applications. Front. Plant Sci. 2020, 11, 33. [Google Scholar] [CrossRef]
- Fang, L.; Yang, T.; Medina-Bolivar, F. Production of prenylated stilbenoids in hairy root cultures of peanut (Arachis hypogaea) and its wild relatives A. ipaensis and A. duranensis via an optimized elicitation procedure. Molecules 2020, 25, 509. [Google Scholar] [CrossRef]
- Nilsson, O.; Olsson, O. Getting to the root: The role of the Agrobacterium rhizogenes rol genes in the formation of hairy roots. Physiol. Plant. 1997, 100, 463–473. [Google Scholar] [CrossRef]
- Jouanin, L.; Guerche, P.; Pamboukdjian, N.; Tourneur, C.; Delbart, F.C.; Tourneur, J. Structure of T-DNA in plants regenerated from roots transformed by Agrobacterium rhizogenes strain A4. Mol. Gen. Genet. 1987, 206, 387–392. [Google Scholar] [CrossRef]
- Jeon, Y.H.; Choi, S.W. Isolation, identification, and quantification of tyrosinase and α-glucosidase inhibitors from UVC-irradiated mulberry (Morus alba L.) leaves. Prev. Nutr. Food Sci. 2019, 24, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; He, Z.D.; Jiang, R.W.; Ye, W.C.; Xu, H.X.; Pui-Hay But, P. Antiviral flavonoids from the root bark of Morus alba L. Phytochemistry 2003, 62, 1235–1238. [Google Scholar] [CrossRef]
- Singab, A.N.; El-Beshbishy, H.A.; Yonekawa, M.; Nomura, T.; Fukai, T. Hypoglycemic effect of Egyptian Morus alba root bark extract: Effect on diabetes and lipid peroxidation of streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2005, 100, 333–338. [Google Scholar] [CrossRef]
- Yang, T.; Fang, L.; Sanders, S.; Jayanthi, S.; Rajan, G.; Podicheti, R.; Medina-Bolivar, F. Stilbenoid prenyltransferases define key steps in the diversification of peanut phytoalexins. J. Biol. Chem. 2018, 293, 28–46. [Google Scholar] [CrossRef]
- Gu, X.D.; Sun, M.Y.; Zhang, L.; Fu, H.W.; Cui, L.; Chen, R.Z.; Zhang, D.W.; Tian, J.K. UV-B induced changes in the secondary metabolites of Morus alba L. leaves. Molecules 2010, 15, 2980–2993. [Google Scholar] [CrossRef]
- Giri, C.C.; Zaheer, M. Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: Recent trends and a sky eye view appraisal. Plant Cell Tissue Organ Cult. 2016, 126, 1–18. [Google Scholar] [CrossRef]
- Yang, T.; Fang, L.; Nopo-Olazabal, C.; Condori, J.; Nopo-Olazabal, L.; Balmaceda, C.; Medina-Bolivar, F. Enhanced production of resveratrol, piceatannol, arachidin-1, and arachidin-3 in hairy root cultures of peanut co-treated with methyl jasmonate and cyclodextrin. J. Agric. Food Chem. 2015, 63, 3942–3950. [Google Scholar] [CrossRef]
- Sharma, A.R.; Gajurel, G.; Ahmed, I.; Roedel, K.; Medina-Bolivar, F. Induction of the prenylated stilbenoids arachidin-1 and arachidin-3 and their semi-preparative separation and purification from hairy root cultures of peanut (Arachis hypogaea L.). Molecules 2022, 27, 6118. [Google Scholar] [CrossRef]
- Gajurel, G.; Hasan, R.; Medina-Bolivar, F. Antioxidant assessment of prenylated stilbenoid-rich extracts from elicited hairy root cultures of three cultivars of peanut (Arachis hypogaea). Molecules 2021, 26, 6778. [Google Scholar] [CrossRef] [PubMed]
- Gajurel, G.; Nopo-Olazabal, L.; Hendrix, E.; Medina-Bolivar, F. Production and secretion of isowighteone in hairy root cultures of pigeon pea (Cajanus cajan) co-treated with multiple elicitors. Plants 2022, 11, 834. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Tewary, P.K. Induction of hairy roots from hypocotyls of mulberry (Morus indica L.) by Japanese wild strains of Agrobacterium rhizogenes. J. Sericult. Sci. Jpn. 2000, 69, 13–19. [Google Scholar]
- Nguyen, H.T.M.; Tran, T.Q.; Bui, A.L.; Quach, P.N.D. Hairy root culture of white mulberry (Morus alba L.) for a source of tyrosinase inhibitors. J. Biol. Res. 2020, 93, 8611. [Google Scholar] [CrossRef]
- Jinhong, Y. Establishment of plantlet regeneration conditions for mulberry hairy root. J. Henan Agric. Sci. 2013, 42, 113–115. [Google Scholar]
- Inyai, C.; Yusakul, G.; Komaikul, J.; Kitisripanya, T.; Likhitwitayawuid, K.; Sritularak, B.; Putalun, W. Improvement of stilbene production by mulberry Morus alba root culture via precursor feeding and co-elicitation. Bioprocess Biosyst. Eng. 2021, 44, 653–660. [Google Scholar] [CrossRef]
- Wang, C.; Zhi, S.; Liu, C.; Xu, F.; Zhao, A.; Wang, X.; Ren, Y.; Li, Z.; Yu, M. Characterization of stilbene synthase genes in mulberry (Morus atropurpurea) and metabolic engineering for the production of resveratrol in Escherichia coli. J. Agric. Food Chem. 2017, 65, 1659–1668. [Google Scholar] [CrossRef]
- Wei, H.; Zhu, J.J.; Liu, X.Q.; Feng, W.H.; Wang, Z.M.; Yan, L.H. Review of bioactive compounds from root barks of Morus plants (Sang-Bai-Pi) and their pharmacological effects. Cogent Chem. 2016, 2, 1212320. [Google Scholar] [CrossRef]
- Kamal, M.; Shakya, K.A.; Jawaid, T. Benzofurans: A new profile of biological activities. Int. J. Med. Pharm. Sci. 2011, 1, 1–15. [Google Scholar]
- Miao, Y.-H.; Hu, Y.-H.; Yang, J.; Liu, T.; Sun, J.; Wang, X.-J. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019, 9, 27510–27540. [Google Scholar] [CrossRef]
- Yang, T.; Fang, L.; Rimando, A.M.; Sobolev, V.; Mockaitis, K.; Medina-Bolivar, F. A stilbenoid-specific prenyltransferase utilizes dimethylallyl pyrophosphate from the plastidic terpenoid pathway. Plant Physiol. 2016, 171, 2483–2498. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Condori, J.; Sivakumar, G.; Hubstenberger, J.; Dolan, M.C.; Sobolev, V.S.; Medina-Bolivar, L.F. Induced biosynthesis of resveratrol and the prenylated stilbenoids arachidin-1 and arachidin-3 in hairy root cultures of peanut: Effects of culture medium and growth stage. Plant Physiol. Biochem. 2010, 48, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Medina-Bolivar, F.; Condori, J.; Rimando, A.M.; Hubstenberger, J.; Shelton, K.; O’Keefe, S.F.; Bennett, S.; Dolan, M.C. Production and secretion of resveratrol in hairy root cultures of peanut. Phytochemistry 2007, 68, 1992–2003. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, L.; Sharma, A.R.; Aniemena, C.; Roedel, K.; Henry, F.; Moussou, P.; Samuga, A.; Medina-Bolivar, F. Elicitation of Stilbenes and Benzofuran Derivatives in Hairy Root Cultures of White Mulberry (Morus alba). Plants 2023, 12, 175. https://doi.org/10.3390/plants12010175
Fang L, Sharma AR, Aniemena C, Roedel K, Henry F, Moussou P, Samuga A, Medina-Bolivar F. Elicitation of Stilbenes and Benzofuran Derivatives in Hairy Root Cultures of White Mulberry (Morus alba). Plants. 2023; 12(1):175. https://doi.org/10.3390/plants12010175
Chicago/Turabian StyleFang, Lingling, Amit Raj Sharma, Chineche Aniemena, Krystian Roedel, Florence Henry, Philippe Moussou, Anita Samuga, and Fabricio Medina-Bolivar. 2023. "Elicitation of Stilbenes and Benzofuran Derivatives in Hairy Root Cultures of White Mulberry (Morus alba)" Plants 12, no. 1: 175. https://doi.org/10.3390/plants12010175
APA StyleFang, L., Sharma, A. R., Aniemena, C., Roedel, K., Henry, F., Moussou, P., Samuga, A., & Medina-Bolivar, F. (2023). Elicitation of Stilbenes and Benzofuran Derivatives in Hairy Root Cultures of White Mulberry (Morus alba). Plants, 12(1), 175. https://doi.org/10.3390/plants12010175